Combined Abiotic Stresses Repress Defense and Cell Wall Metabolic Genes and Render Plants More Susceptible to Pathogen Infection
Abstract
:1. Introduction
2. Results
2.1. Heat and Combined Osmotic-Heat Stresses Weaken Arabidopsis Resistance to Pseudomonas syringae (Pst)
2.2. Combined Osmotic-Heat Stress Highly Enhances Disease Susceptibility of Arabidopsis against Botrytis Cinerea (Bc)
2.3. Osmotic and Heat Stresses Decrease the Expression of Many Defense and Cell Wall Related Genes
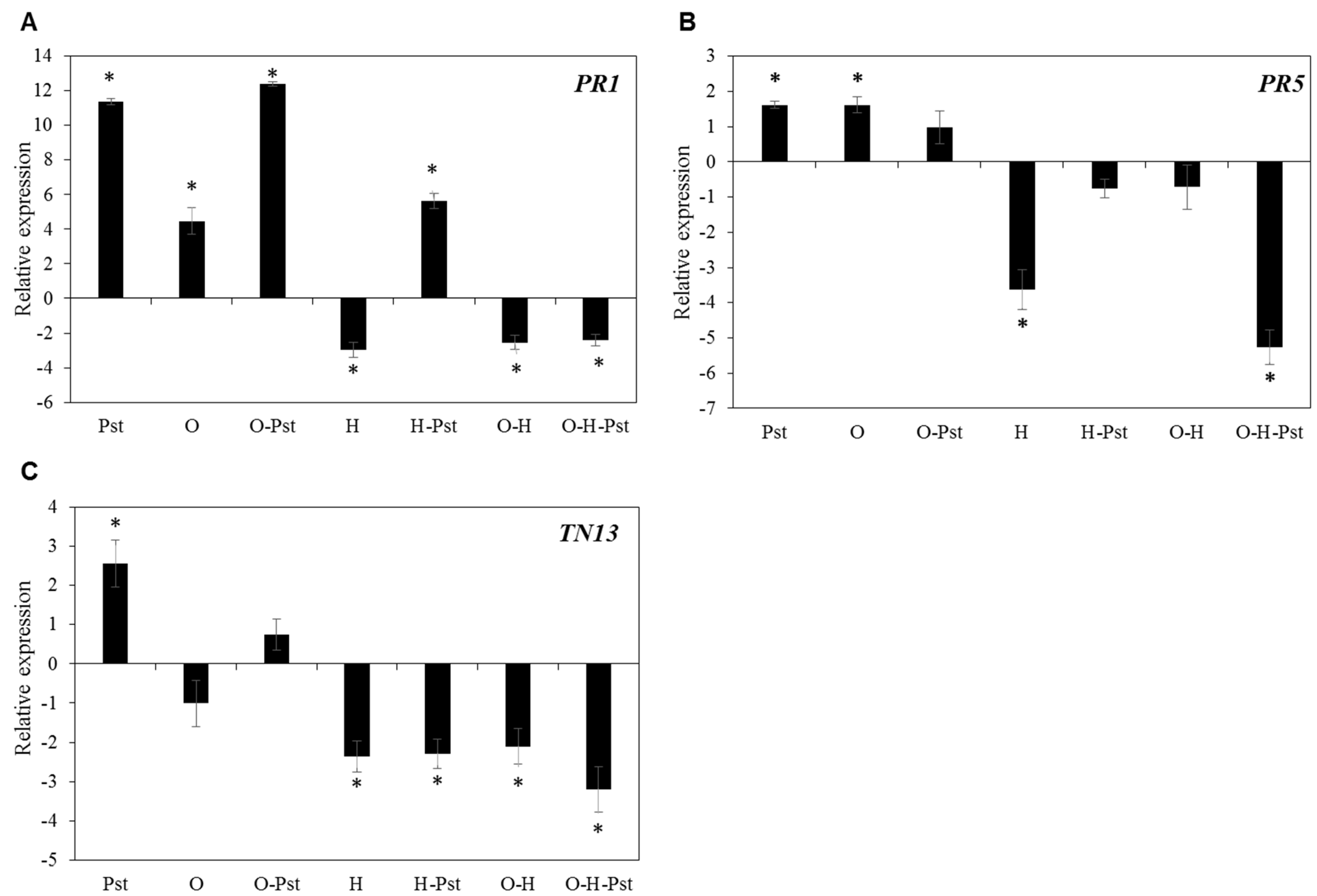
2.4. Heat Stress Dominates the Inhibition of Transcriptional Response of Defense Genes against Pst Infection
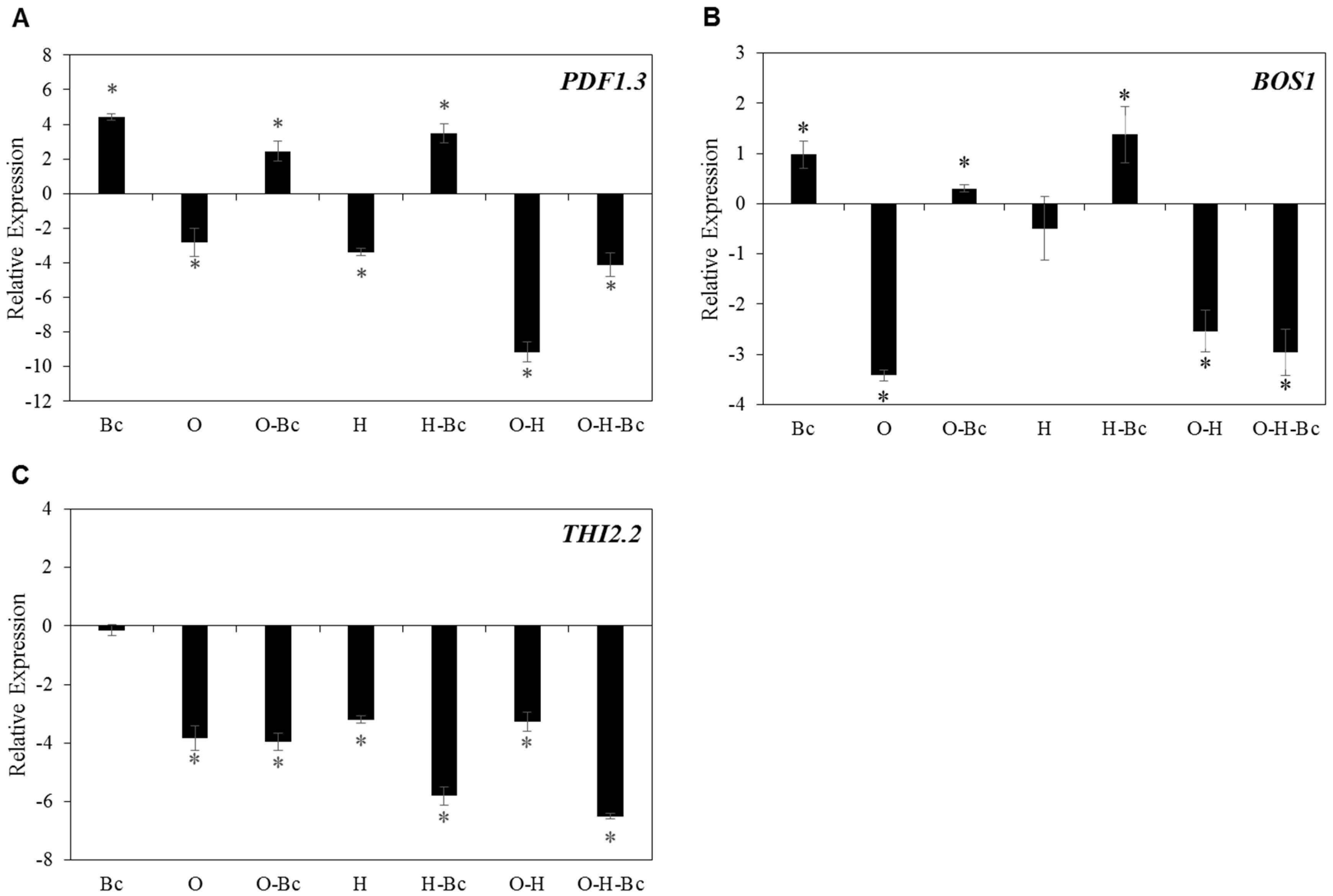
2.5. Combined Osmotic-Heat Stress Downregulate the Expression of Defense Related Genes against Bc Infection
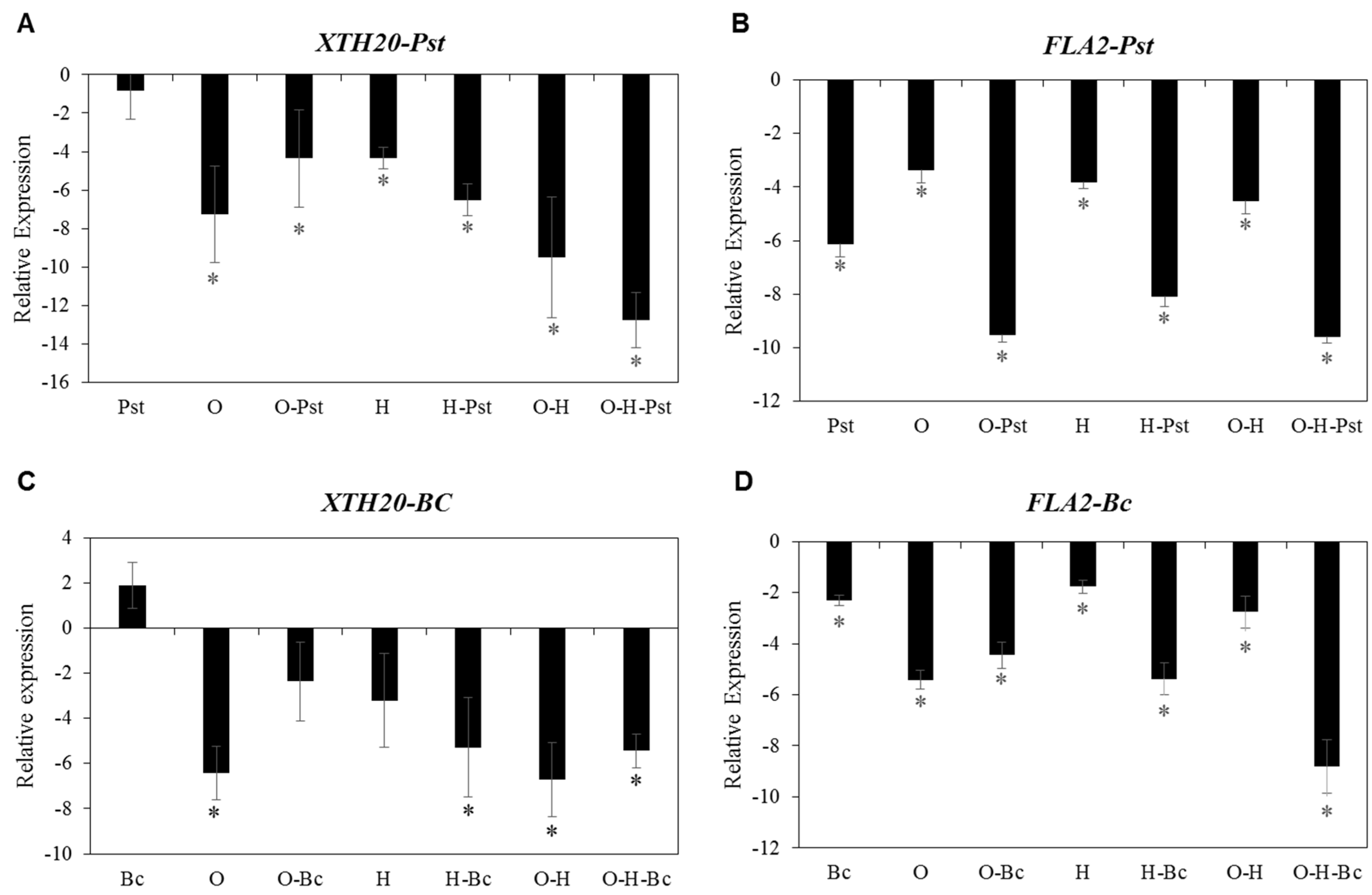
2.6. Impaired Disease Resistance against Both PST and BC under Combined Abiotic Stress Is Associated with Reduced Expression of Cell Wall-Related Genes
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Abiotic Stress Treatments
4.2. Inoculum Preparation and Biotic Stress Treatments
4.3. Quantification of Pathogen Infection
4.4. Real Time RT-PCR Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis; Intergovernmental Panel on Climate Change: Cambridge, UK, 2013. [Google Scholar]
- Becklin, K.M.; Anderson, J.T.; Gerhart, L.M.; Wadgymar, S.M.; Wessinger, C.A.; Ward, J.K. Examining plant physiological responses to climate change through an evolutionary lens. Plant Physiol. 2016, 172, 635–649. [Google Scholar] [CrossRef]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically rethinking agriculture for the 21st century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef] [Green Version]
- Swetha, B.; Singiri, J.R.; Novoplansky, N.; Grandhi, R.; Srinivasan, J.; Khadka, J.; Galis, I.; Grafi, G. Single and combined salinity and heat stresses impact yield and dead pericarp priming activity. Plants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Schlenker, W.; Roberts, M.J. Nonlinear temperature effects indicate severe damages to US crop yields under climate change. Proc. Nat. Acad. Sci. USA 2009, 106, 15594–15598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaderi, M.M.; Reid, D.M. Crop responses to elevated carbon dioxide and temperature. In Climate Change and Crops; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–18. [Google Scholar]
- Parry, M.; Parry, M.L.; Canziani, O.; Palutikof, J.; Van der Linden, P.; Hanson, C. (Eds.) Climate Change 2007-Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef] [Green Version]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacquard, S.; Spaepen, S.; Garrido-Oter, R.; Schulze-Lefert, P. Interplay between innate immunity and the plant microbiota. Annu. Rev. Phytopathol. 2017, 55, 565–589. [Google Scholar] [CrossRef]
- Tippmann, H.F.; Schlüter, U.; Collinge, D.B. Common themes in biotic and abiotic stress signalling in plants. In Floriculture, Ornamental and Plant Biotechnology; Global Science Books: London, UK, 2006; pp. 52–67. [Google Scholar]
- Bostock, R.M.; Pye, M.F.; Roubtsova, T.V. Predisposition in plant disease: Exploiting the nexus in abiotic and biotic stress perception and response. Annu. Rev. Phytopathol. 2014, 52, 517–549. [Google Scholar] [CrossRef] [Green Version]
- Sewelam, N.; Oshima, Y.; Mitsuda, N.; Ohme-Takagi, M. A step towards understanding plant responses to multiple environmental stresses: A genome-wide study. Plant Cell Environ. 2014, 37, 2024–2035. [Google Scholar] [CrossRef]
- Bai, Y.; Kissoudis, C.; Yan, Z.; Visser, R.G.; van der Linden, G. Plant behaviour under combined stress: Tomato responses to combined salinity and pathogen stress. Plant J. 2018, 93, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Sewelam, N.; Brilhaus, D.; Bräutigam, A.; Alseekh, S.; Fernie, A.R.; Maurino, V.G. Molecular plant responses to combined abiotic stresses put a spotlight on unknown and abundant genes. J. Exp. Bot. 2020, 71, 5098–5112. [Google Scholar] [CrossRef]
- Chan, Z. Expression profiling of ABA pathway transcripts indicates crosstalk between abiotic and biotic stress responses in Arabidopsis. Genomics 2012, 100, 110–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Bohnert, H.J. Integration of Arabidopsis thaliana stress-related transcript profiles, promoter structures, and cell-specific expression. Genome Biol. 2007, 8, R49. [Google Scholar] [CrossRef] [Green Version]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-microbe interactions facing environmental challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Duveiller, E.; Singh, R.; Nicol, J. The challenges of maintaining wheat productivity: Pests, diseases, and potential epidemics. Euphytica 2007, 157, 417–430. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [Green Version]
- Shakya, S.K.; Goss, E.M.; Dufault, N.S.; van Bruggen, A.H.C. Potential effects of diurnal temperature oscillations on potato late blight with special reference to climate change. Phytopathology 2015, 105, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Chatterjee, A.; Cui, Y.; Chatterjee, A.K. Elevated temperature enhances virulence of Erwinia carotovora subsp. carotovora strain EC153 to plants and stimulates production of the quorum sensing signal, N-acyl homoserine lactone, and extracellular proteins. Appl. Environ. Microbiol. 2005, 71, 4655–4663. [Google Scholar] [CrossRef] [Green Version]
- Moury, B.; Selassie, K.G.; Marchoux, G.; Daubèze, A.M.; Palloix, A. High temperature effects on hypersensitive resistance to tomato spotted wilt tospovirus (TSWV) in pepper (Capsicum chinense Jacq.). Eur. J. Plant Pathol. 1998, 104, 489–498. [Google Scholar] [CrossRef]
- Király, L.; Hafez, Y.M.; Fodor, J.; Király, Z. Suppression of tobacco mosaic virus-induced hypersensitive-type necrotization in tobacco at high temperature is associated with downregulation of NADPH oxidase and superoxide and stimulation of dehydroascorbate reductase. J. Gen. Virol. 2008, 89, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, Z.; Zhu, Y.; Hua, J. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol. Plant.-Microbe Interact. 2009, 22, 498–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnova, A.; Li, H.; Weingart, H.; Aufhammer, S.; Burse, A.; Finis, K.; Schenk, A.; Ullrich, M.S. Thermoregulated expression of virulence factors in plant-associated bacteria. Arch. Microbiol. 2001, 176, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Castroverde, C.D.M.; Velásquez, A.C.; Hubbard, E.; Pulman, J.A.; Yao, J.; Childs, K.L.; Tsuda, K.; Montgomery, B.L.; He, S.Y. Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat. Commun. 2017, 8, 1808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 16, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Mauch-Mani, B.; Mauch, F. The role of abscisic acid in plant–pathogen interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef]
- Jiang, C.J.; Shimono, M.; Sugano, S.; Kojima, M.; Yazawa, K.; Yoshida, R.; Inoue, H.; Hayashi, N.; Sakakibara, H.; Takatsuji, H. Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice–Magnaporthe grisea interaction. Mol. Plant. Microbe Interact. 2010, 23, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.K. Necrotroph. Encycl. Plant Pathol. 2001, 2, 676–677. [Google Scholar]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The plant cell wall: A complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 2016, 7, 984. [Google Scholar] [CrossRef] [Green Version]
- Janda, M.; Lamparová, L.; Zubíková, A.; Burketová, L.; Martinec, J.; Krčková, Z. Temporary heat stress suppresses PAMP-triggered immunity and resistance to bacteria in Arabidopsis thaliana. Mol. Plant Pathol. 2019, 20, 1005–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangappa, S.N.; Berriri, S.; Kumar, S.V. PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr. Biol. 2017, 27, 243–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, C.; Lüdke, D.; Klenke, M.; Quathamer, A.; Valerius, O.; Braus, G.H.; Wiermer, M. The truncated NLR protein TIR-NBS13 is a MOS6/IMPORTIN-α3 interaction partner required for plant immunity. Plant J. 2017, 92, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Achuo, E.A.; Prinsen, E.; Höfte, M. Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol. 2006, 55, 178–186. [Google Scholar] [CrossRef]
- Moffat, C.S.; Ingle, R.A.; Wathugala, D.L.; Saunders, N.J.; Knight, H.; Knight, M.R. ERF5 and ERF6 play redundant roles as positive regulators of JA/Et-mediated defense against Botrytis cinerea in Arabidopsis. PLoS ONE 2012, 7, e35995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengiste, T.; Chen, X.; Salmeron, J.; Dietrich, R. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 2003, 15, 2551–2565. [Google Scholar] [CrossRef] [Green Version]
- Epple, P.; Apel, K.; Bohlmann, H. Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum. Plant Cell 1997, 9, 509–520. [Google Scholar]
- Donoghue, M.T.; Keshavaiah, C.; Swamidatta, S.H.; Spillane, C. Evolutionary origins of Brassicaceae specific genes in Arabidopsis thaliana. BMC Evol. Biol. 2011, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Pitaksaringkarn, W.; Matsuoka, K.; Asahina, M.; Miura, K.; Sage-Ono, K.; Ono, M.; Yokoyama, R.; Nishitani, K.; Ishii, T.; Iwai, H.; et al. XTH 20 and XTH 19 regulated by ANAC 071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J. 2014, 80, 604–614. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, J.S.; Tsai, A.Y.L.; Xue, H.; Voiniciuc, C.; Šola, K.; Seifert, G.J.; Mansfield, S.D.; Haughn, G.W. SALT-OVERLY SENSITIVE5 mediates Arabidopsis seed coat mucilage adherence and organization through pectins. Plant Physiol. 2014, 165, 991–1004. [Google Scholar] [CrossRef] [Green Version]
- Seifert, G.J.; Xue, H.; Acet, T. The Arabidopsis thaliana FASCICLIN LIKE ARABINOGALACTAN PROTEIN 4 gene acts synergistically with abscisic acid signalling to control root growth. Ann. Bot. 2014, 114, 1125–1133. [Google Scholar] [CrossRef] [Green Version]
- Bu, Q.; Jiang, H.; Li, C.B.; Zhai, Q.; Zhang, J.; Wu, X.; Sun, J.; Xie, Q.; Li, C. Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid-signaled defense responses. Cell Res. 2008, 18, 756–767. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; El-Shetehy, M.; Shine, M.B.; Yu, K.; Navarre, D.; Wendehenne, D.; Kachroo, A.; Kachroo, P. Free radicals mediate systemic acquired resistance. Cell Rep. 2014, 7, 348–355. [Google Scholar] [CrossRef]
- L’haridon, F.; Besson-Bard, A.; Binda, M.; Serrano, M.; Abou-Mansour, E.; Balet, F.; Schoonbeek, H.J.; Hess, S.; Mir, R.; Léon, J.; et al. A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity. PLoS Pathog. 2011, 7, e1002148. [Google Scholar] [CrossRef]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef] [Green Version]


| Gene Locus | Gene Name | Abiotic Stress Treatment | ||
|---|---|---|---|---|
| O | H | O-H | ||
| AT3G22231 | PCC1 (pathogen and circadian controlled 1) | −5.16 | −14.93 | −27.67 |
| AT1G33960 | AIG1 (AVRRPT2-INDUCED GENE 1) | −3.99 | −5.91 | −8.38 |
| AT1G65390 | PP2-A5 (phloem protein 2 A5) | −2.04 | −5.24 | −8.12 |
| AT1G33950 | AIG1 (avirulence induced gene family protein) | −6.02 | −5.06 | −6.19 |
| AT1G13609 | Defensin-like (DEFL) family protein | −11.13 | −2.69 | −6.07 |
| AT1G59780 | NB-ARC domain-containing disease resistance protein | −2.12 | −5.25 | −5.42 |
| AT3G48080 | Alpha/beta-Hydrolases superfamily protein | −1.67 | −2.87 | −4.29 |
| AT2G14580 | PRB1 (basic pathogenesis-related protein 1) | −1.79 | −1.93 | −4.29 |
| AT2G14610 | PR1 (pathogenesis-related gene 1) | −3.32 | −3.08 | −4.10 |
| AT5G25910 | RLP52 (receptor like protein 52) | 1.98 | −3.12 | −3.83 |
| AT5G44430 | PDF1.2c (plant defensin 1.2C) | −5.81 | −4.11 | −3.81 |
| AT5G44420 | PDF1.2 (plant defensin 1.2) | −6.00 | −4.52 | −3.69 |
| AT3G04210 | TN13 (Disease resistance protein, TIR-NBS class) | −1.43 | −2.83 | −3.42 |
| AT5G24200 | PR-protein, Alpha/beta-Hydrolases superfamily protein | −2.66 | −5.08 | −3.36 |
| AT2G26010 | PDF1.3 (plant defensin 1.3) | −8.33 | −3.34 | −3.24 |
| AT3G48720 | DCF (HXXXD-type acyl-transferase family protein) | −1.42 | −2.94 | −3.19 |
| AT2G26020 | PDF1.2b (plant defensin 1.2b) | −2.24 | −3.77 | −2.27 |
| AT5G36910 | THI2.2 (thionin 2.2) | −2.05 | 1.06 | −1.96 |
| AT1G75040 | PR5 (pathogenesis-related gene 5) | 3.11 | −2.62 | 1.94 |
| AT3G06490 | MYB108 or BOS1 (Botrytis-susceptible1) | −1.82 | −1.20 | −1.53 |
| Gene Locus | Gene Name/Function | Abiotic Stress Treatment | ||
|---|---|---|---|---|
| O | H | O-H | ||
| AT5G57550 | XTH25 (xyloglucan endotransglucosylase/hydrolase 25) | −4.94 | −1.94 | −10.34 |
| AT4G13410 | ATCSLA15 (encodes a gene similar to cellulose synthase) | −2.03 | −6.30 | −8.47 |
| AT5G48070 | XTH20 (xyloglucan endotransglucosylase/hydrolase 20) | −1.44 | −8.72 | −7.18 |
| AT4G15320 | CSLB06 (cellulose synthase-like B6) | −1.69 | −6.94 | −5.40 |
| AT1G19940 | GH9B5 (glycosyl hydrolase 9B5) | −1.16 | −4.64 | −4.73 |
| AT2G20870 | Cell wall protein precursor | −1.31 | −2.51 | −4.40 |
| AT2G45220 | Plant invertase/pectin methylesterase inhibitor | −2.43 | −2.19 | −3.66 |
| AT4G12730 | FLA2 (FASCICLIN-like arabinogalactan 2) | −1.51 | −2.28 | −3.00 |
| AT1G14080 | FUT6 (fucosyltransferase 6) | −1.43 | −3.88 | −2.97 |
| AT5G06870 | PGIP2 (polygalacturonase inhibiting protein 2) | −2.03 | −1.98 | −2.85 |
| AT4G13210 | Pectin lyase-like superfamily protein | −1.07 | −2.62 | −2.82 |
| AT3G27400 | Pectin lyase-like superfamily protein | −1.06 | −3.16 | −2.80 |
| AT5G26670 | Pectinacetylesterase family protein | −1.20 | −2.41 | −2.73 |
| AT2G26440 | Plant invertase/pectin methylesterase inhibitor | −0.81 | −2.46 | −2.72 |
| AT4G01630 | EXPA17 (expansin A17) | −0.98 | −4.18 | −2.71 |
| AT5G45280 | Pectinacetylesterase family protein | −1.14 | −2.00 | −2.69 |
| AT2G04780 | FLA7 (FASCICLIN-like arabinoogalactan 7) | −1.11 | −2.27 | −2.35 |
| AT4G24780 | Pectin lyase-like superfamily protein | −1.06 | −2.20 | −1.96 |
| AT1G24070 | CSLA10 (cellulose synthase-like A10) | −1.37 | −2.18 | −1.68 |
| AT1G35230 | AGP5 (arabinogalactan protein 5) | 2.88 | −2.78 | −1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sewelam, N.; El-Shetehy, M.; Mauch, F.; Maurino, V.G. Combined Abiotic Stresses Repress Defense and Cell Wall Metabolic Genes and Render Plants More Susceptible to Pathogen Infection. Plants 2021, 10, 1946. https://doi.org/10.3390/plants10091946
Sewelam N, El-Shetehy M, Mauch F, Maurino VG. Combined Abiotic Stresses Repress Defense and Cell Wall Metabolic Genes and Render Plants More Susceptible to Pathogen Infection. Plants. 2021; 10(9):1946. https://doi.org/10.3390/plants10091946
Chicago/Turabian StyleSewelam, Nasser, Mohamed El-Shetehy, Felix Mauch, and Veronica G. Maurino. 2021. "Combined Abiotic Stresses Repress Defense and Cell Wall Metabolic Genes and Render Plants More Susceptible to Pathogen Infection" Plants 10, no. 9: 1946. https://doi.org/10.3390/plants10091946







