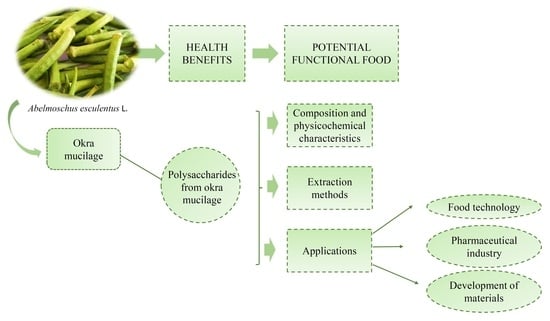
Okra (Abelmoschus esculentus L.) as a Potential Functional Food Source of Mucilage and Bioactive Compounds with Technological Applications and Health Benefits
Abstract
:1. Introduction
2. Composition, Cost, and Main Uses of Okra
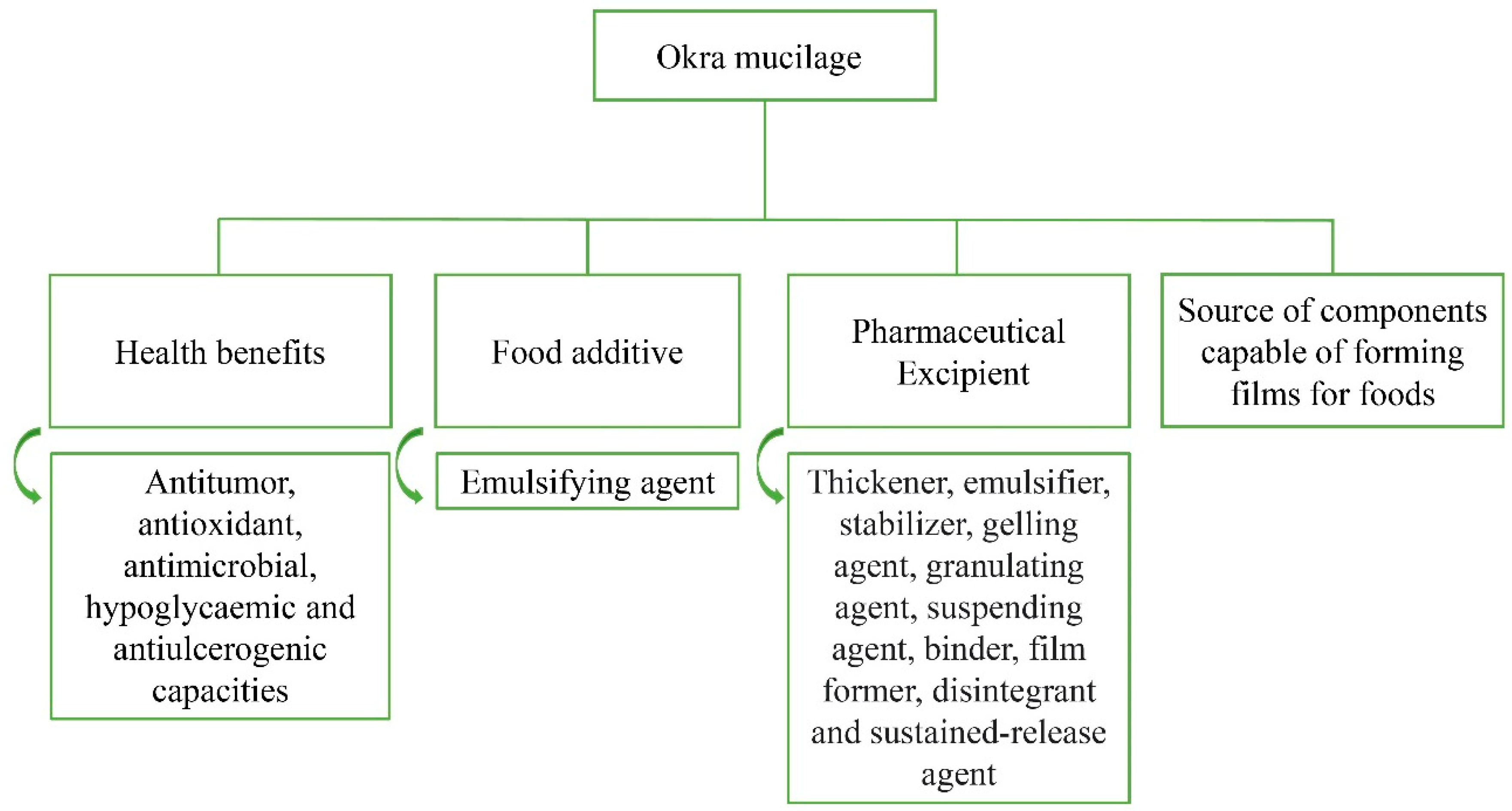
3. Beneficial Properties of Okra Mucilage to Health and Its Relevance for Considering Okra as a Functional Food
4. Composition and Physicochemical Characteristics of Okra Mucilage
5. Extraction Methods
6. Applications of Okra Mucilage
7. Food Technology
8. Pharmaceutical Technology
9. Development of Materials
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Durazzo, A.; Lucarini, M.; Novellino, E.; Souto, E.B.; Daliu, P.; Santini, A. Abelmoschus esculentus (L.): Bioactive components’ beneficial properties—Focused on antidiabetic role—For sustainable health applications. Molecules 2019, 24, 38. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T. Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): A literature-based review. Phytother. Res. 2019, 33, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Gemede, H.F.; Woldegiorgis, A.Z.; Retta, N.; Haki, G.D. Nutritional quality and health benefits of okra (Abelmoschus esculentus): A review. Am. J. Food Sci. Nutr. 2015, 4, 208–215. [Google Scholar] [CrossRef]
- Wankhade, P.K.; Sapkal, R.S.; Sapkal, V.S. Drying characteristics of okra slices on drying in hot air dryer. Procedia Eng. 2013, 51, 371–374. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Abidi, A.B.; Chauhan, V.; Tiwari, B.K. An overview on okra (Abelmoschus esculentus) and it’s importance as a nutritive vegetable in the world. Biol. Sci. 2014, 4, 227–233. [Google Scholar]
- Bencharsi, S. Okra (Abelmoschus esculentus (L.) Moench) as a valuable vegetable of the world. Ratar. Povrt. 2012, 49, 105–112. [Google Scholar]
- Bendale, V.W.; Kadam, S.R.; Bhave, S.G.; Mehta, J.L.; Pethe, U.B. Genetic variability and correlation studies in okra. Orissa J. Hortic. 2003, 31, 2. [Google Scholar]
- Nawaz, A.; Ali, H.; Sufyan, M.; Dildar, M.G.; Arif, M.J.; Ali, A.; Qasim, M.; Islam, W.; Ali, N.; Bodla, I.; et al. In-vitro assessment of food consumption, utilization indices and losses promises of leafworm, Spodoptera litura (Fab.), on okra crop. J. Asia Pac. Entomol. 2020, 23, 60–66. [Google Scholar] [CrossRef]
- Moulana, S.; Prasad, V.V.; Bahadur, V. Effect of different levels of cycocel (CCC) on two different cultivars of okra (Abelmoschus esculantus L.) under Prayagraj Agro climatic conditions. Int. J. Chem. Stud. 2020, 8, 133–136. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, R.; Wang, H.; Chen, C.; Tu, Z. Structural properties, bioactivities, and applications of polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: A review. J. Agric. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef]
- Al-Shawi, A.A.A.; Hameed, M.F.; Hussein, K.A.; Thawini, H.K. Review on the “Biological Applications of Okra Polysaccharides and Prospective Research”. Future J. Pharm. Sci. 2021, 7, 102. [Google Scholar] [CrossRef]
- Barcellos, M.D.; Lionello, R.L. Consumer market for functional foods in south Brazil. Int. J. Food Syst. Dyn. 2011, 2, 126–144. [Google Scholar] [CrossRef]
- Bonciu, E. Aspects of the involvement of biotechnology in functional food and nutraceuticals. Sci. Pap. Ser. A Agron. 2020, 63, 261–266. [Google Scholar]
- Costa, M.F.N.; Araújo, B.C.; Silva Primo, M.G.; Nogueira, T.R.; Rodrigues, G.P. Alimentos funcionais: Conhecimento e consumo por usuários de restaurante self-service em capital do nordeste brasileiro. Rev. Eletrônica Acervo Saúde 2019, 11, 2369–2379. [Google Scholar] [CrossRef]
- Da Cruz, G.F.R.; Ferreira, M.C.O.; Da Silva, J.G.; Cucato, J.S.T. O comportamento do consumidor de alimentos funcionais. In Proceedings of the VI Simpósio Internacional de Gestão de Projetos, Inovação e Sustentabilidade, São Paulo, Brazil, 14 November 2017. [Google Scholar]
- Adelakun, O.E.; Oyelade, O.J.; Ade-Omowaye, B.I.O.; Adeyemi, I.A.; Venter, M.V. Chemical composition and the antioxidative properties of Nigerian Okra Seed (Abelmoschus esculentus Moench) Flour. Food Chem. Toxicol. 2009, 47, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Adetuyi, F.; Ajala, L.; Ibrahim, T. Effect of the addition of defatted okra seed (Abelmoschus esculentus) flour on the chemical composition, functional properties and Zn bioavailability of plantain (Musa paradisiacal Linn) flour. J. Microbiol. Biotechnol. Food Sci. 2021, 2, 69–82. [Google Scholar]
- Olawuyi, I.F.; Lee, W.Y. Structural characterization, functional properties and antioxidant activities of polysaccharide extract obtained from okra leaves (Abelmoschus esculentus). Food Chem. 2021, 354, 129437. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, Q.; Zhao, J.; Mao, G.; Feng, W.; Chen, Y.; Li, Q.; Wu, X.; Yang, L.; Zhao, T. Purification, structural elucidation and physicochemical properties of a polysaccharide from Abelmoschus esculentus L (okra) flowers. Int. J. Biol. Macromol. 2020, 155, 740–750. [Google Scholar] [CrossRef]
- Fauza, A.; Al-Baarri, A.N.M.; Djamiatun, K. Potency of Okra flour (Abelmoschus esculentus) in improving adiponectin level and total antioxidant capacity of high fat diet streptozotocin rat model. Potravin. Slovak J. Food Sci. 2017, 13, 644–650. [Google Scholar] [CrossRef] [Green Version]
- IBGE. Sidra. Censo Agropecuário 2017. Available online: https://sidra.ibge.gov.br/tabela/6954 (accessed on 3 May 2021).
- Programa Brasileiro de Modernização do Mercado de Hortigranjeiro. Preços mais comum no atacado em todas as Ceasas por unidade da federação. Available online: http://www.ceasa.gov.br/precos.php?TIP=1&P01=6&P02=1&P03=37&P04=0 (accessed on 6 May 2021).
- Romdhane, M.H.; Chahdoura, H.; Barros, L.; Dias, M.I.; Corrêa, R.C.G.; Morales, P.; Ciudad-Mulero, M.F.H.; Flamini, G.C.F.R.; Majdoub, H.; Ferreira, I.C.F.R. Chemical composition, nutritional value and biological evaluation of Tunisian Okra pods. Molecules 2020, 25, 4739. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. FoodData Central; Agricultural Research Service; USDA: Washington, DC, USA, 2019. Available online: https://fdc.nal.usda.gov/index.html (accessed on 18 May 2021).
- Kumar, D.S.; Kumar, A.P.; Rao, S.B.; Nadendla, R. A review on: Abelmoschus esculentus (okra). Int. Res. J. Pharm. App. Sci. 2013, 3, 129–132. [Google Scholar]
- Petropoulos, S.; Fernandes, A.; Barros, L.; Ferreira, I.C.F.R. Chemical composition, nutritional value and antioxidant properties of Mediterranean okra genotypes in relation to harvest stage. Food Chem. 2018, 242, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus Esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules 2020, 26, 696. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qi, J.; Luo, J.; Qin, W. Okra in food field: Nutritional value, health benefits and effects of processing methods on quality. Food Rev. Int. 2021, 37, 67–90. [Google Scholar] [CrossRef]
- Ofori, J.; Tortoe, C.; Agbenorhevi, J.K. Physicochemical and functional properties of dried okra (Abelmoschus esculentus L.) seed flour. Food Sci. Nutr. 2020, 8, 4291–4296. [Google Scholar] [CrossRef] [PubMed]
- Oyelade, O.J.; Ade-omowaye, B.I.O.; Adeomi, V.F. Influence of variety on protein, fat contents and some physical characteristics of okra seeds. J. Food Eng. 2003, 57, 111–114. [Google Scholar] [CrossRef]
- Gerrano, A.S. Agronomic performance, nutritional phenotyping and trait associations of Okra (Abelmoschus esculentus) Genotypes in South Africa. Rediscov. Landrac. A Resour. Future 2018, 69. [Google Scholar] [CrossRef] [Green Version]
- Brito, M.M.; Ribeiro, L.N.; Araújo, M.A.M.; Moreira-Araújo, R.S.R. Desenvolvimento de bolo enriquecido com farinha de quiabo (Hibiscus esculentus L.). Hig. Aliment. 2017, 31, 125–129. [Google Scholar]
- Oliveira, T.W.N.; Damasceno, A.N.C.; Oliveira, V.A.; Silva, C.E.O.; Barros, N.V.S.; Medeiros, M.M.L.; Araújo, I.M.S.; Medeiros, S.R.A. Caracterização físico-química e sensorial de biscoitos tipo cookie elaborados com farinha de berinjela (Solanum melongena L.) e quiabo (Abelmoschus esculentus L. Moench). Braz. J. Dev. 2020, 6, 14259–14277. [Google Scholar] [CrossRef]
- Rindiani, R.; Kumalasari, P. Steamed cake with okra flour substitution as an alternative to snack for a fibre source. IOP Conf. Ser. Earth Environ. Sci. 2021, 672, 012048. [Google Scholar] [CrossRef]
- Anastasakis, K.; Kalderis, D.; Diamadopoulos, E. Flocculation behavior of mallow and okra mucilage in treating wastewater. Desalination 2009, 249, 786–791. [Google Scholar] [CrossRef]
- Adetuyi, F.O.; Dada, I.B.O. Nutritional, phytoconstituent and antioxidant potential of mucilage extract of Okra (Abelmoschus esculentus), water leaf (Talinum triangulare) and Jews mallow (Corchorus olitorius). Int. Food Res. J. 2014, 21, 2345–2353. [Google Scholar]
- Chukwuma, C.I.; Islam, S.; Amonsou, E.O. A comparative study on the physicochemical, anti-oxidative, anti-hyperglycemic and anti-lipidemic properties of amadumbe (Colocasia esculenta) and okra (Abelmoschus esculentus) mucilage. J. Food Biochem. 2018, 42, 5. [Google Scholar] [CrossRef]
- KuruwitaArachchige, S.V.; Deepthi, I.; Uluwaduge, D.; Premakumara, S. Cardio protective activity of Abelmoschus esculentus (Okra). Int. J. Food Sci. Nutr. 2018, 3, 39–43. [Google Scholar]
- Trakoolpolpruek, T.; Moonmangmee, S.; Chanput, W. Structure-dependent immune modulating activity of okra polysaccharide on THP-1 macrophages. Bioact. Carbohydr. Diet. Fibre 2019, 17, 100173. [Google Scholar] [CrossRef]
- Ameena, K.; Dilip, C.; Saraswathi, R.; Krishnan, P.N.; Sankar, C.; Simi, S.P. Isolation of the mucilages from Hibiscus rosasinensis linn. and Okra (Abelmoschus esculentus linn.) and studies of the binding effects of the mucilages. Asian Pac. J. Trop. Med. 2010, 7, 539–543. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wanh, C.; Peng, C. Active subfractions of Abelmoschus esculentus substantially prevent free fatty acid-induced β cell apoptosis via inhibiting dipeptidyl peptidase-4. PLoS ONE 2017, 12, e0180285. [Google Scholar] [CrossRef] [Green Version]
- Ortaç, D.; Cemek, M.; Karaca, T.; Büyükokuroglu, M.; Özdemir, Z.Ö.; Kocaman, A.T.; Gönes, S. In vivo anti-ulcerogenic effect of okra (Abelmoschus esculentus) on ethanol-induced acute gastric mucosal lesions. Pharm. Bio. 2018, 56, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.; Lin, H.; Lin, C.; Wang, C.; Huang, C. Abelmoschus esculentus subfractions improved nephropathy with regulating dipeptidyl peptidase-4 and type 1 glucagon-like peptide receptor in type 2 diabetic rats. J. Food Drug Anal. 2019, 27, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, M.; Wen, X.; Chen, X.; He, Z.; Ni, Y. Optimization of ultrasound-assisted extraction of okra (Abelmoschus esculentus (L.) Moench) polysaccharides based on response surface methodology and antioxidant activity. Int. J. Biol. Macromol. 2018, 114, 1056–1063. [Google Scholar] [CrossRef]
- Nie, X.; Li, H.; Lin, S.; Hu, R.; Li, H.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.; Qin, W. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Wahyuningsih, S.P.A.; Pramudya, M.; Putri, I.P.; Winarni, D.; Savira, N.I.I.; Darmanto, W. Crude polysaccharides from okra pods (Abelmoschus esculentus) grown in Indonesia enhance the immune response due to bacterial infection. Adv. Pharmacol. Sci. 2018, 2018, 8505383. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, J.; Wang, J.; Yan, T.; Xu, F.; Wu, B.; Xiao, F.; Bi, K.; Niu, J.; Jia, Y. The anti-nephritic activity of a polysaccharide from okra (Abelmoschus esculentus (L.) Moench) via modulation of AMPK-Sirt1-PGC-1α signaling axis mediated anti-oxidative in type 2 diabetes model mice. Int. J. Biol. Macromol. 2019, 140, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xiang, J.; Zheng, G.; Yan, R.; Min, X. Preliminary characterization and anti-hyperglycemic activity of a pectic polysaccharide from okra (Abelmoschus esculentus (L.) Moench). J. Funct. Foods 2018, 41, 19–24. [Google Scholar] [CrossRef]
- Daliu, P.; Annunzianta, G.; Tenore, G.C.; Santini, A. Abscisic acid identification in Okra, Abelmoschus esculentus L. (Moench): Perspective nutraceutical use for the treatment of diabetes. Nat. Prod. Res. 2019, 34, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.H.; Miao, F.; Zhang, X.; Wang, Q.; Lei, N.; Guo, L. Therapeutic effect of okra extract on gestational diabetes mellitus rats induced by streptozotocin. Asian Pac. J. Trop. Med. 2015, 8, 1038–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matazu, K.I.; Ismaila, M.; Bilbis, L.; Abbas, A.Y. Formulation of okra-based antidiabetic nutraceutical from Abelmoschus esculentus (L.) Moench (Ex-maradi Variety) and evaluation of its effect on alloxan-induced diabetic rats. Int. J. Curr. Res. Rev. 2018, 10, 11–16. [Google Scholar] [CrossRef]
- Liao, B.; Zhu, D.; Thakur, K.; Li, L.; Zhang, J.; Wei, Z. Thermal and antioxidant properties of polysaccharides sequentially extracted from mulberry leaves (Morus alba L.). Molecules 2017, 22, 2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Zi, W.; Pei, Z.; Liu, S. Characterization of polysaccharides and their antioxidant properties from Plumula nelumbinis. Saudi. Pharm. J. 2018, 26, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. In vitro antioxidant and antibacterial activity of sulfated polysaccharides isolated from Spatoglossum asperum. Carbohydr. Polym. 2017, 170, 296–304. [Google Scholar] [CrossRef]
- Slima, S.B.; Ktari, N.; Trabelsi, I.; Moussa, H.; Makni, I.; Salah, R. Purification, characterization and antioxidant properties of a novel polysaccharide extracted from Sorghum bicolor (L.) seeds in sausage. Int. J. Biol. Macromol. 2018, 106, 168–178. [Google Scholar] [CrossRef]
- Messing, J.; Thöle, C.; Niehues, M.; Shevtsova, A.; Glocker, E.; Borén, T.; Hensel, A. Antiadhesive properties of Abelmoschus esculentus (okra) immature fruit extract against Helicobacter pylori adhesion. PLoS ONE 2014, 9, e0084836. [Google Scholar] [CrossRef] [Green Version]
- Kontogiorgos, V.; Margelou, I.; Georgiadis, N.; Ritzoulis, C. Rheological characterization of okra pectins. Food Hydrocoll 2012, 29, 356–362. [Google Scholar] [CrossRef]
- Alamri, M.S.; Mohamed, A.A.; Hussain, S. Effects of alkaline-soluble okra gum on rheological and thermal properties of systems with wheat or corn starch. Food Hydrocoll. 2013, 30, 541–551. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, W.; Wang, B.; Hui, A.; Du, B.; Wang, T.; Meng, L.; Bian, H.; Wu, Z. Purification and anti-fatigue activity of polysaccharide fractions from okra (Abelmoschus esculentus (L.) Moench). Food Funct. 2018, 9, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, T.; Bai, S.Q.; Mao, G.H.; Zou, Y.; Feng, W.W.; Wang, W.; Huang, J.; Wu, X.S.; Yang, L.Q.; et al. Water-soluble polysaccharides from leaves of abelmoschus esculentus: Purification, characterization, and antioxidant activity. Chem. Nat. Compd. 2017, 53, 412–416. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, Y. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydr. Polym. 2014, 108, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Nie, X.; Shen, D.; Li, H.; Zhao, L.; Zhang, Q.; Lin, D.; Qin, W. Phenolic Compounds, Antioxidant Activities, and Inhibitory Effects on Digestive Enzymes of Different Cultivars of Okra (Abelmoschus esculentus). Molecules 2020, 25, 1276. [Google Scholar] [CrossRef] [Green Version]
- Nampuak, C.; Tongkhao, K. Okra mucilage powder: A novel functional ingredient with antioxidant activity and antibacterial mode of action revealed by scanning and transmission electron microscopy. Int. J. Food Sci. Technol. 2020, 55, 569–577. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.J.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, W.; Liu, C.; Li, T.; Liang, R.; Luo, S. Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Moreno, L.; Wosiacki, G.; Scheer, A.P. Pectina: Da matéria-prima ao produto final. Polímeros 2012, 22, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Farooq, U.; Malviya, R.; Sharma, P.K. Extraction and characterization of okra mucilage as pharmaceutical excipient. Acad. J. Plant. Sci. 2013, 6, 168–172. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Verhoef, R.P.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Characterization of cell wall polysaccharides from okra (Abelmoschus esculentus (L.) Moench). Carbohydr. Res. 2009, 344, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.; Liu, W.; Su, Y.; Han, Q.; Zhao, L.; Zhang, Q.; Lin, D.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Cahyana, A.H.; Kam, N. Study on the stability of antioxidant and anti α-glucosidase activities using soaking treatment in okra (Abelmoschus esculentus L.) mucilage extraction. Chem. Int. 2017, 3, 202–211. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Brito-de La Fuente, E.; Gómex-Aldapa, C.A.; Aragon-Piña, A.; Toro-Vazquez, J.F. Structural characteristics of gels formed by mixtures of carrageenan and mucilage gum from Opuntia ficus indica. Carbohydr. Polym. 2006, 63, 299–309. [Google Scholar] [CrossRef]
- Cornelia, M.; Narania, K.; Cahyana, H.; Sutyono, E. Encapsulation of soursop (Annona muricata Linn.) leaf tea extract using natural mucilage. Reaktor 2019, 19, 26–33. [Google Scholar] [CrossRef]
- Hussain, A.; Qureshi, F.; Abbas, N.; Arshad, M.S.; Ali, E. An Evaluation of the Binding Strength of Okra Gum and the Drug Release Characteristics of Tablets Prepared from It. Pharmaceutics 2017, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Köse, M.D.; Bayraktar, O.; Helvacioglu, M.; Engin, B. Preparation and characterization of biopolymer based bioactive mucoadhesive films with turmeric extract. J. Pharm. Appl. Chem. 2018, 4, 41–45. [Google Scholar] [CrossRef]
- Navamanisubramanian, R.; Nerella, R.D.C.; Seetharaman, S. Use of okra mucilage and chitosan acetate in verapamil hydrochloride buccal patches development; in vitro and ex vivo characterization. J. Young Pharm. 2017, 9, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Noorlaila, A.; Aziah, S.; And, R.; Norizzah, A.R. Emulsifying properties of extracted okra (Abelmoschus esculentus L.) mucilage of different maturity index and its application in coconut milk emulsion. Int. Food Res. J. 2015, 22, 782–787. [Google Scholar]
- Xu, K.; Guo, M.; Du, J. Okra polysaccharide: Effect on the texture and microstructure of set yoghurt as a new natural stabilizer. Int. J. Biol. Macromol. 2019, 133, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J.; Bai, L.; Chung, C. Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Yuenaan, P.; Sajjaanantakul, T.; Goff, H.D. Effect of okra cell wall and polysaccharide on physical properties and stability of ice cream. J. Food Sci. 2014, 79, E1522–E1527. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.S.; Sofian-Seng, N.-S.; Yusop, S.M.; Kasim, K.F.; Razali, N.S.M. Functionality of Okra Gum as a Novel Carbohydrate-based Fat Replacer in Ice Cream. Food Sci. Technol. Res. 2018, 24, 519–530. [Google Scholar] [CrossRef]
- Cui, L.; Fan, J.; Sun, Y.; Zhu, Z.; Yi, J. The prooxidant activity of salts on the lipid oxidation of lecithin-stabilized oil-in-water emulsions. Food Chem. 2018, 252, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Datsomor, D.N.; Agbenorhevi, J.; Kpodo, F.; Oduro, I.N. Okra pectin as lecithin substitute in chocolate. Sci. Afr. 2019, 3, e00070. [Google Scholar] [CrossRef]
- Araujo, S.S.F.P.; Silva, L.M.A.; Feitosa, B.; Silva, A.L. Mucilagem de quiabo Abelmoschus esculentus (L.) Moench como aditivo natural em molho de tomate. Res., Soc. Dev. 2020, 9, 5. [Google Scholar] [CrossRef]
- Simeoni, C.P.; Etchepare, M.A.; Menezes, C.R.; Fries, L.M.; Menezez, C.R.; Fries, L.M.; Menezes, M.F.C.; Stefanello, F.S. Microencapsulação de probióticos: Inovação tecnológica na indústria de alimentos. Rev. Eletrônica Gestão Educ. Tecnol. Ambient. 2014, 18, 66–75. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Etchepare, M.A.; Menezes, M.F.S.C.; Menezes, C.R.; Fries, L.L.M. Encapsulação: Alternativa para a aplicação de microrganismos probióticos em alimentos termicamente processados. Ciênc. Nat. 2015, 37, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.J.; Omura, M.H.; Cedran, M.F.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Garcia, S. Effect of natural polymers on the survival of Lactobacillus casei encapsulated in alginate microspheres. J. Microencapsul. 2017, 34, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, E.; Garcia, S. Eficiência de materiais encapsulantes naturais e comerciais na liberação controlada de probiótico encapsulado. Braz. J. Food Technol. 2013, 16, 107–115. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Babrekar, L.; Kharpude, S.; Chaudhari, J. Synthesis and characterization of psyllium seed mucilage grafted with N, N -methylene bisacrylamide. Int. J. Biol. Macromol. 2017, 103, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, A.; Nishad, D.K.; Khanna, K.; Sharma, B.G.; Kakkar, D.; Bhatnagar, A. Development and Gamma Scintigraphy Study of Trigonella foenum-graecum (Fenugreek) Polysaccharide-Based Colon Tablet. AAPS PharmSciTech 2018, 19, 2564–2571. [Google Scholar] [CrossRef]
- Choudhary, P.D.; Pawar, H.A. Recently Investigated Natural Gums and Mucilages as Pharmaceutical Excipients: An Overview. J. Pharm. 2014, 2014, 204849. [Google Scholar] [CrossRef] [Green Version]
- Ghori, M.U.; Alba, K.; Smith, A.M.; Conway, B.R.; Kontogiorgos, V. Okra extracts in pharmaceutical and food applications. Food Hydrocoll. 2014, 42, 342–347. [Google Scholar] [CrossRef]
- Nagpal, M.; Aggarwal, G.; Jain, U.K.; Madan, J. Okra fruit gum-chitosan impregnated polymer network films: Formulation and substantial depiction. Innovare Acad. Sci. 2017, 10, 2019–2022. [Google Scholar] [CrossRef] [Green Version]
- Pushpamalar, J.; Veeramachineni, A.K.; Owh, C.; Loh, X.J. Biodegradable Polysaccharides for Controlled Drug Delivery. ChemPlusChem 2016, 81, 504–514. [Google Scholar] [CrossRef]
- Medeiros, D.C.; Faria, T.; Lemos-Senna, E. Estudo de formulação de microesferas de acetobutirato de celulose com diferentes concentrações de Poloxamer 188. Semin. Ciênc. Biol. Saúde 2013, 34, 159–166. [Google Scholar] [CrossRef]
- Ghumman, S.A.; Bashir, S.; Noreen, S.; Khan, A.M.; Riffat, S.; Abbas, M. Polymeric microspheres of okra mucilage and alginate for the controlled release of oxcarbazepine: In vitro & in vivo evaluation. Int. J. Biol. Macromol. 2018, 111, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Palei, N.; Mamidi, S.; Rajangam, J. Formulation and evaluation of lamivudine sustained release tablet using okra mucilage. J. App. Pharm. Sci. 2016, 6, 069–075. [Google Scholar] [CrossRef] [Green Version]
- Sinha, P.; Ubaidulla, U.; Nayak, A.K. Okra (Hibiscus esculentus) gum-alginate blend mucoadhesive beads for controlled glibenclamide release. Int. J. Biol. Macromol. 2015, 72, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mazumder, R. Formulation and evaluation of losartan potassium compression coated tablets by okra gum as a binder. Int. J. Pharm. Sci. Res. 2019, 10, 9. [Google Scholar] [CrossRef]
- Hussein, A.H. Formulation and evaluation of sustained release tablets of pentoxifylline using okra extract as a novel retardant. Int. J. Pharm. Pharm. Sci. 2015, 7, 204–208. [Google Scholar]
- Newton, A.M.J.; Indana, V.L.; Kumar, J. Chronotherapeutic drug delivery of tamarind gum, chitosan and okra gum controlled release colon targeted directly compressed propranolol HCl matrix tablets and in-vitro evaluation. Int. J. Biol. Macromol. 2015, 79, 290–299. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, D.; Brar, V. Bioadhesive okra polymer based buccal patches as platform for controlled drug delivery. Int. J. Biol. Macromol. 2014, 70, 408–419. [Google Scholar] [CrossRef]
- Rajamma, A.J.; Yogesha, H.N.; Sateesha, S.B. Natural gums as sustained release carriers: Development of gastroretentive drug delivery system of ziprasidone HCl. Daru 2012, 20, 58. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, Y.; Riaz, H.; Barkat, K.; Yousaf, H.; Malik, A.R.; Raza, S.A. Fabrication of HPMC and Hibiscus esculentus (okra) gum based microspheres loaded with sulfasalazine and dexamethasone. J. Polym. Res. 2019, 26, 130. [Google Scholar] [CrossRef]
- Newton, A.M.J.; Swathi, P.; Kumar, N.; Kumar, K.M. A comparative study of Okra gum on controlled release kinetics and other formulation characteristics of tramadol HCl extended release matrix tablets vs synthetic hydrophilic polymers. Int. J. Drug Deliv. 2014, 6, 339–350. [Google Scholar]
- Zaharuddin, N.D.; Noordin, M.I.; Kadivar, A. The Use of Hibiscus esculentus(okra) gum in sustaining the selease of propranolol hydrochloride in a solid oral dosage form. Biomed. Res. Int. 2014, 2014, 735891. [Google Scholar] [CrossRef] [Green Version]
- Alalor, C.A.; Uhumwangho, M.U.; Iwuagwu, M.A. Evaluation of ciprofloxacin floating-bioadhesive tablet formulated with okra gum as multifunctional polymer. UK J. Pharm. Biosci. 2018, 6, 01–11. [Google Scholar] [CrossRef]
- Tawari, P.D.; Umekar, M.J.; Taksande, J.B. Formulation and evaluation of metformin sustained release matrix tablet using okra (Abelmoschus Esculentus L.) polysaccharide. Res. Pharm. 2018, 2, 13–19. [Google Scholar]
- Mistry, A.K.; Nagda, C.D.; Nagda, D.C.; Dixit, B.C.; Dixit, R.B. Formulation and In Vitro Evaluation of Ofloxacin Tablets using Natural Gums as Binders. Sci. Pharm. 2014, 82, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Mane, K.V.; Manthen, M.U.; Mhamane, S.S. Evaluation and formulation of okra extract (mucilage) containing moisturizing hair conditioner. Int. J. Res. Eng. Sci. Manag. 2019, 2, 330–332. [Google Scholar]
- Galus, S.; Kadzinska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Ma, D.; Jiang, Y.; Ahmed, S.; Qin, W.; Liu, Y. Physical and antimicrobial properties of edible films containing Lactococcus lactis. Int. J. Biol. Macromol. 2019, 141, 378–386. [Google Scholar] [CrossRef]
- Pajak, P.; Przetaczek-Roŝnowska, I.; Juszczak, L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int. J. Biol. Macromol. 2019, 138, 441–449. [Google Scholar] [CrossRef]
- Araújo, A.; Galvão, A.; Silva Filho, C.; Mendes, F.; Oliveira, M.; Barbosa, F.; Sousa Filho, M.; Bastos, M. Okra mucilage and corn starch bio-based film to be applied in food. Polym. Test. 2018, 71, 352–361. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A. Nanocomposite films based on CMC, okra mucilage and ZnO nanoparticles: Physico mechanical and antibacterial properties. Carbohydr. Polym. 2018, 181, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, M.A.P.; Mottin, A.C.; Ayres, E. Preparation and characterization of okra mucilage (Abelmoschus esculentus) edible Films. Macromol. Symp. 2016, 357, 90–100. [Google Scholar] [CrossRef]

| Part of Vegetable | Monosaccharide Composition | Extraction | Yield (%) | Purification | Molecular Weight (Da) | References |
|---|---|---|---|---|---|---|
| Leaves | D-ara, D-xyl, D-glu, D-man, and D-gal, Gal, and Xyl | Boiling water (1:15, w/v) for 3 h | 13.0–15.2 | Chromatography–mass spectrometry | 1.9 × 105–1.6 × 106 | [16] |
| Flowers | Gal, Rha, and GalA | Deionized water (1:40, w/v) at 100 °C for 3 h | 15.33 | Ion-exchange chromatography | 2.741 × 105 | [19] |
| Pods | Glu, Man, Gal, Ara, Xyl, and Fuc | Ultrasonic extractor using distilled water | 10.35 | Anion-exchange chromatography | 1.92 × 105 | [44] |
| Pods | Ara, Gal, Rha, and GalA | Deionized water (1:10, w/w) at 75 °C for 2 h | 1.1 | Anion-exchange chromatography | 2.99 × 106 | [45] |
| Pods | Rha, GalA, Gal, GlcA, Glu, and Ara | Distilled water (1:20, w/v) at 100 °C for 4 h | 7.9 | Anion-exchange chromatography | 5.80 × 105 | [49] |
| Leaves | Ara, Gal, Rha, GalA, and Glu | Ultrasonic extractor using distilled water | 3.11 | High-performance liquid chromatography | 26.9 × 103 | [61] |
| Matrix | Drug | Reference |
|---|---|---|
| Tablet | Naproxen sodium | [74] |
| Mucoadhesive films | Turmeric extract | [75] |
| Bioadhesive patches | Verapamil hydrochloride | [76] |
| Mucoadhesive beads | Glibenclamide | [99] |
| Tablets | Losartan potassium | [100] |
| Tablets | Pentoxifylline | [101] |
| Tablets | Tramadol HCl | [102] |
| Mucoadhesive films | Zolmitriptan | [103] |
| Tablet | Ziprasidone HCl | [104] |
| Microspheres | Sulfasalazine and dexamethasone | [105] |
| Tablet | Propranolol HCl | [106] |
| Tablet | Propranolol HCl | [107] |
| Floating bioadhesive tablet | Ciprofloxacin | [108] |
| Tablet | Metformin | [109] |
| Tablet | Ofloxacin | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a Potential Functional Food Source of Mucilage and Bioactive Compounds with Technological Applications and Health Benefits. Plants 2021, 10, 1683. https://doi.org/10.3390/plants10081683
Dantas TL, Alonso Buriti FC, Florentino ER. Okra (Abelmoschus esculentus L.) as a Potential Functional Food Source of Mucilage and Bioactive Compounds with Technological Applications and Health Benefits. Plants. 2021; 10(8):1683. https://doi.org/10.3390/plants10081683
Chicago/Turabian StyleDantas, Thamires Lacerda, Flávia Carolina Alonso Buriti, and Eliane Rolim Florentino. 2021. "Okra (Abelmoschus esculentus L.) as a Potential Functional Food Source of Mucilage and Bioactive Compounds with Technological Applications and Health Benefits" Plants 10, no. 8: 1683. https://doi.org/10.3390/plants10081683