Molecular Evaluation of Kyoho Grape Leaf and Berry Characteristics Influenced by Different NPK Fertilizers
Abstract
:1. Introduction
2. Results
2.1. Fruit Biochemical Attributes
2.2. Yield Parameters
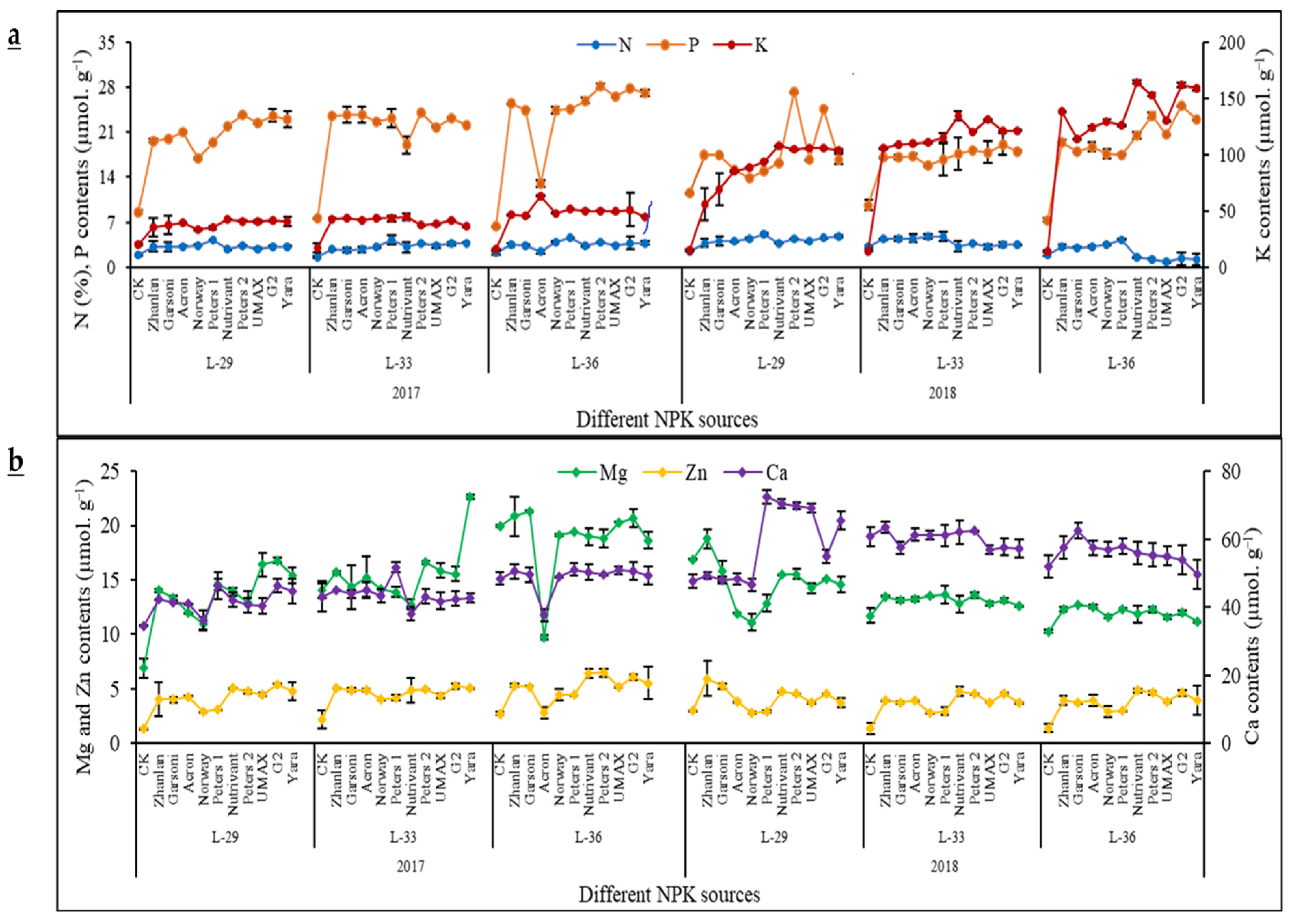
2.3. Macro- and Micro-Nutrients Contents in Grape Leaves
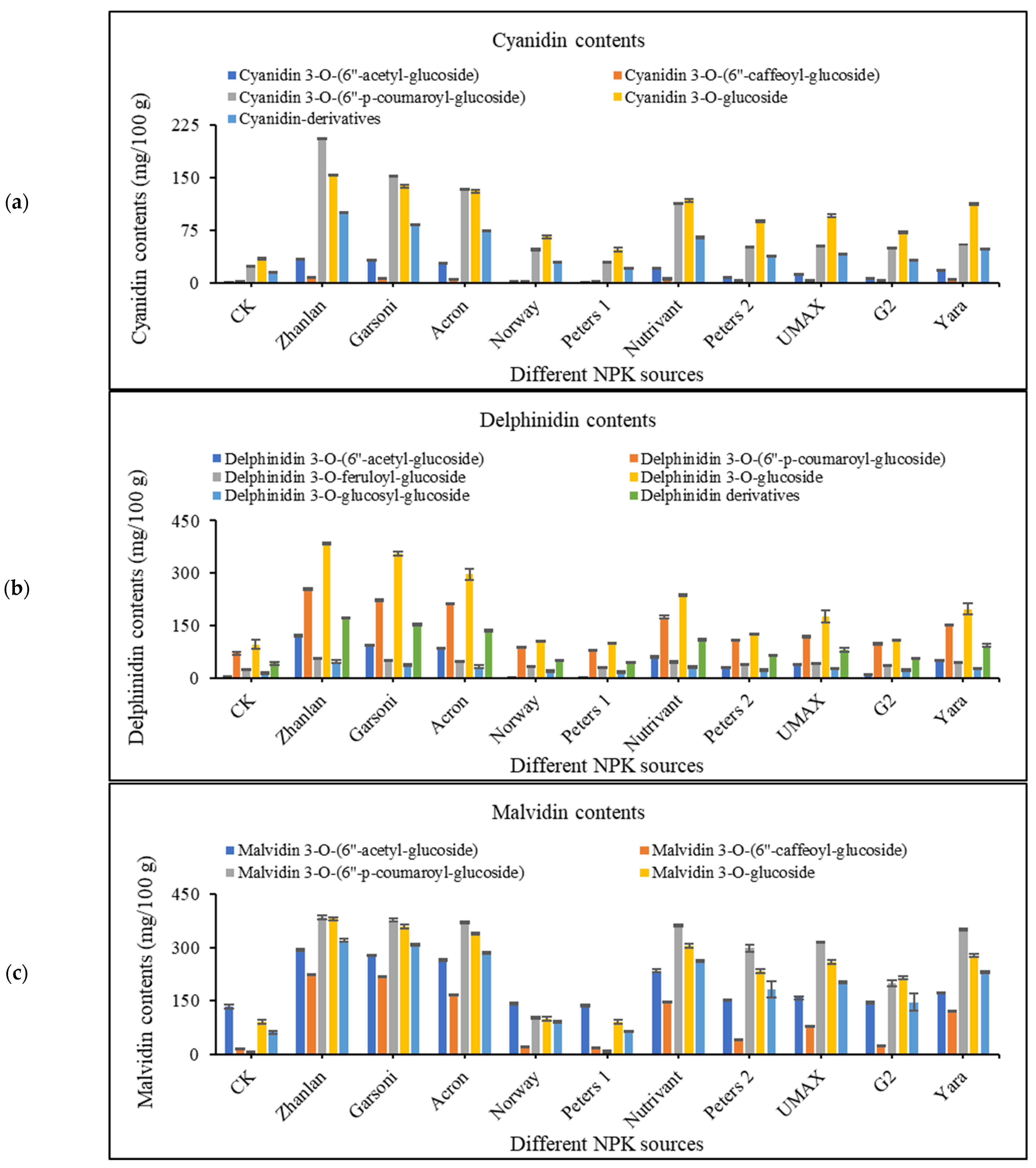
2.4. Anthocyanin Contents in Grape Peel
2.4.1. Cyanidin Contents
2.4.2. Delphinidin Contents
2.4.3. Malvidin Contents
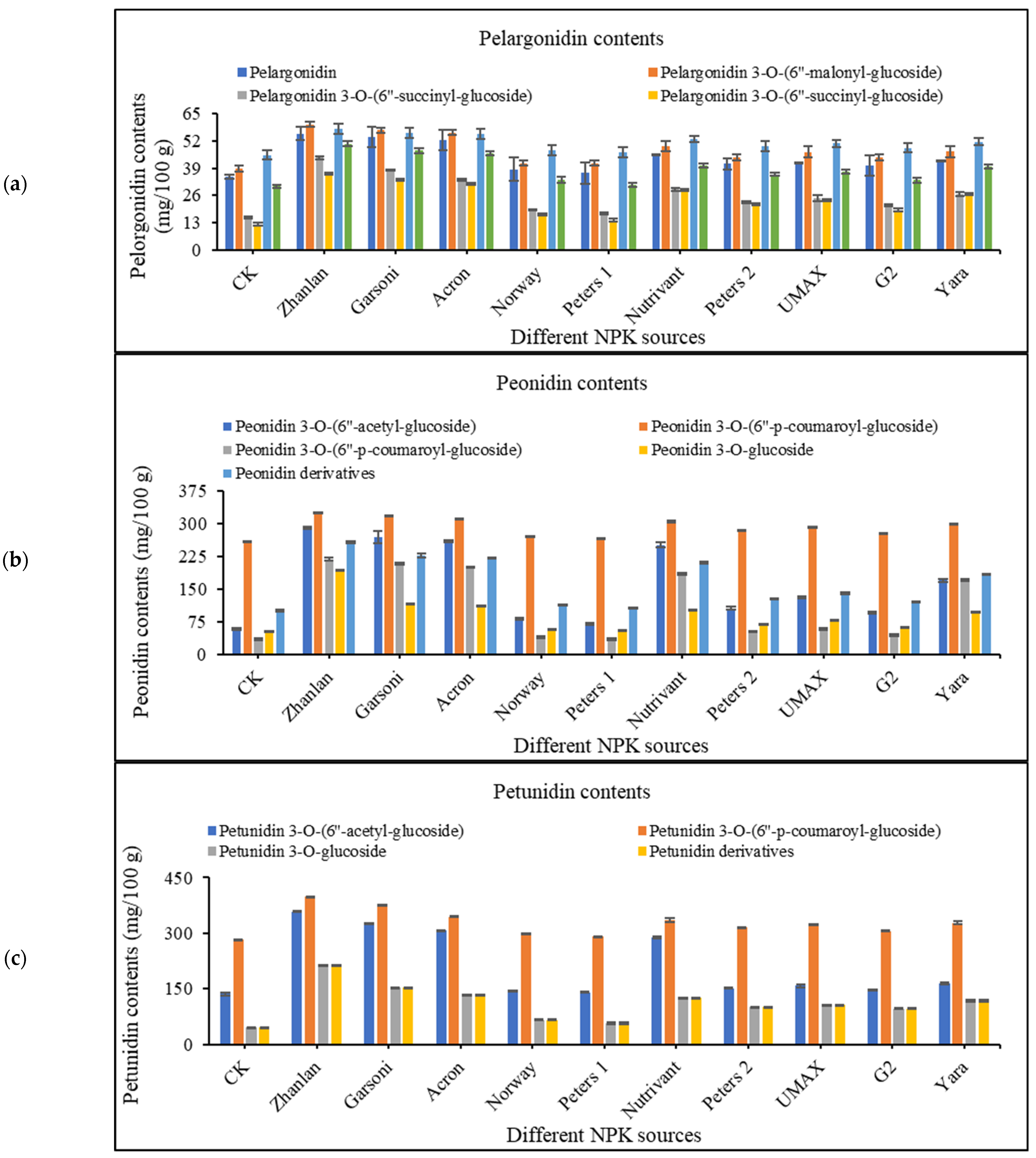
2.4.4. Pelargonidin Contents
2.4.5. Peonidin Contents
2.4.6. Petunidin Contents
2.5. Relative Expression of Different Genes Understudy
2.5.1. Relative Expression of Nitrogen Pathway Genes
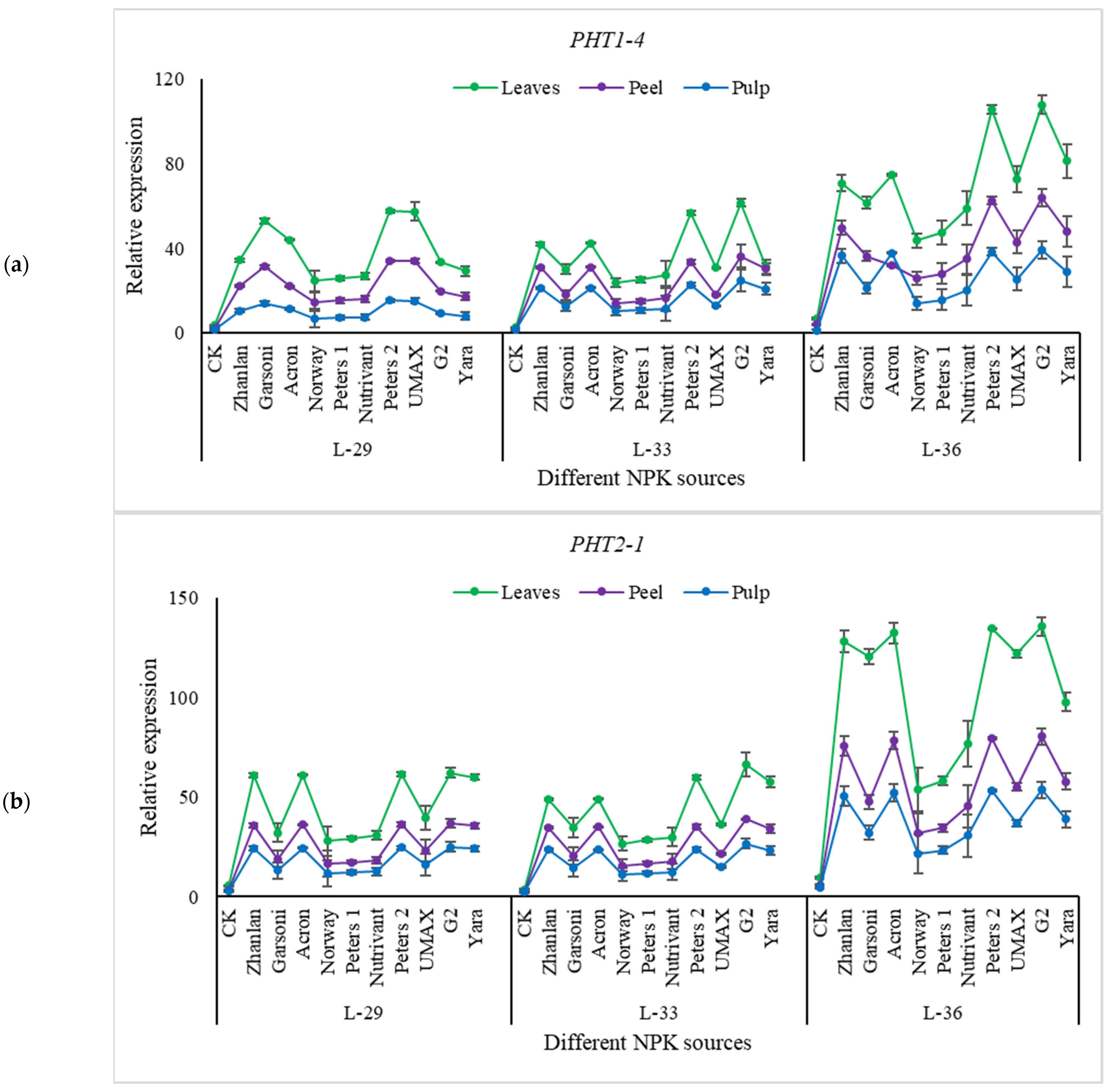
2.5.2. Relative Expression of Phosphorus Pathway Genes
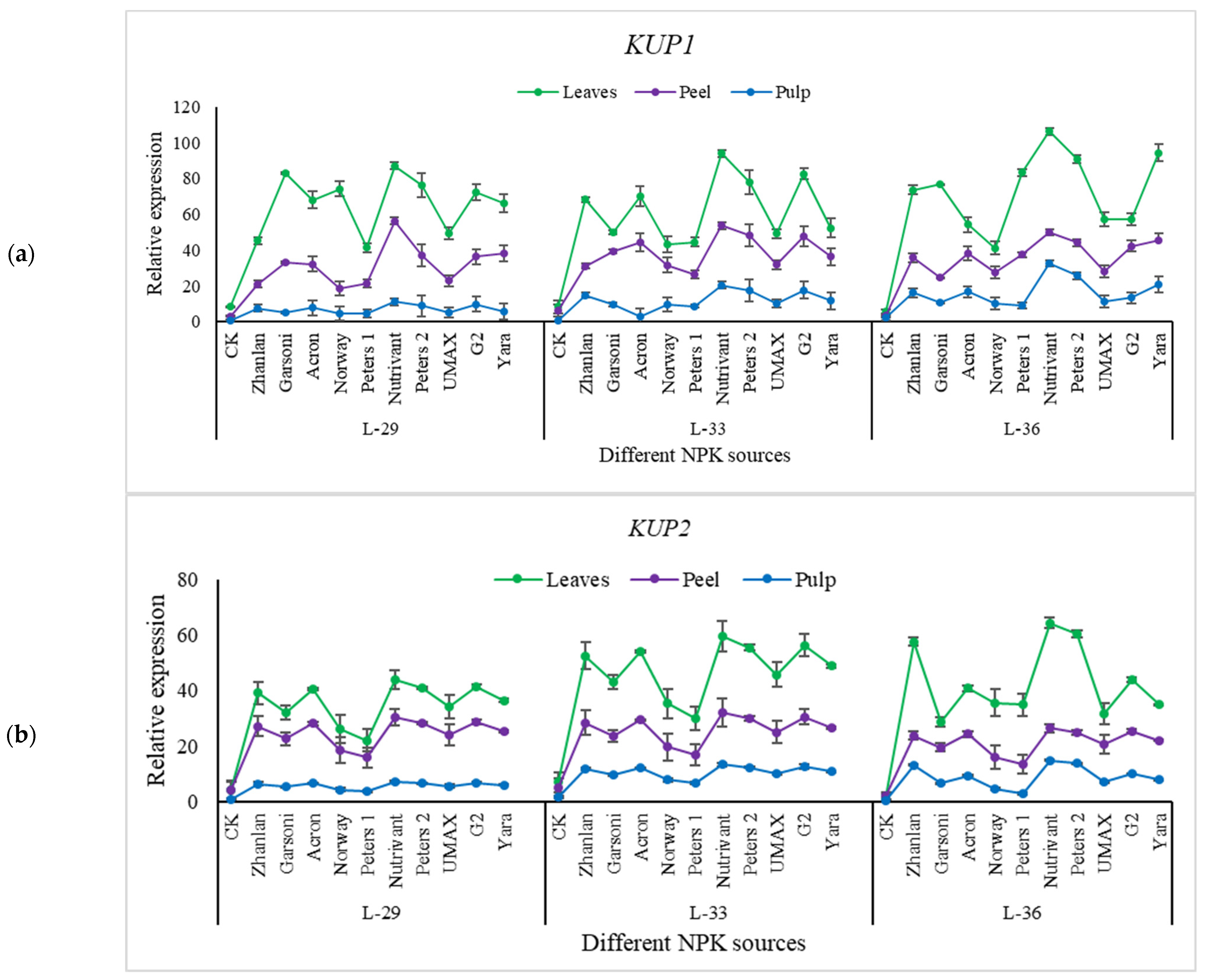
2.5.3. Relative Expression of Potassium Pathway Genes
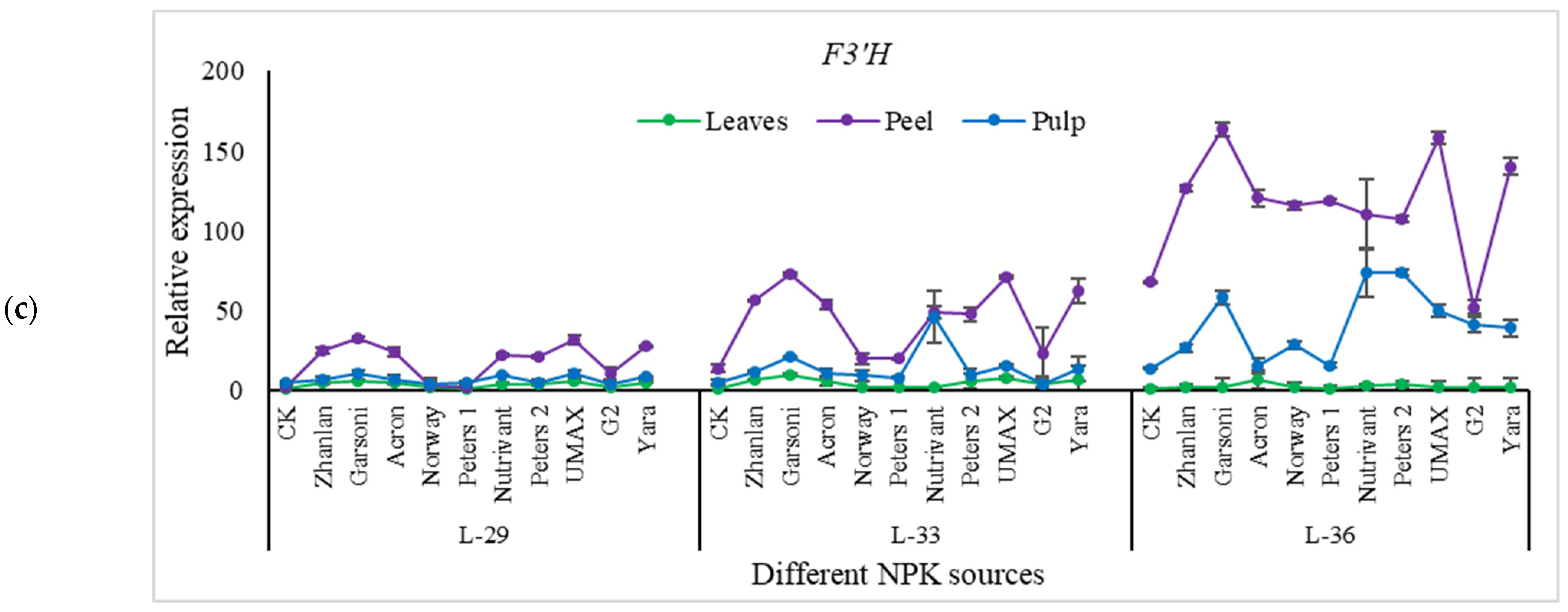
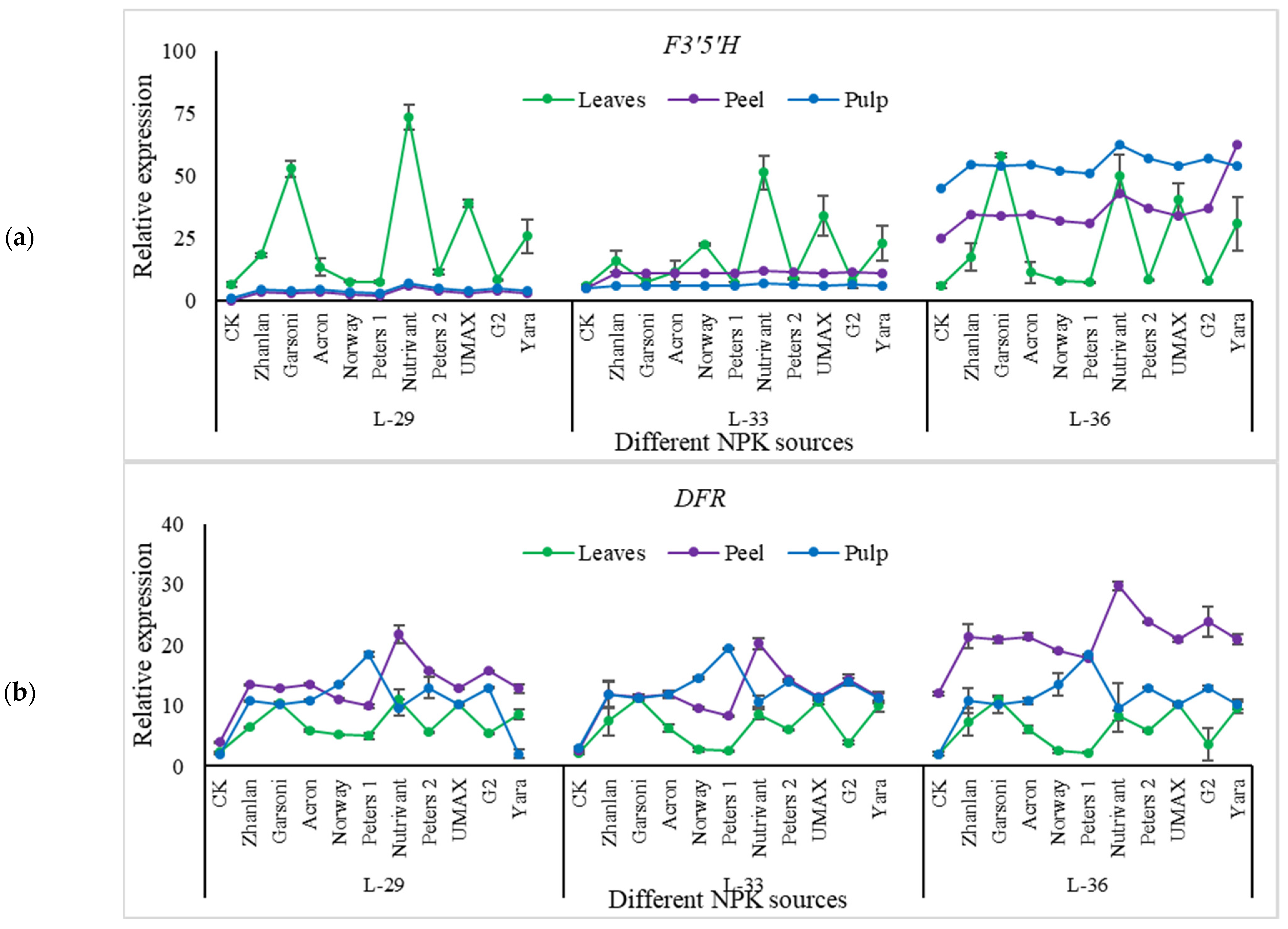
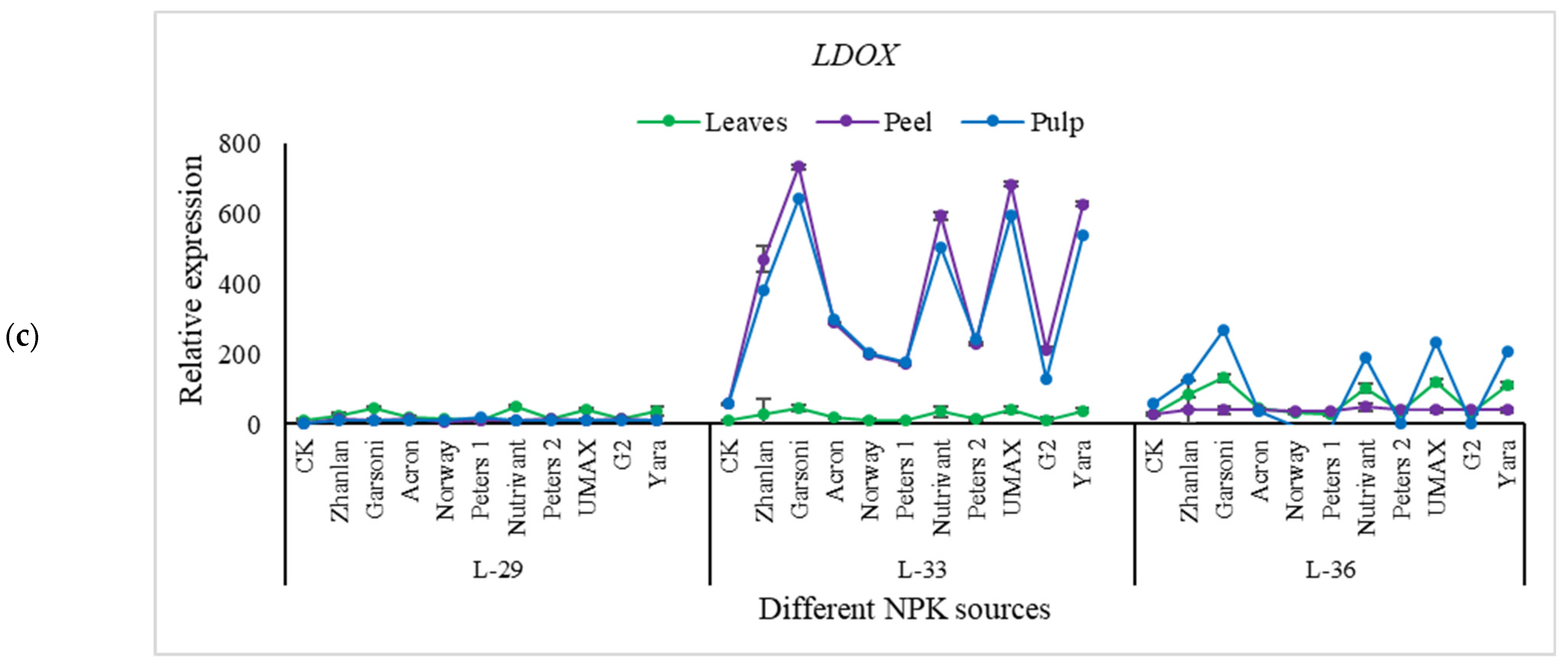
2.5.4. Relative Expressions of Anthocyanin Structural Genes
3. Discussion
3.1. Various NPK Sources
3.2. Chlorophyll and Carotenoids Contents
3.3. Leaf Macro- and Micro-Nutrients Levels
3.4. Berry Yield
3.5. Fruit Biochemical Attributes
3.6. Anthocyanin Contents
3.7. Anthocyanin and NPK Pathway Genes
4. Materials and Methods
4.1. Experimental Design
4.2. Yield Components
4.3. Nutrients Determination in Leaves (ICP-OES)
4.4. Nitrogen Determination in Leaves
4.5. Biochemical Parameters
4.6. Extraction of Fruit Skin’s Individual Anthocyanins by UPLC
4.7. Total RNA Isolation, cDNA Synthesis, and Gene Expression
4.7.1. RNA Isolation and cDNA Synthesis
4.7.2. qPCR and Gene Expression Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunetto, G.; Ceretta, C.A.; de Melo, G.W.B.; Girotto, E.; Ferreira, P.A.A.; Lourenzi, C.R. Contribution of nitrogen from urea applied at different rates and times on grapevine nutrition. Sci. Hortic. 2016, 207, 1–6. [Google Scholar] [CrossRef] [Green Version]
- FAO. World Fertilizer Trends and Outlook to 2020; Food and Agriculture Organization of United Nations: Rome, Italy, 2017; p. 38. [Google Scholar]
- Costa, L.D.; Malnoy, M.; Gribaudo, I. Breeding next generation tree fruits: Technical and legal challenges. Hortic. Res. 2017, 4, 1–11. [Google Scholar]
- Mccarty, J.P.; Zedler, J.B.; Munn, T. Restoration, Ecosystem Edited by Professor Harold A Mooney and Dr Josep G Canadell Restoration, Ecosystem. Earth 2002, 2, 532–539. [Google Scholar]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen Utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Hawke, M.A.; Maun, M.A. Some aspects of nitrogen, phosphorus, and potassium nutrition of three colonizing beach species. Can. J. Bot. 1988, 66, 1490–1496. [Google Scholar] [CrossRef]
- Zaman, M.; Kurepin, L.V.; Catto, W.; Pharis, R.P. Enhancing crop yield with the use of N-based fertilizers co-applied with plant hormones or growth regulators. J. Sci. Food Agric. 2014, 95, 1777–1785. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, G.; Chen, J. Effect of different fertilizations on the quantitative characters and yield of adzuki bean. J. Beijing Univ. Agric. 2012, 27, 4–6. [Google Scholar]
- Bethlenfalvay, G.J.; Pacovsky, R.S.; Bayne, H.G.; Stafford, A.E. Interactions between nitrogen fixation, mycorrhizal colonization, and host-plant growth in the Phaseolus-Rhizobium-Glomus symbiosis. Plant Physiol. 1982, 70, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Yanni, Y.G.; Rizk, R.Y.; Abd El-Fattah, F.K.; Squartini, A.; Corich, V.; Giacomini, A.; de Bruijn, F.; Rademaker, J.L.; Maya-Flores, J.; Ostrom, P.H.; et al. The beneficial plant growth-promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Funct. Plant Biol. 2001, 28, 845–870. [Google Scholar] [CrossRef]
- Hamman, R.A.; Dami, I.E. Effects of Irrigation on Wine Grape Growth and Fruit Quality. HortTechnology 2000, 10, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Webb, L.B.; Whetton, P.H.; Barlow, E.W.R. Potential impacts of projected greenhouse gas-induced climate change on Australian viticulture. Wine Ind. J. 2005, 21, 1–4. [Google Scholar]
- Stefanello, L.O.; Schwalbert, R.; Schwalbert, R.A.; De Conti, L.; de Souza Kulmann, M.S.; Garlet, L.P.; Silveira, M.L.R.; Sautter, C.K.; de Melo, G.W.B.; Rozane, D.E.; et al. Nitrogen supply method affects growth, yield and must composition of young grape vines (Vitis vinifera L. cv Alicante Bouschet) in southern Brazil. Sci. Hortic. 2020, 261, 108910. [Google Scholar] [CrossRef]
- Karimi, E.; Abdolzadeh, A.; Sadeghipour, H.R.; Aminei, A. The potential of glauconitic sandstone as a potassium fertilizer for olive plants. Arch. Agron. Soil Sci. 2012, 58, 983–993. [Google Scholar] [CrossRef]
- Zahoor, R.; Dong, H.; Abid, M.; Zhao, W.; Wang, Y.; Zhou, Z. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017, 137, 73–83. [Google Scholar] [CrossRef]
- Thalooth, A.T.; Tawfik, M.M.; Mohamed, H.M. A comparative study on the effect of foliar application of zinc, potassium and magnesium on growth, yield and some chemical constituents of mungbean plants grown under water stress conditions. World J. Agric. Sci. 2006, 2, 37–46. [Google Scholar]
- Jie, L. Effects of the different density and fertilizer application methods on the yield of mung bean. Hortic. Seed 2011, 6, 81–83. [Google Scholar]
- Gil, M.I.; Tudela, J.A.; Luna, M.C.; Allende, A. Water management and its effect on the postharvest quality of fresh-cut vegetables. Stewart Postharvest Rev. 2013, 9, 1–8. [Google Scholar]
- Zafar, Z.U.; Athar, H.-U.-R. Influence of different phosphorus regimes on disease resistance in two cotton (Gossypium hirsutum L.) cultivars differing in resistance to cotton leaf curl virus (clcuv). Pak. J. Bot. 2013, 45, 617–627. [Google Scholar]
- Jin, J.; Lauricella, D.; Armstrong, R.; Sale, P.; Tang, C. Phosphorus application and elevated CO2 enhance drought tolerance in field pea grown in a phosphorus-deficient vertisol. Ann. Bot. 2014, 116, 975–985. [Google Scholar] [CrossRef] [Green Version]
- Hansel, F.D.; Amado, T.J.; Diaz, D.A.R.; Rosso, L.H.; Nicoloso, F.T.; Schorr, M. Phosphorus Fertilizer Placement and Tillage Affect Soybean Root Growth and Drought Tolerance. Agron. J. 2017, 109, 2936–2944. [Google Scholar] [CrossRef] [Green Version]
- Mamedov, M.I. Effect of the fertilizing system on the yield and quality of grapes. Russ. Agric. Sci. 2015, 41, 434–436. [Google Scholar] [CrossRef]
- Wickert, E.; Marcondes, J.; Lemos, M.V.; Lemos, E.G. Nitrogen assimilation in Citrus based on CitEST data mining. Genet. Mol. Biol. 2007, 30, 810–818. [Google Scholar] [CrossRef]
- Wang, C.; Han, J.; Shangguan, L.; Yang, G.; Kayesh, E.; Zhang, Y.; Leng, X.; Fang, J. Depiction of Grapevine Phenology by Gene Expression Information and a Test of its Workability in Guiding Fertilization. Plant Mol. Biol. Rep. 2014, 32, 1070–1084. [Google Scholar] [CrossRef]
- Davies, C.; Shin, R.; Liu, W.; Thomas, M.R.; Schachtman, D. Transporters expressed during grape berry (Vitis vinifera L.) development are associated with an increase in berry size and berry potassium accumulation. J. Exp. Bot. 2006, 57, 3209–3216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanlauwe, B.; Kihara, J.; Chivenge, P.; Pypers, P.; Coe, R.; Six, J. Agronomic use efficiency of N fertilizer in maize-based systems in sub-Saharan Africa within the context of integrated soil fertility management. Plant Soil 2011, 339, 35–50. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, V.N.; Srivastav, L.K.; Banwasi, R. Evaluations of soil fertility status of available major nutrients (N, P & K) and micro nutrients (Fe, Mn, Cu & Zn) in Vertisol of Kabeerdham District of Chhattisgarh, India. Int. J. Interdiscip. Multidiscip. Stud. 2014, 1, 72–79. [Google Scholar]
- Rusinamhodzi, L.; Corbeels, M.; Zingore, S.; Nyamangara, J.; Giller, K. Pushing the envelope? Maize production intensification and the role of cattle manure in recovery of degraded soils in smallholder farming areas of Zimbabwe. Field Crop. Res. 2013, 147, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Hua, H.M. Studies on effect of multiple factors of nitrogen, phosphorus and potassium in small red bean. Sci. Technol. Tianjin Agric. For. 1993, 2, 3–5. [Google Scholar]
- Wu, D.; Qin, L.; Zou, Y.; Mou, H.; Zhang, J.; Wei, S.; Li, X.; Lin, G. Influences of different fertilizer treatments on the yield and quality of wild grape variety Yeniang 2 (Vitis quinquangularis Rehd.). J. South. Agric. 2013, 44, 96–100. [Google Scholar]
- Delgado, R.; Martín, P.; Del Álamo, M.; González, M. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Bhargava, B.S.; Sumner, M.E. Proposal for sampling grape (Vitis vinifera L.) petioles for nutritional diagnosis. Commun. Soil Sci. Plant Anal. 1987, 18, 581–591. [Google Scholar] [CrossRef]
- Li, S.J.; Zhong, Z.; Liu, X.R.; Xie, J.Z.; Li, G.X. Studies on the standard fertilizing formula and specific fertilizers for apple trees in organic orchards. J. Fruit Sci. 2007, 6, 030. [Google Scholar]
- Marangoni, B.; Toselli, M.; Venturi, A.; Fontana, M.; Scudellari, D. Effects of vineyard soil management and fertilization on grape diseases and wine quality. IOBC WPRS Bull. 2001, 24, 353–358. [Google Scholar]
- Romero, I.; García-Escudero, E.; Martín, I. Effects of Leaf Position on Blade and Petiole Mineral Nutrient Concentration of Tempranillo Grapevine (Vitis vinifera L.). Am. J. Enol. Vitic. 2010, 61, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, S.J.; Pickering, G.J. Optimizing grape quality through soil management practices. J. Food Agric. Environ. 2003, 1, 190–197. [Google Scholar]
- Soubeyrand, E.; Basteau, C.; Hilbert, G.; van Leeuwen, C.; Delrot, S.; Gomès, E. Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv. Cabernet-Sauvignon berries. Phytochemistry 2014, 103, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey Iii, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Bojović, B.; Stojanović, J. Some wheat leaf characteristics in dependence of fertilization. Kragujev. J. Sci. 2006, 28, 139–146. [Google Scholar]
- Waraich, E.A.; Ahmad, Z.; Ahmad, R.; Ullah, S.; Ashraf, M.Y. Foliar Applied Phosphorous Enhanced Growth, Chlorophyll Contents, Gas Exchange Attributes and PUE in Wheat (Triticum aestivum L.). J. Plant Nutr. 2015, 38, 1929–1943. [Google Scholar] [CrossRef]
- Shubhra, S.; Dayal, J.; Goswami, C.; Munjal, R. Influence of Phosphorus Application on Water Relations, Biochemical Parameters and Gum Content in Cluster Bean Under Water Deficit. Biol. Plant. 2004, 48, 445–448. [Google Scholar] [CrossRef]
- Dutt, S.; Sharma, S.D.; Kumar, P. Inoculation of apricot seedlings with indigenous arbuscular mycorrhizal fungi in optimum phosphorus fertilization for quality growth attributes. J. Plant Nutr. 2013, 36, 15–31. [Google Scholar] [CrossRef]
- Çelekli, A.; Yavuzatmaca, M.; Bozkurt, H. Modeling of biomass production by Spirulina platensis as function of phosphate concentrations and pH regimes. Bioresour. Technol. 2009, 100, 3625–3629. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, B.P.R.; Singh, P.K. Effect of phosphorus and nitrogen on growth, chlorophyll, amino nitrogen, soluble sugar contents and algal heterocysts of water fern Azolla pinnata. Biol. Plant. 1979, 21, 401–406. [Google Scholar] [CrossRef]
- Liang, X.-L.; Lin, Y.-C.; Nian, H.; Xie, L.-X. The effect of low phosphorus stress on main physiological traits of different maize genotypes. Acta Agron. Sin. 2005, 5, 667–669. [Google Scholar]
- Barlow, K.; Bond, W.; Holzapfel, B.; Smith, J.; Hutton, R. Nitrogen concentrations in soil solution and surface run-off on irrigated vineyards in Australia. Aust. J. Grape Wine Res. 2009, 15, 131–143. [Google Scholar] [CrossRef]
- Bravo, K.; Toselli, M.; Baldi, E.; Marcolini, G.; Sorrenti, G.; Quartieri, M.; Marangoni, B. Effect of organic fertilization on carbon assimilation and partitioning in bearing nectarine trees. Sci. Hortic. 2012, 137, 100–106. [Google Scholar] [CrossRef]
- El-Khawaga, A.S.; Maklad, M.F. Effect of combination between Bio and chemical fertilization on vegetative growth, yield and quality of Valencia orange fruits. Hortsci. J. Suez Canal Univ. 2013, 1, 269–279. [Google Scholar]
- Ernani, P.R.; Rogeri, D.A.; Proença, M.M.; Dias, J. Addition of nitrogen had no effect on yield and quality of apples in an high density orchard carrying a dwarf rootstock. Rev. Bras. Frutic. 2008, 30, 1113–1118. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.H.; Tai, T.N. Effects of Combination between Nitrogen and Potassium Fertilization on Yield and Quality of Valencia Orange Fruits. J. Agric. Sci. 2019, 12, 38. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Razek, E.; Treutter, D.; Saleh, M.M.S.; El-Shammaa, M.; Abdel-Hamid, N.; Abou-Rawash, M. Effect of nitrogen and potassium fertilization on productivity and fruit quality of ‘crimson seedless’ grape. Agric. Biol. J. N. Am. 2011, 2, 330–340. [Google Scholar] [CrossRef]
- Ramming, D.W.; Tarailo, R.; Badr, S.A. ‘Crimson Seedless’: A New Late-maturing, Red Seedless Grape. HortScience 1995, 30, 1473–1474. [Google Scholar] [CrossRef] [Green Version]
- Guilpart, N.; Metay, A.; Gary, C. Grapevine bud fertility and number of berries per bunch are determined by water and nitrogen stress around flowering in the previous year. Eur. J. Agron. 2014, 54, 9–20. [Google Scholar] [CrossRef]
- Rahimi, A.; Kordlaghari, K.P.; Kelidari, A. Effects of nitrogen and phosphorus fertilizers on yield and yield components of bean (Phaseolus vulgaris L.) grown in Boyerahmad region of Iran. Res. Crops 2012, 13, 118–122. [Google Scholar]
- Fageria, N.K.; Melo, L.C. Agronomic Evaluation of Dry Bean Genotypes for Potassium Use Efficiency. J. Plant Nutr. 2014, 37, 1899–1912. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.P.B.; Da Costa, J.G.C. Potassium-use efficiency in common bean genotypes. J. Plant Nutr. 2001, 24, 1937–1945. [Google Scholar] [CrossRef]
- Hu, H.; Xiao, J.; Guo, X.; Jie, X.; Liu, S.; Hua, D.; Lu, J.; Liu, F. Effect of N, P and K fertilization on yield and nutrient quality of alfalfa in Yellow River beach regions. J. Hunan Agric. Univ. 2009, 35, 178–188. [Google Scholar]
- Yin, Z.-C.; Guo, W.-Y.; Liang, J.; Xiao, H.-Y.; Hao, X.-Y.; Hou, A.-F.; Zong, X.-X.; Leng, T.-R.; Wang, Y.-J.; Wang, Q.-Y.; et al. Effects of multiple N, P, and K fertilizer combinations on adzuki bean (Vigna angularis) yield in a semi-arid region of northeastern China. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Beltran, G.; Esteve-Zarzoso, B.; Rozès, N.; Mas, A.; Guillamón, J.M. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 2005, 53, 996–1002. [Google Scholar] [CrossRef]
- Delgado, R.; María, R.G.; Martin, P. Interaction effects of nitrogen and potassium fertilization on anthocyanin composition and chromatic features of Tempranillo grapes. OENO One 2006, 40, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Curtin, C.; Franco, C. Towards manipulation of post-biosynthetic events in secondary metabolism of plant cell cultures. Enzym. Microb. Technol. 2002, 30, 688–696. [Google Scholar] [CrossRef]
- Hilbert, G.; Soyer, J.P.; Molot, C.; Giraudon, J.; Milin, M.; Gaudillere, J.P. Effects of nitrogen supply on must quality and anthocyanin accumulation in berries of cv. Merlot. VITIS-J. Grapevine Res. 2015, 42, 69. [Google Scholar]
- Shehata, W.; Aldaej, M.I.; Alturki, S.M.; Ghazzawy, H. Effect of Ammonium Nitrate on Antioxidants Production of Date Palm (Phoenix dactylifera L.) in vitro. Biotechnology 2014, 13, 116–125. [Google Scholar] [CrossRef]
- Zahedzadeh, F.; Kakavand, F.; Mahna, N. Effects of carbohydrate, light, nitrogen and magnesium on in vitro production of anthocyanin in apple. Int. J. Biosci. 2015, 6, 250–260. [Google Scholar]
- Dai, Z.; Ollat, N.; Gomès, E.; Decroocq, S.; Tandonnet, J.-P.; Bordenave, L.; Pieri, P.; Hilbert, G.; Kappel, C.; van Leeuwen, C.; et al. Ecophysiological, Genetic, and Molecular Causes of Variation in Grape Berry Weight and Composition: A Review. Am. J. Enol. Vitic. 2011, 62, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Deis, L.; Cavagnaro, B.; Bottini, R.; Wuilloud, R.; Silva, M.F. Water deficit and exogenous ABA significantly affect grape and wine phenolic composition under in field and in-vitro conditions. Plant Growth Regul. 2011, 65, 11–21. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [Green Version]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar]
- Boss, P.K.; Davies, C. Molecular biology of anthocyanin accumulation in grape berries. In Grapevine Molecular Physiology & Biotechnology; Springer: Dordrecht, The Netherlands, 2009; pp. 263–292. [Google Scholar]
- Deluc, L.; BoGS, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.-M.; Robinson, S.P.; Barrieu, F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hichri, I.; Heppel, S.C.; Pillet, J.; Léon, C.; Czemmel, S.; Delrot, S.; Lauvergeat, V.; Bogs, J. The Basic Helix-Loop-Helix Transcription Factor MYC1 Is Involved in the Regulation of the Flavonoid Biosynthesis Pathway in Grapevine. Mol. Plant 2010, 3, 509–523. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Larbat, R.; Olsen, K.M.; Slimestad, R.; Løvdal, T.; Bénard, C.; Verheul, M.; Bourgaud, F.; Robin, C.; Lillo, C. Influence of repeated short-term nitrogen limitations on leaf phenolics metabolism in tomato. Phytochemistry 2012, 77, 119–128. [Google Scholar] [CrossRef]
- Scheible, W.-R.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Palacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.; Stitt, M. Genome-Wide Reprogramming of Primary and Secondary Metabolism, Protein Synthesis, Cellular Growth Processes, and the Regulatory Infrastructure of Arabidopsis in Response to Nitrogen. Plant Physiol. 2004, 136, 2483–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.-L.; Shi, M.-Z.; Xie, D.-Y. Regulation of anthocyanin biosynthesis by nitrogen in TTG1–GL3/TT8–PAP1-programmed red cells of Arabidopsis thaliana. Planta 2012, 236, 825–837. [Google Scholar] [CrossRef]
- Saad, K.R.; Kumar, G.; Giridhar, P.; Shetty, N.P. Differential expression of anthocyanin biosynthesis genes in Daucus carota callus culture in response to ammonium and potassium nitrate ratio in the culture medium. 3 Biotech 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Shi, M.-Z.; Xie, D.-Y. Features of anthocyanin biosynthesis in pap1-D and wild-type Arabidopsis thaliana plants grown in different light intensity and culture media conditions. Planta 2010, 231, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Løvdal, T.; Olsen, K.M.; Slimestad, R.; Verheul, M.; Lillo, C. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 2010, 71, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Hudson, D.; Schofield, A.; Tsao, R.; Yang, R.; Gu, H.; Bi, Y.-M.; Rothstein, S.J. Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. J. Exp. Bot. 2008, 59, 2933–2944. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Tedesco, M.J.; Volkweiss, S.J.; Bohnen, H.; Gianello, C.; Bissani, C. Análise de solos, plantas e outros materiais. Bol. Técnico 1995, 5, 174. [Google Scholar]
- Chemists, A. Official Methods of Analysis: Changes in official methods of analysis made at the annual meeting. Assoc. Off. Anal. Chem. 1990, 15, 132–137. [Google Scholar]
- Zhang, K.; Liu, Z.; Guan, L.; Zheng, T.; Jiu, S.; Zhu, X.; Jia, H.; Fang, J. Changes of anthocyanin component biosynthesis in “Summer Black” grape berries after the red flesh mutation. J. Agric. Food Chem. 2018, 66, 9209–9218. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Li, J.-H.; Fan, P.-G.; Chen, S.; Fang, J.-B.; Li, S.-H.; Wu, B.-H. Anthocyanin Accumulation in Various Organs of a Teinturier Cultivar (Vitis vinifera L.) during the Growing Season. Am. J. Enol. Vitic. 2012, 63, 177–184. [Google Scholar] [CrossRef]







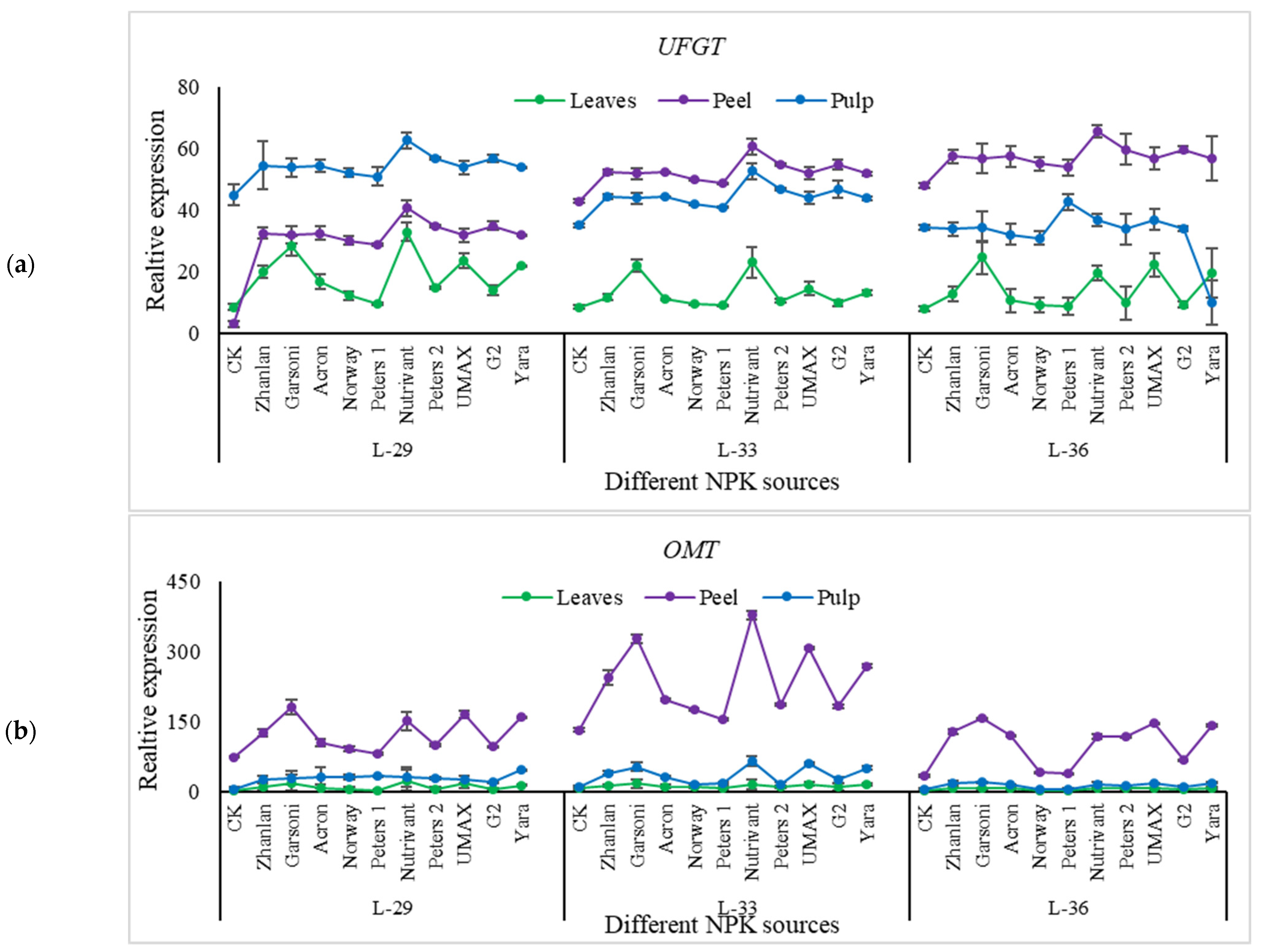
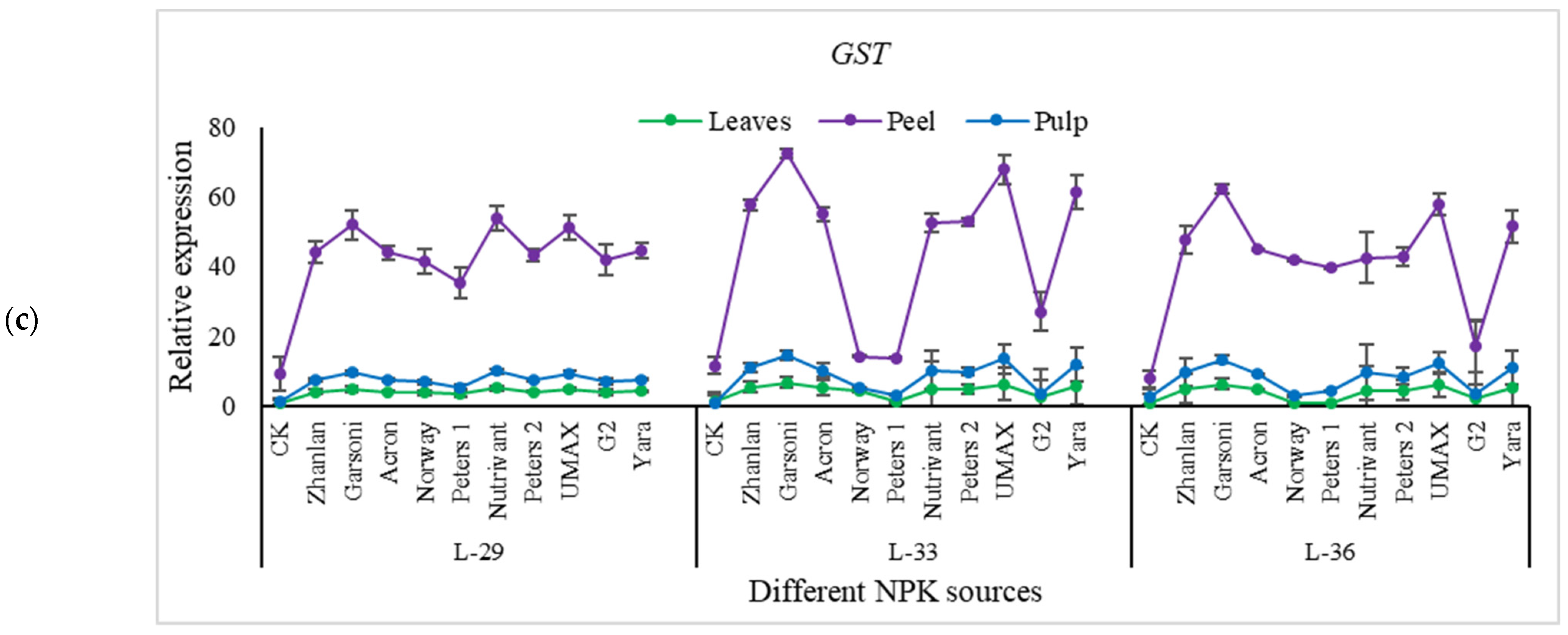

| Fertilizer Source | pH of Grape Berries | |
|---|---|---|
| 2017 | 2018 | |
| CK | 5.6 ± 0.036 e | 5.98 ± 0.008 c |
| Zhanlan | 5.39 ± 0.026 fg | 5.84 ± 0.028 d |
| Garsoni | 5.14 ± 0.052 i | 5.74 ± 0.050 d |
| Acron | 4.93 ± 0.017 j | 5.48 ± 0.061 f |
| Norway | 4.69 ± 0.005 lm | 5.08 ± 0.026 i |
| Peters1 | 4.62 ± 0.008 m | 4.93 ± 0.036 j |
| Nutrivant | 6.26 ± 0.052 b | 6.42 ± 0.037 a |
| Peters 2 | 4.80 ± 0.017 k | 5.37 ± 0.010 g |
| UMAX | 5.82 ± 0.028 d | 6.06 ± 0.024 c |
| G2 | 4.73 ± 0.006 kl | 5.24 ± 0.049 h |
| Yara | 6.02 ± 0.045 c | 6.27 ± 0.013 b |
| Fertilizer Source | Clusters per Plant | Mean | |
|---|---|---|---|
| 2017 | 2018 | ||
| CK | 13.77 NS | 19.433 | 16.6 NS |
| Zhanlan | 14.43 | 15.767 | 15.1 |
| Garsoni | 15.10 | 16.433 | 15.767 |
| Acron | 15.43 | 15.767 | 15.6 |
| Norway | 18.77 | 14.1 | 16.433 |
| Peters1 | 14.10 | 13.767 | 13.933 |
| Nutrivant | 16.10 | 16.767 | 16.433 |
| Peters 2 | 15.77 | 16.767 | 16.267 |
| UMAX | 12.77 | 18.1 | 15.433 |
| G2 | 16.10 | 13.1 | 14.6 |
| Yara | 11.10 | 14.1 | 12.6 |
| Mean | 14.86NS | 15.83 | |
| Fertilizers Trade Name | N | P | K | |
|---|---|---|---|---|
| 1 | CK | 0 | 0 | 0 |
| 2 | Zhanlan | 16 | 16 | 16 |
| 3 | Garsoni | 15 | 15 | 15 |
| 4 | Acron | 16 | 15 | 15 |
| 5 | Norway | 21 | 7 | 12 |
| 6 | Peters 1 | 30 | 10 | 10 |
| 7 | Nutrivant | 14 | 14 | 30 |
| 8 | Peters 2 | 20 | 20 | 20 |
| 9 | UMAX | 15 | 15 | 15 |
| 10 | G2 | 20 | 20 | 20 |
| 11 | Yara | 15 | 15 | 15 |
| Gene Name | Sequence of Forward (5′-3′) | Sequence of Reverse Primers (5′-3′) | |
|---|---|---|---|
| 1 | NR | ACCGACAGCATCCTCATCAA | AGGTGTTGGGAGATGATGCA |
| 2 | GS | AGAAGATTGACTGGTCGCCA | CTTTTCAGTGTCACGGCCAA |
| 3 | GDH | CTTTCGGCTTGGCATCAACT | ATACCGGCCACACTTTGTTG |
| 4 | AS | GAATTGCACTTCATTGGCGC | GCTGTCACTGCCAACATGAA |
| 5 | HKT1 | GGTTCCTACTGTTCTGGGCT | CGCCTGTATGCCTTGAGTTC |
| 6 | NIR | ATGCCTGCCACAAAGAAAGG | ACAAACTGGGAGGACATCGT |
| 7 | PHT1–4 | GTACTCTTGCCGGACAGCTC | GCCAGAACCTGAAGAAGCAC |
| 8 | PHT2–1 | CAAGGCTTTAGCCTGTGGAG | CATGGGCAAATGACATGAAG |
| 9 | PHO-1 | ACCCAAGTTGACGCCATT | GAGCAGACTATGTTGCGAATG |
| 10 | PAP | ACCTGATTATTCTGCTTTCCG | TCCTGCTTCCCAGCTTACTT |
| 11 | SIRK | GCTTTCAGCCTCCTGTTCAC | CACCAATCCAGGTTCGTTCT |
| 12 | SORK | AGGAAGCAACGAAGACGAAA | CACGAGCCTGCATAGTTCAA |
| 13 | KUP1 | TGAGCTTTGAAACATGGGAAGACT | TTCTTGTTACCAAGCCTTCCGG |
| 14 | KUP2 | ATGCTTCCTGCCATTTCCACATA | GGTTGGCATGGTTTATATCGTCTG |
| 15 | PAL | GCCAATCCTGTCACCAACCAT | TGATGGCAGTCCATTGTTGT |
| 16 | CHS | GTTCTGTTTGGATTTGGACCAG | GTGAGTCGATTGTGTAGCAAGG |
| 17 | F3′H | GCCTCCGTTGCTGCTCAGTT | GAGAAGAGGTGGACGGAGCAAATC |
| 18 | F3′5′H | AAACCGCTCAGACCAAAACC | ACTAAGCCACAGGAAACTAA |
| 19 | DFR | GAAACCTGTAGATGGCAGGA | GGCCAAATCAAACTACCAGA |
| 20 | LDOX | AGGGAAGGGAAAACAAGTAG | ACTCTTTGGGGATTGACTGG |
| 21 | UFGT | GGGATGGTAATGGCTGTGG | ACATGGGTGGAGAGTGAGTT |
| 22 | OMT | CTCTGCAGGCGCCTCTATTA | CCCAAAACAGAGTCTGGACA |
| 23 | GST | ACTTGGTGAAGGAAGCTGGA | TTGGAAAGGTGCATACATGG |
| 24 | UBIQUITIN1 | TCTGAGGCTTCGTGGTGGTA | AGGCGTGCATAACATTTGCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiaz, M.; Wang, C.; Zia Ul Haq, M.; Haider, M.S.; Zheng, T.; Mengqing, G.; Jia, H.; Jiu, S.; Fang, J. Molecular Evaluation of Kyoho Grape Leaf and Berry Characteristics Influenced by Different NPK Fertilizers. Plants 2021, 10, 1578. https://doi.org/10.3390/plants10081578
Fiaz M, Wang C, Zia Ul Haq M, Haider MS, Zheng T, Mengqing G, Jia H, Jiu S, Fang J. Molecular Evaluation of Kyoho Grape Leaf and Berry Characteristics Influenced by Different NPK Fertilizers. Plants. 2021; 10(8):1578. https://doi.org/10.3390/plants10081578
Chicago/Turabian StyleFiaz, Muhammad, Chen Wang, Muhammad Zia Ul Haq, Muhammad Salman Haider, Ting Zheng, Ge Mengqing, Haifeng Jia, Songtao Jiu, and Jinggui Fang. 2021. "Molecular Evaluation of Kyoho Grape Leaf and Berry Characteristics Influenced by Different NPK Fertilizers" Plants 10, no. 8: 1578. https://doi.org/10.3390/plants10081578
APA StyleFiaz, M., Wang, C., Zia Ul Haq, M., Haider, M. S., Zheng, T., Mengqing, G., Jia, H., Jiu, S., & Fang, J. (2021). Molecular Evaluation of Kyoho Grape Leaf and Berry Characteristics Influenced by Different NPK Fertilizers. Plants, 10(8), 1578. https://doi.org/10.3390/plants10081578





