Pressurized Hot Water Extraction of Okra Seeds Reveals Antioxidant, Antidiabetic and Vasoprotective Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Okra Materials
2.2. PHWE System and Heating under Reflux
2.3. LC/UV/MS Profiling of Samples
2.4. Antioxidant Activity Assays
2.5. Alpha-Glucosidase Assay
2.6. Cell-Culture and Cytoprotective Effects
2.7. RNA Extraction and Quantitative Real Time PCR
2.8. Statistical Analysis
3. Results and Discussion
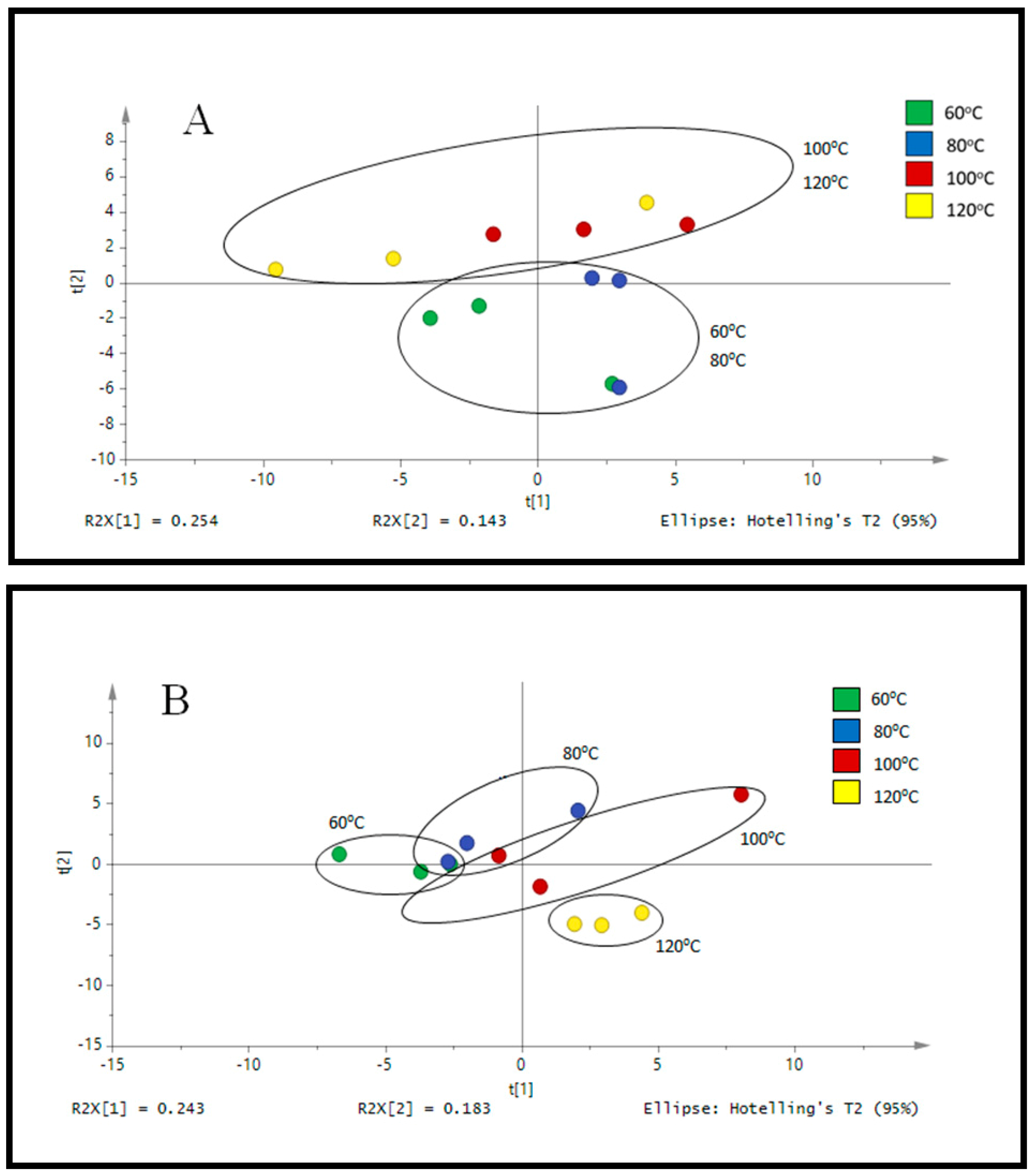
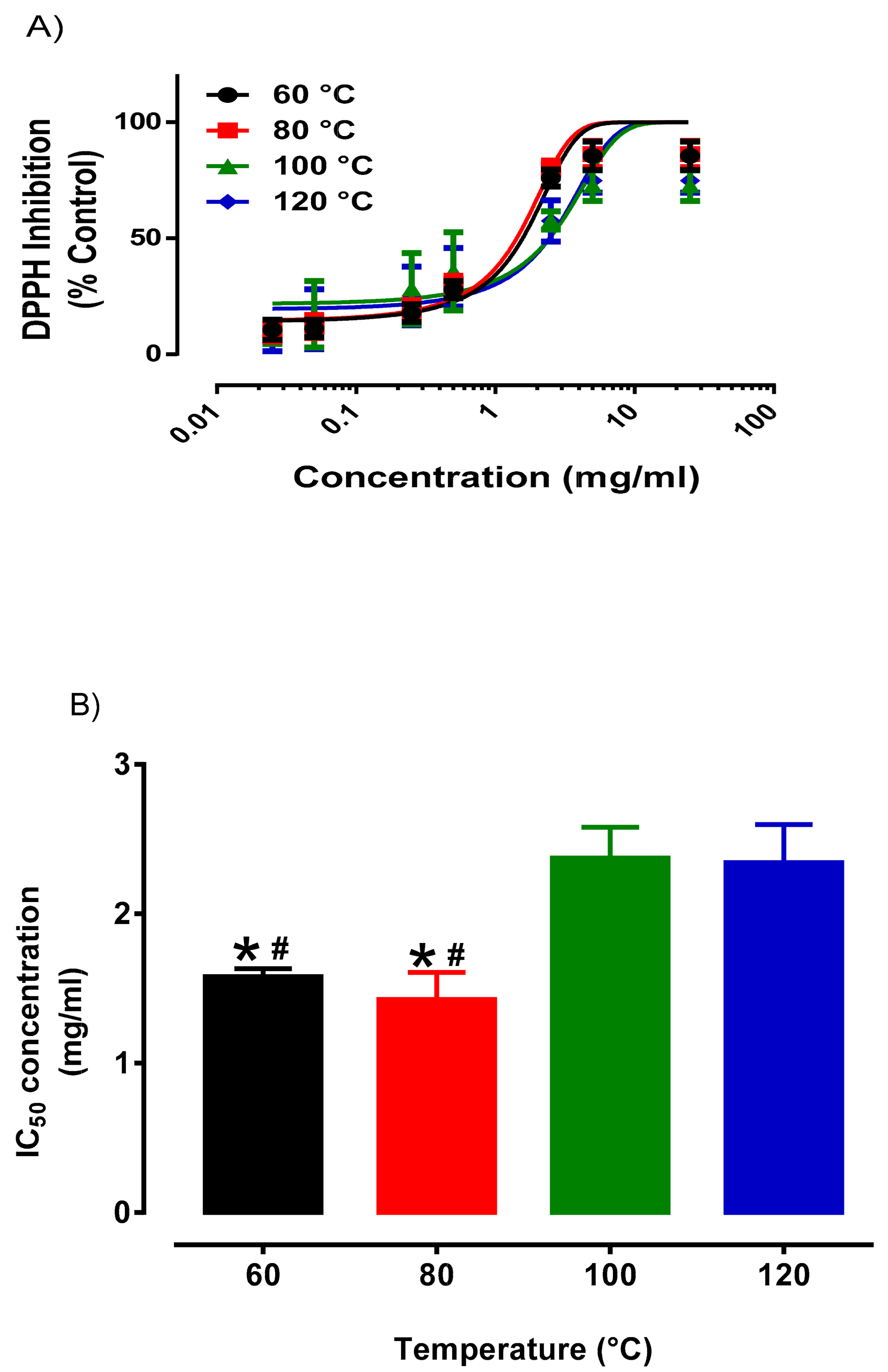
3.1. Optimization of PHWE for Okra
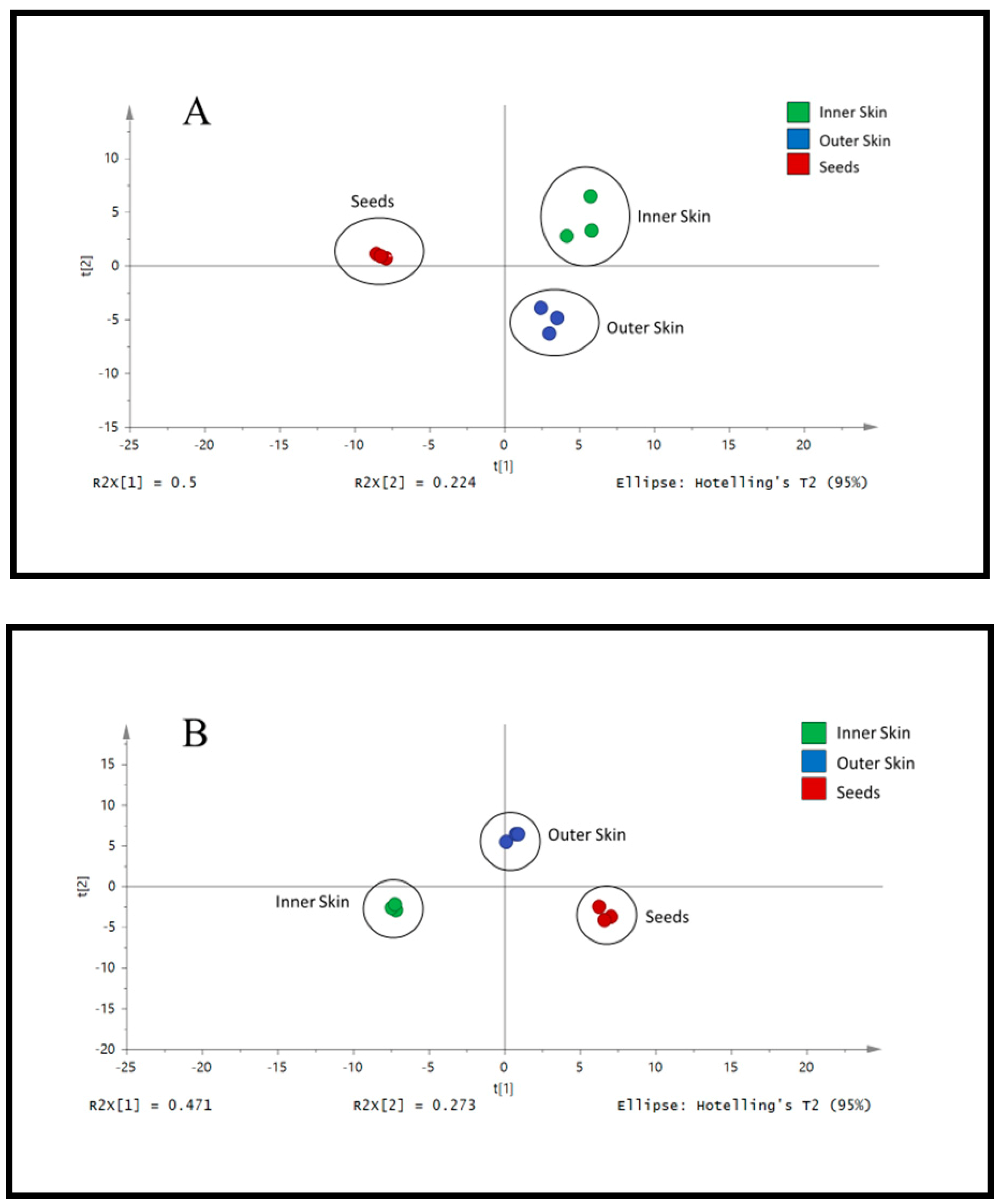
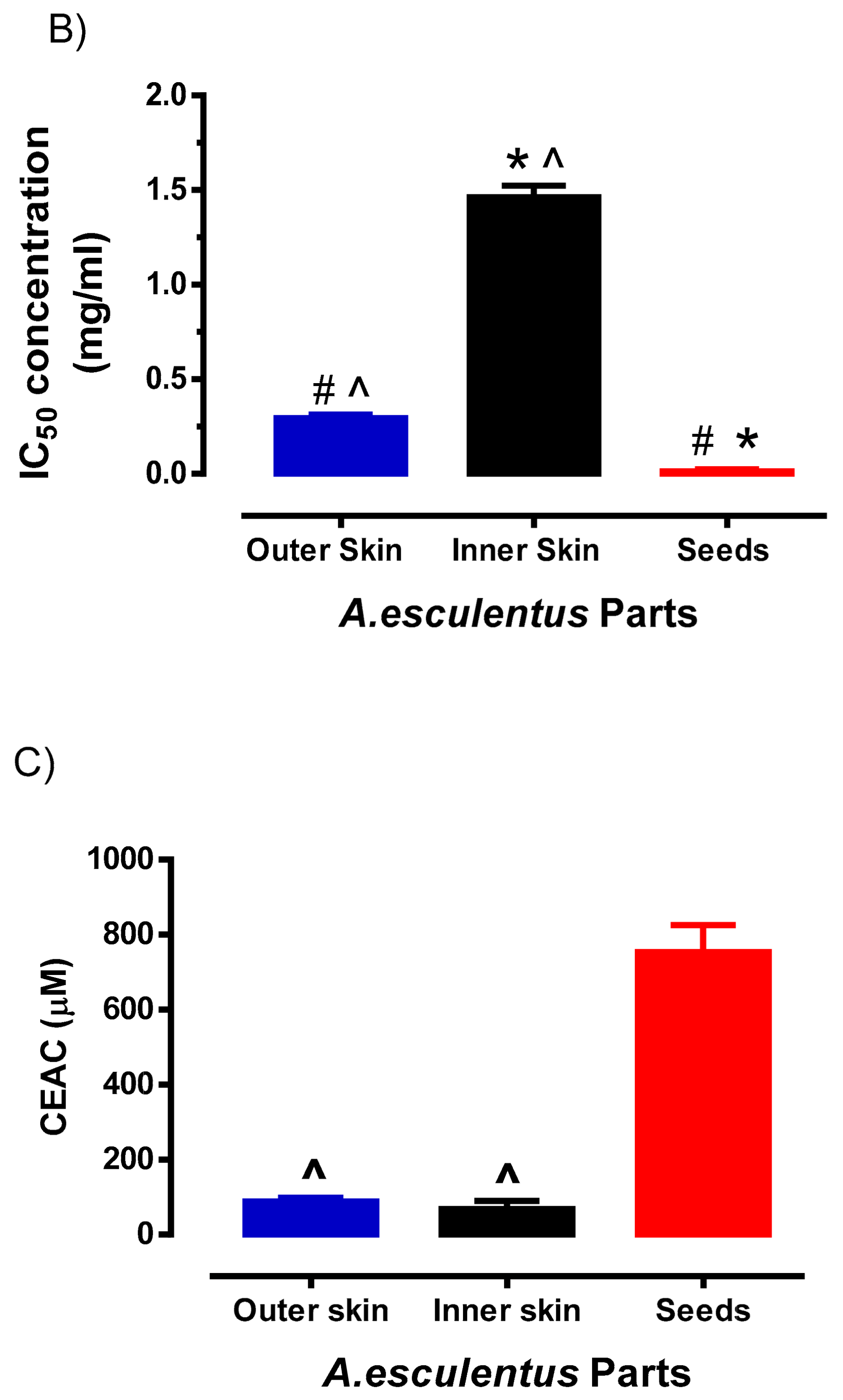
3.2. Analysis of Different Parts of Okra by LC/UV/MS
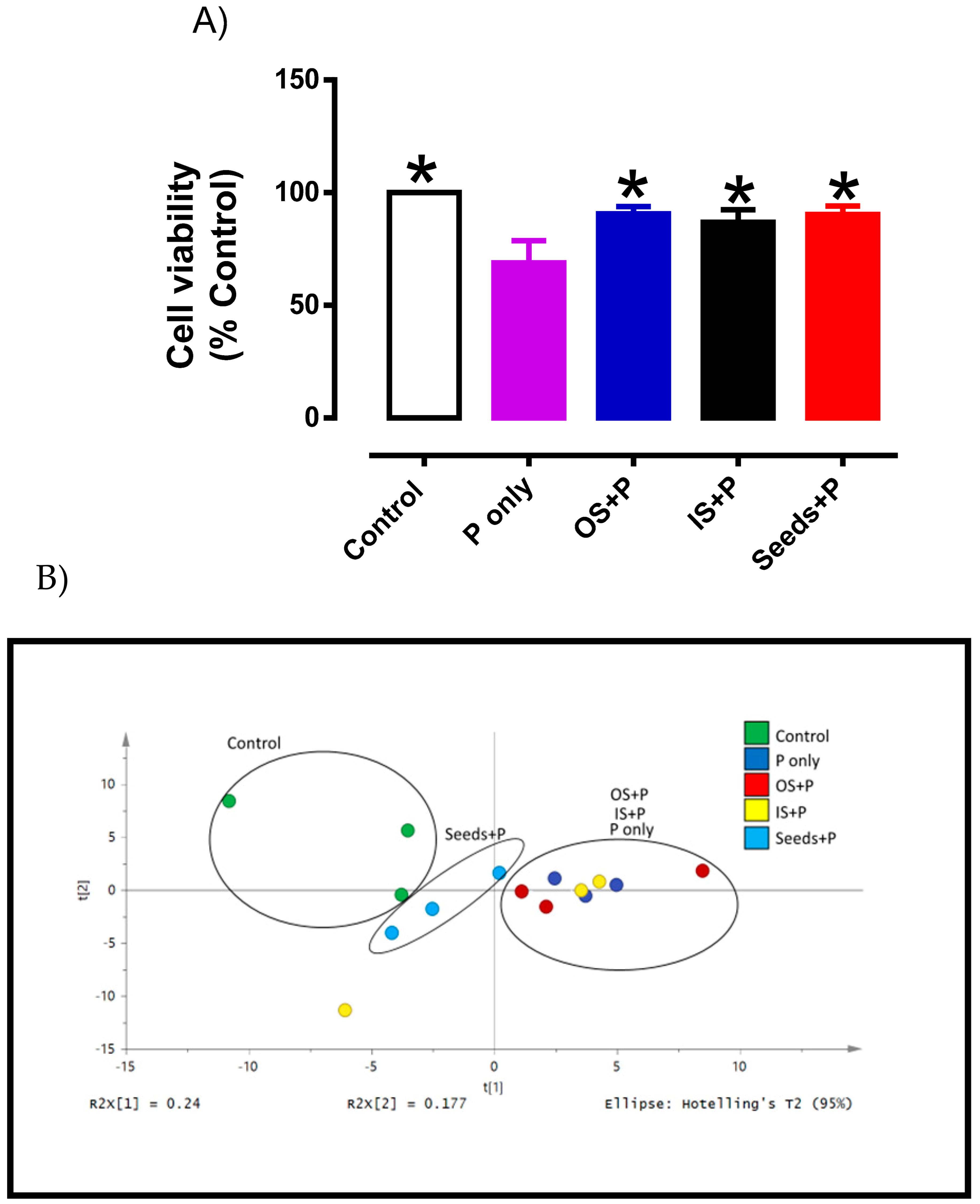
3.3. Cytoprotective Effects of Okra Extracts in Endothelial Cells
3.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, C.H.; Chyau, C.C.; Wang, C.J.; Lin, H.T.; Huang, C.N.; Ker, Y.B. Abelmoschus esculentus fractions potently inhibited the pathogenic targets associated with diabetic renal epithelial to mesenchymal transition. Food Funct. 2016, 7, 728–740. [Google Scholar] [CrossRef]
- Karakoltsidis, P.A.; Constantinides, S.M. Okra seeds. New protein source. J. Agric. Food Chem. 1975, 23, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P. Identification and quantification of polyphenolic compounds from okra seeds and skins. Food Chem. 2008, 110, 1041–1045. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, J.; Liu, B.; Yan, T.; Xu, F.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) Improves Antioxidant Capacity via PI3K/AKT Pathways and Nrf2 Translocation in a Type 2 Diabetes Model. Molecules 2019, 24, 1906. [Google Scholar] [CrossRef] [Green Version]
- Xia, F.; Zhong, Y.; Li, M.; Chang, Q.; Liao, Y.; Liu, X.; Pan, R. Antioxidant and anti-fatigue constituents of okra. Nutrients 2015, 7, 8846–8858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C.F.R. The chemical composition, nutritional value and antimicrobial properties of Abelmoschus esculentus seeds. Food Funct. 2017, 8, 4733–4743. [Google Scholar] [CrossRef]
- Shen, D.D.; Li, X.; Qin, Y.L.; Li, M.T.; Han, Q.H.; Zhou, J.; Lin, S.; Zhao, L.; Zhang, Q.; Qin, W.; et al. Physicochemical properties, phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of okra (Abelmoschus esculentus) fruit at different maturation stages. J. Food Sci. Technol. 2019, 56, 1275–1286. [Google Scholar] [CrossRef]
- Leo, C.H.; Woodman, O.L. Flavonols in the Prevention of Diabetes-induced Vascular Dysfunction. J. Cardiovasc. Pharmacol. 2015, 65, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yu, W.; Li, Y.; Prasad, N.; Tang, Z. Antioxidant activity of extract and its major constituents from okra seed on rat hepatocytes injured by carbon tetrachloride. Biomed. Res. Int. 2014, 2014, 341291. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, V.; Ramachandran, S.; Naveen, K.R.; Panneerselvam, K. Investigation of in vivo antioxidant property of Abelmoschus esculentus (L) moench fruit seed and peel powders in streptozotocin-induced diabetic rats. J. Ayurveda Integr. Med. 2012, 3, 188–193. [Google Scholar] [PubMed] [Green Version]
- Marshall, S.A.; Qin, C.X.; Jelinic, M.; O’Sullivan, K.; Deo, M.; Walsh, J.; Li, M.; Parry, L.J.; Ritchie, R.H.; Leo, C.H. The Novel Small-molecule Annexin-A1 Mimetic, Compound 17b, Elicits Vasoprotective Actions in Streptozotocin-induced Diabetic Mice. Int. J. Mol. Sci. 2020, 21, 1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, I.; Badoer, E.; Alahmadi, E.; Leo, C.H.; Woodman, O.L.; Stebbing, M.J. Microglia Are Selectively Activated in Endocrine and Cardiovascular Control Centres in Streptozotocin-Induced Diabetic Rats. J. Neuroendocrinol. 2014, 26, 413–425. [Google Scholar] [CrossRef]
- Ng, H.H.; Jelinic, M.; Parry, L.J.; Leo, C.H. Increased superoxide production and altered nitric oxide-mediated relaxation in the aorta of young but not old male relaxin-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H285–H296. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.H.; Leo, C.H.; Parry, L.J. Serelaxin (recombinant human relaxin-2) prevents high glucose-induced endothelial dysfunction by ameliorating prostacyclin production in the mouse aorta. Pharmacol. Res. 2016, 107, 220–228. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus Esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Li, M.; Wen, X.; Chen, X.; He, Z.; Ni, Y. Optimization of ultrasound-assisted extraction of okra (Abelmoschus esculentus (L.) Moench) polysaccharides based on response surface methodology and antioxidant activity. Int. J. Biol. Macromol. 2018, 114, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- EMA. CPMP/ICH/283/95 Impurities: Guideline for Residual Solvents & CVMP/VICH/502/99 Guideline on Impurities: Residual Solvents; European Medicines Agency: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Heng, M.Y.; Katayama, S.; Mitani, T.; Ong, E.S.; Nakamura, S. Solventless extraction methods for immature fruits: Evaluation of their antioxidant and cytoprotective activities. Food Chem. 2017, 221, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.C.; Tan, S.N.; Yong, J.W.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Hawrył, A.; Hawrył, M.; Waksmundzka-Hajnos, M. Liquid chromatography fingerprint analysis and antioxidant activity of selected lavender species with chemometric calculations. PLoS ONE 2019, 14, e0218974. [Google Scholar] [CrossRef]
- Zhou, S.T.; Luan, K.; Ni, L.L.; Wang, Y.; Yuan, S.M.; Che, Y.H.; Yang, Z.Z.; Zhang, C.G.; Yang, Z.B. A Strategy for Quality Control of Vespa magnifica (Smith) Venom Based on HPLC Fingerprint Analysis and Multi-Component Separation Combined with Quantitative Analysis. Molecules 2019, 24, 2920. [Google Scholar] [CrossRef] [Green Version]
- Ong, E.S.; Pek, C.J.N.; Tan, J.C.W.; Leo, C.H. Antioxidant and Cytoprotective Effect of Quinoa (Chenopodium quinoa Willd.) with Pressurized Hot Water Extraction (PHWE). Antioxidants 2020, 9, 1110. [Google Scholar] [CrossRef]
- ICH Expert Working Group. ICH Harmonised Tripartite Guideline Impurities in New Drug Products Q3B(R2). In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Brussels, Belgium, 25 October 2006; Available online: http://academy.gmp-compliance.org/guidemgr/files/ICH%20Q3B%20R2.PDF (accessed on 2 August 2021).
- Law, W.S.; Huang, P.Y.; Ong, E.S.; Ong, C.N.; Li, S.F.; Pasikanti, K.K.; Chan, E.C. Metabonomics investigation of human urine after ingestion of green tea with gas chromatography/mass spectrometry, liquid chromatography/mass spectrometry and 1H NMR spectroscopy. Rapid Commun. Mass Spectrom. 2008, 22, 2436–2446. [Google Scholar] [CrossRef]
- Ong, E.S.; Chor, C.F.; Zou, L.; Ong, C.N. A multi-analytical approach for metabolomic profiling of zebrafish (Danio rerio) livers. Mol. Biosyst. 2008, 5, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.S.; Zou, L.; Li, S.; Cheah, P.Y.; Eu, K.W. Metabolic profiling in colorectal cancer reveals signature metabolic shifts during tumorigenesis. Mol. Cell. Proteom. 2010. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.H.; Jelinic, M.; Ng, H.H.; Tare, M.; Parry, L.J. Time-dependent activation of prostacyclin and nitric oxide pathways during continuous i.v. infusion of serelaxin (recombinant human H2 relaxin). Br. J. Pharmacol. 2016, 173, 1005–1017. [Google Scholar] [CrossRef] [Green Version]
- Leo, C.H.; Jelinic, M.; Gooi, J.H.; Tare, M.; Parry, L.J. A vasoactive role for endogenous relaxin in mesenteric arteries of male mice. PLoS ONE 2014, 9, e107382. [Google Scholar] [CrossRef]
- Leo, C.H.; Jelinic, M.; Parkington, H.C.; Tare, M.; Parry, L.J. Acute intravenous injection of serelaxin (recombinant human relaxin-2) causes rapid and sustained bradykinin-mediated vasorelaxation. J. Am. Heart Assoc. 2014, 3, e000493. [Google Scholar] [CrossRef] [Green Version]
- Leo, C.H.; Ng, H.H.; Marshall, S.A.; Jelinic, M.; Rupasinghe, T.; Qin, C.; Roessner, U.; Ritchie, R.H.; Tare, M.; Parry, L.J. Relaxin reduces endothelium-derived vasoconstriction in hypertension: Revealing new therapeutic insights. Br. J. Pharmacol. 2020, 177, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Doreddula, S.K.; Bonam, S.R.; Gaddam, D.P.; Desu, B.S.; Ramarao, N.; Pandy, V. Phytochemical analysis, antioxidant, antistress, and nootropic activities of aqueous and methanolic seed extracts of ladies finger (Abelmoschus esculentus L.) in mice. Sci. World J. 2014, 2014, 519848. [Google Scholar] [CrossRef] [Green Version]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Ho, T.T.; Murthy, H.N.; Park, S.Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, G. Analysis of Secondary Metabolites from Plant Endophytic Fungi. Methods Mol. Biol. 2018, 1848, 25–38. [Google Scholar]
- Tohge, T.; Perez de Souza, L.; Fernie, A.R. On the natural diversity of phenylacylated-flavonoid and their in planta function under conditions of stress. Phytochem. Rev. 2018, 17, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Yang, W.T.; Cho, K.M.; Lee, J.H. Comparative analysis of isoflavone aglycones using microwave-assisted acid hydrolysis from soybean organs at different growth times and screening for their digestive enzyme inhibition and antioxidant properties. Food Chem. 2020, 305, 125462. [Google Scholar] [CrossRef] [PubMed]
- Ratseewo, J.; Meeso, N.; Siriamornpun, S. Changes in amino acids and bioactive compounds of pigmented rice as affected by far-infrared radiation and hot air drying. Food Chem. 2020, 306, 125644. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Esmaeilzadeh, D.; Razavi, B.M.; Hosseinzadeh, H. Effect of Abelmoschus esculentus (okra) on metabolic syndrome: A review. Phytother. Res. 2020, 34, 2192–2202. [Google Scholar] [CrossRef]
- Huang, C.N.; Wang, C.J.; Lin, C.L.; Lin, H.T.; Peng, C.H. The nutraceutical benefits of subfractions of Abelmoschus esculentus in treating type 2 diabetes mellitus. PLoS ONE 2017, 12, e0189065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, X.R.; Li, H.Y.; Du, G.; Lin, S.; Hu, R.; Li, H.Y.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.T.; et al. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Marshall, S.A.; Leo, C.H.; Senadheera, S.N.; Girling, J.E.; Tare, M.; Parry, L.J. Relaxin deficiency attenuates pregnancy-induced adaptation of the mesenteric artery to angiotensin II in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R847–R857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, H.H.; Leo, C.H.; Parry, L.J.; Ritchie, R.H. Relaxin as a Therapeutic Target for the Cardiovascular Complications of Diabetes. Front. Pharmacol. 2018, 9, 501. [Google Scholar] [CrossRef]
- Leo, C.H.; Jelinic, M.; Ng, H.H.; Marshall, S.A.; Novak, J.; Tare, M.; Conrad, K.P.; Parry, L.J. Vascular actions of relaxin: Nitric oxide and beyond. Br. J. Pharmacol. 2017, 174, 1002–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langston-Cox, A.; Leo, C.H.; Tare, M.; Wallace, E.M.; Marshall, S.A. Sulforaphane improves vascular reactivity in mouse and human arteries after “preeclamptic-like” injury. Placenta 2020, 101, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Anthonisz, J.; Leo, C.H.; Kahlberg, N.; Velagic, A.; Li, M.; Jap, E.; Woodman, O.L.; Parry, L.J.; Horowitz, J.D.; et al. NO• resistance, induced in the myocardium by diabetes is circumvented by the NO redox sibling, nitroxyl. Antioxid. Redox Signal 2020, 32, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Hyötyläinen, T.; Orešič, M. Systems Biology Strategies to Study Lipidomes in Health and Disease. Prog. Lipid Res. 2014, 55, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.H.; Hart, J.L.; Woodman, O.L. Impairment of both nitric oxide-mediated and EDHF-type relaxation in small mesenteric arteries from rats with streptozotocin-induced diabetes. Br. J. Pharmacol. 2011, 162, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leo, C.H.; Hart, J.L.; Woodman, O.L. 3′,4′-dihydroxyflavonol restores endothelium dependent relaxation in small mesenteric artery from rats with type 1 and type 2 diabetes. Eur. J. Pharmacol. 2011, 659, 193–198. [Google Scholar] [CrossRef]
- Leo, C.H.; Hart, J.L.; Woodman, O.L. 3′,4′-dihydroxyflavonol reduces superoxide and improves nitric oxide function in diabetic rat mesenteric arteries. PLoS ONE 2011, 6, e20813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, H.H.; Leo, C.H.; Prakoso, D.; Qin, C.; Ritchie, R.H.; Parry, L.J. Serelaxin treatment reverses vascular dysfunction and left ventricular hypertrophy in a mouse model of Type 1 diabetes. Sci. Rep. 2017, 7, 39604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, K.; Chio, T.I.; Sackett, D.L.; Bane, S.L. Detection of Oxidative Stress-Induced Carbonylation in Live Mammalian Cells. Free Radic. Biol. Med. 2015, 84, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leo, C.H.; Jelinic, M.; Ng, H.H.; Parry, L.J.; Tare, M. Recent developments in relaxin mimetics as therapeutics for cardiovascular diseases. Curr. Opin. Pharmacol. 2019, 45, 42–48. [Google Scholar] [CrossRef]
- Leo, C.H.; Jelinic, M.; Ng, H.H.; Tare, M.; Parry, L.J. Serelaxin: A novel therapeutic for vascular diseases. Trends Pharmacol. Sci. 2016, 37, 498–507. [Google Scholar] [CrossRef]
- Gou, L.; Liu, G.; Ma, R.; Regmi, A.; Zeng, T.; Zheng, J.; Zhong, X.; Chen, L. High fat-induced inflammation in vascular endothelium can be improved by Abelmoschus esculentus and metformin via increasing the expressions of miR-146a and miR-155. Nutr. Metab. 2020, 17, 35. [Google Scholar] [CrossRef]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Vauchel, P.; Carolina Colli, C.; Pradal, D.; Philippot, M.; Decossin, S.; Dhulster, P.; Dimitrov, K. Comparative LCA of ultrasound-assisted extraction of polyphenols from chicory grounds under different operational conditions. J. Clean. Prod. 2018, 196, 1116–1123. [Google Scholar] [CrossRef]



| Polyphenolic Compounds | tR (min) | MS- (m/z) | MS/MS (m/z) | Quantitative Analysis (Normalised % Peak Intensity) | ||
|---|---|---|---|---|---|---|
| Outer Skin (OS) | Inner Skin (IS) | Seeds | ||||
| p-courmaryol-hexose | 0.923 | 326 | 147, 164 | 0.31 ± 0.04 # | 0.84 ± 0.09 *^ | 0.16 ± 0.02 # |
| Sinapoyl-feruloyl | 2.86 | 399 | 193, 207 | 3.51 ± 0.24 #^ | 2.89 ± 0.04 *^ | 0.084 ± 0.005 *# |
| Quercetin-3-O-glucose-xylose | 3.421 | 595 | 300 | 5.08 ± 0.63 #^ | 0.81 ± 0.15 *^ | 6.80 ± 0.30 *# |
| Quercetin 3-O-(malonyl)gluose | 3.877 | 549 | 301 | 0.15 ± 0.21 | 0.52 ± 0.03 ^ | 7.57 ± 0.39 *# |
| Kaempferol 3-O-glucose | 3.97 | 447 | 285 | ND | ND | 0.559 ± 0.001 *# |
| Sinapoyl-hexose | 4.584 | 385 | 193, 176 | 0.36 ± 0.17 | 0.82 ± 0.28 ^ | 0.11 ± 0.01 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, E.S.; Oh, C.L.Y.; Tan, J.C.W.; Foo, S.Y.; Leo, C.H. Pressurized Hot Water Extraction of Okra Seeds Reveals Antioxidant, Antidiabetic and Vasoprotective Activities. Plants 2021, 10, 1645. https://doi.org/10.3390/plants10081645
Ong ES, Oh CLY, Tan JCW, Foo SY, Leo CH. Pressurized Hot Water Extraction of Okra Seeds Reveals Antioxidant, Antidiabetic and Vasoprotective Activities. Plants. 2021; 10(8):1645. https://doi.org/10.3390/plants10081645
Chicago/Turabian StyleOng, Eng Shi, Christina Liu Ying Oh, Joseph Choon Wee Tan, Su Yi Foo, and Chen Huei Leo. 2021. "Pressurized Hot Water Extraction of Okra Seeds Reveals Antioxidant, Antidiabetic and Vasoprotective Activities" Plants 10, no. 8: 1645. https://doi.org/10.3390/plants10081645







