An Assessment of the Phytoremediation Potential of Planted and Spontaneously Colonized Woody Plant Species on Chronosequence Fly Ash Disposal Sites in Serbia—Case Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical and Chemical Characteristics of Fly Ash
2.2. The Concentration of Examined Trace Elements in Fly Ash and Their Bioavailability
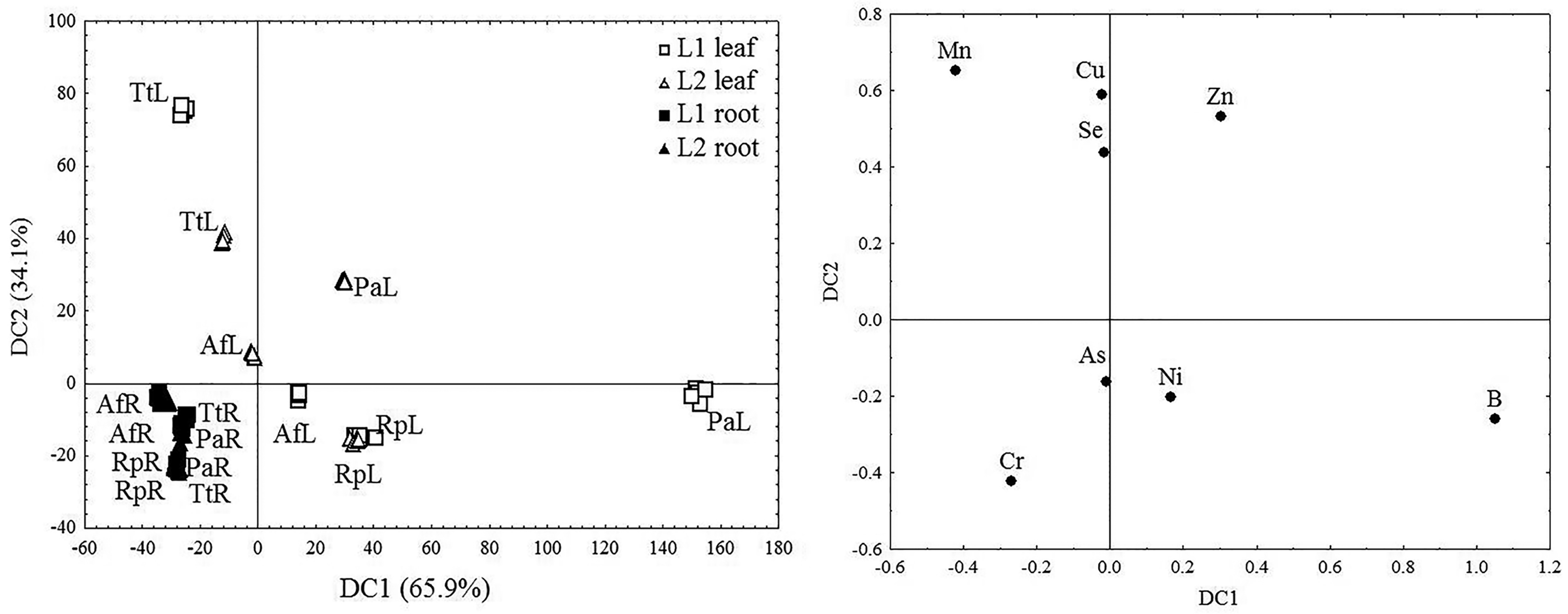
2.3. The Accumulation of Examined Trace Elements in the Roots and Leaves of Four Woody Plant Species
2.4. An Assessment of the Phytoremediation Potential of the Examined Woody Plant Species Based on Biological Indices
3. Materials and Methods
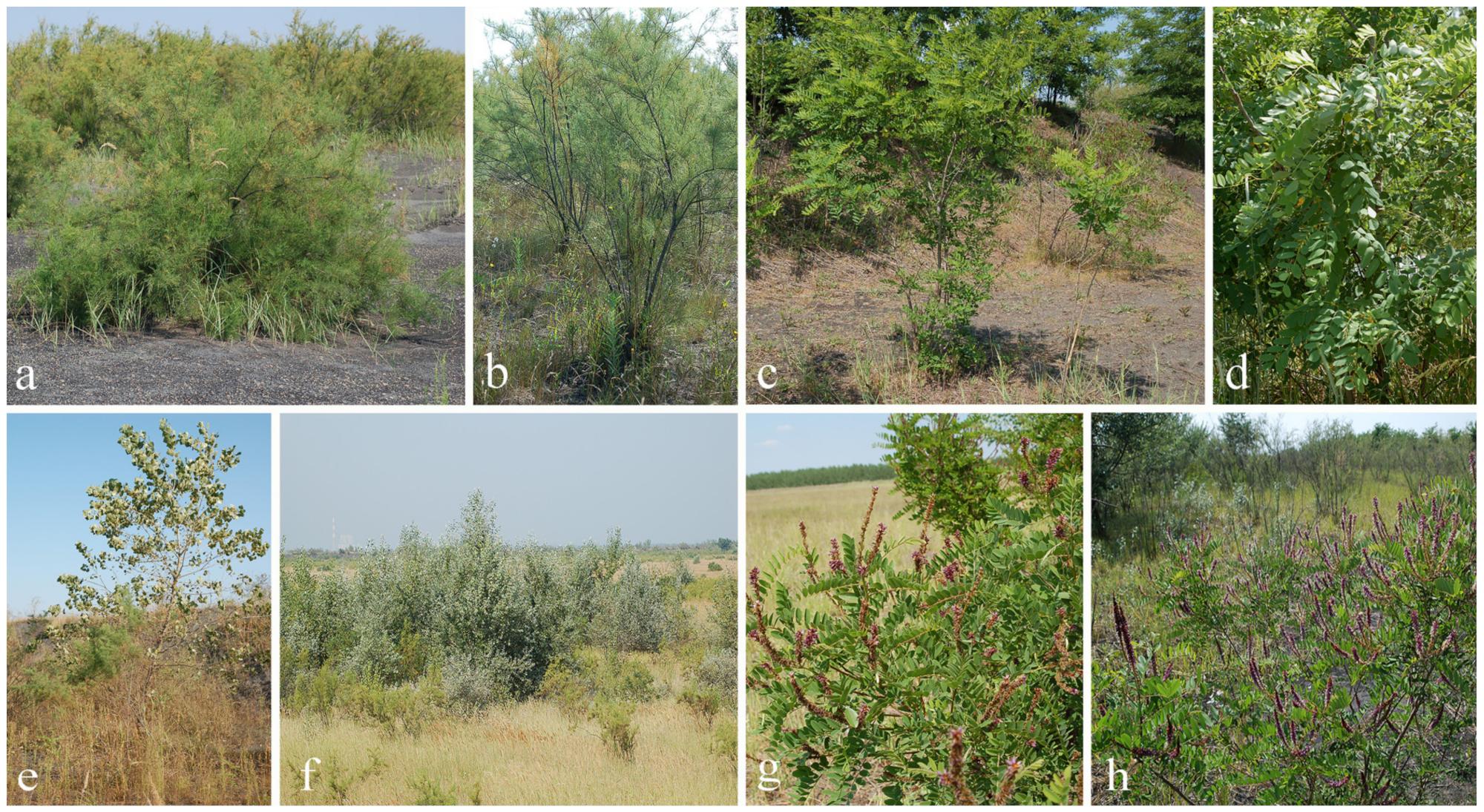
3.1. Study Sites Description
3.2. Plant Species Description
3.3. Sample Collection and Preparation
3.4. Physical and Chemical Analysis of Fly Ash
3.5. Pseudototal and Bioavailable Concentration of Trace Elements in Fly Ash
3.6. Estimation of Trace Elements in Plants
3.7. Chemicals
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, S.; Zhao, Z.; Jiang, S.; Sun, J.; Pan, H.; Jiang, L. Comparison and Summary of Relevant Standards for Comprehensive Utilization of Fly Ash at Home and Abroad. IOP Conf. Ser. Earth Environ. Sci. 2021, 621, 012006. [Google Scholar] [CrossRef]
- Asokan, P.; Saxena, M.; Asolekar, S.R. Coal combustion residues—Environmental implications and recycling potentials. Resour. Conserv. Recycl. 2005, 43, 239–262. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Popławska, A.; Kołodziej, B.; Ciarkowska, K.; Gambuś, F.; Bryk, M.; Babula, J. Application of ash and municipal sewage sludge as macronutrient sources in sustainable plant biomass production. J. Environ. Manag. 2020, 264, 110450. [Google Scholar] [CrossRef]
- Antonkiewicz, J. Effect of sewage sludge and furnace waste on the content of selected elements in the sward of legume-grass mixture. J. Elem. 2010, 15, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Gollakota, A.R.K.; Volli, V.; Shu, C.-M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef]
- Weber, J.; Kocowicz, A.; Debicka, M.; Jamroz, E. Changes in soil morphology of podzols affected by alkaline fly ash blown out from the dumping site of an electric power plant. J. Soils Sediments 2017, 17, 1852–1861. [Google Scholar] [CrossRef] [Green Version]
- Ćujić, M.; Dragović, S.; Djordjević, M.; Dragović, R.; Gajić, B. Environmental assessment of heavy metals around the largest coal fired power plant in Serbia. Catena 2016, 139, 44–52. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Ram, L.C.; Masto, R.E. Reclamation of overburden and lowland in coal mining area with fly ash and selective plantation: A sustainable ecological approach. Ecol. Eng. 2014, 71, 479–489. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, K.; Singh, R.P.; Singh, B. Naturally growing Saccharum munja L. on the fly ash lagoons: A potential ecological engineer for the revegetation and stabilization. Ecol. Eng. 2012, 40, 95–99. [Google Scholar] [CrossRef]
- Técher, D.; Laval-Gilly, P.; Bennasroune, A.; Henry, S.; Martinez-Chois, C.; D’Innocenzo, M.; Falla, J. An apprasial of Miscanthus x giganteus cultivation for fy ash revegetation and soil restoration. Ind. Crops Prod. 2012, 36, 427–433. [Google Scholar] [CrossRef]
- Haynes, R.J. Reclamation and revegetation of fy ash disposal sites–challenges and research needs. J. Environ. Manag. 2009, 90, 43–53. [Google Scholar] [CrossRef]
- Iyer, R. The surface chemistry of leaching coal fly ash. J. Hazard. Mater. 2002, 93, 321–329. [Google Scholar] [CrossRef]
- Geré, R.; Carleton, L.E.; Lumpkin, G.R. Micro- and nanochemistry of fly ash from a coal-fired power plant. Am. Mineral. 2003, 88, 1853–1865. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Rana, V.; Kumar, A.; Kumar Maiti, S. Biodiversity variability and metal accumulation strategies in plants spontaneously inhibiting fly ash lagoon. India. Environ. Sci. Pollut. Res. 2017, 24, 22990–23005. [Google Scholar] [CrossRef] [PubMed]
- Kostić, O.; Mitrović, M.; Knežević, M.; Jarić, S.; Gajić, G.; Djurdjević, L.; Pavlović, P. The potential of four woody species for the revegetation of fly ash deposits from the ‘Nikola Tesla-A’ thermoelectric plant (Obrenovac, Serbia). Arch. Biol. Sci. 2012, 64, 145–158. [Google Scholar] [CrossRef]
- Krzaklewski, W.; Pietrzykowski, M.; Woś, B. Survival and growth of alders (Alnus glutinosa (L.) Gaertn. and Alnus incana (L.) Moench) on fly ash technosols at different substrate improvement. Ecol. Eng. 2012, 49, 35–40. [Google Scholar] [CrossRef]
- Mitrović, M.; Pavlović, P.; Lakušić, D.; Djurdjević, L.; Stevanović, B.; Kostić, O.; Gajić, G. The potential of Festuca rubra and Calamagrostis epigejos for the revegetation of fy ash deposits. Sci. Ttotal Environ. 2008, 407, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Sinha, S. Decontamination and/or revegetation of fly ash dykes through naturally growing plants. J. Hazard. Mater. 2008, 153, 1078–1087. [Google Scholar] [CrossRef]
- Pavlović, P.; Mitrović, M.; Djurdjević, L. An ecophysi-ological study of plants growing on the fy ash deposits from the “Nikola tesla—A” thermal power station in Serbia. Environ. Manage. 2004, 33, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Goliński, P.; Krzesłowska, M.; Gąsecka, M.; Magdziak, Z.; Rutkowski, P.; Budzyńska, S.; Waliszewska, B.; Kozubik, T.; Karolewski, Z.; et al. Phytoextraction of potentially toxic elements by six tree species growing on hazardous mining sludge. Environ. Sci. Pollut. Res. 2017, 24, 22183–22195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Stevanović, B.; Mitrović, M.; Pavlović, P. Phytoremediation potential, photosynthetic and antioxidant response to arsenic—Induced stress of Dactylis glomerata L. sown on fly ash deposits. Plants 2020, 9, 657. [Google Scholar] [CrossRef]
- Maiti, D.; Prasad, B. Studies on colonisation of fly ash disposal sites using invasive species and aromatic grasses. J. Environ. Eng. Landsc. Manag. 2017, 25, 251–263. [Google Scholar] [CrossRef]
- Pandey, V.C. Invasive species based efficient green technology for phytoremediation of fly ash deposits. J. Geochem. Explor. 2012, 123, 13–18. [Google Scholar] [CrossRef]
- Čermak, P. Forest reclamation of dumpsites of coal combustion by-products (CCB). J. For. Sci. 2008, 54, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P.J.A. A Preliminary Study of Successional Changes in Vegetation and Soil Development on Unamended Fly Ash (PFA) in Southern England. J. Appl. Ecol. 1992, 29, 728–736. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Gąsecka, M.; Magdziak, Z.; Budzyńska, S.; Szostek, M.; Niedzielski, P.; Budka, A.; Roszyk, E.; Doczekalska, B.; Górska, M.; et al. The Possibility of Using Paulownia elongata S. Y. Hu × Paulownia fortunei Hybrid for Phytoextraction of Toxic Elements from Post-Industrial Wastes with Biochar. Plants 2021, 10, 2049. [Google Scholar] [CrossRef] [PubMed]
- Kostić, O.; Jarić, S.; Gajić, G.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Pedological properties and ecological implications of substrates derived 3 and 11 years after the revegetation of lignite fly ash disposal sites in Serbia. Catena 2018, 163, 78–88. [Google Scholar] [CrossRef]
- Żołnierz, L.; Weber, J.; Gilewska, M.; Strączyńska, S.; Pruchniewicz, D. The spontaneous development of understory vegetation on reclaimed and afforested post-mine excavation filled with fly ash. Catena 2016, 136, 84–90. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, B. Rehabilitation of coal fly ash basind: Current need to use ecological engineering. Ecol. Rng. 2012, 49, 190–192. [Google Scholar] [CrossRef]
- Kostić, O.; Mitrović, M.; Pavlović, P. Impact of Weathering and Revegetation on Pedological Characteristics and Pollutant Dispersion Control at Coal Fly Ash Disposal Sites. In Advances in Understanding Soil Degradation. Innovations in Landscape Research; Saljnikov, E., Mueller, L., Lavrishchev, A., Eulenstein, F., Eds.; Springer: Cham, Switzerland, 2022; pp. 473–505. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Ahirwal, J.; Maiti, S.K.; Das, R. An assessment of metal in fly ash and their translocation and bioaccumulation in perennial grasses growing at the reclaimed opencast mines. Int. J. Environ. Res. 2015, 9, 1089–1096. [Google Scholar] [CrossRef]
- Maiti, S.K.; Jaiswal, S. Bioaccumulation and translocation of metals in the natural vegetation growing on fly ash lagoons: A field study from Santaldih thermal power plant, West Bengal, India. Environ. Monit. Ass. 2008, 136, 355–370. [Google Scholar] [CrossRef]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Mitrović, M.; Pavlović, P. Ecological Potential of Plants for Phytoremediation and Ecorestoration of Fly Ash Deposits and Mine Wastes. Front. Environ. Sci. 2018, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.K.; Herat, S.; Tandon, P. Phytoremediation: Role of Plants in Contaminated Site Management. In Environmental Bioremediation Technologies; Singh, S.N., Tripathi, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 315–330. [Google Scholar]
- Leitenmaier, B.; Küpper, H. Compartmentation and complexation of metals in hyperaccumulator plants. Front. Plant Sci. 2013, 4, 374. [Google Scholar] [CrossRef] [Green Version]
- Pilon-Smits, E.; LeDuc, D.L. Phytoremediation of selenium using transgenic plants. Curr. Opin. Biotech. 2009, 20, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, K.; Xu, J.; Yhang, Z.; Ma, T.; Lu, X.; Zang, J.; Zhu, Q. Lead toxicity, uptake and translocation in different rice cultivars. Plant Sci. 2004, 165, 793–802. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, Toxicity, and Detoxification in Plants. In Reviews of Environmental Contamination and Toxicology; (Continuation of Residue Reviews); Whitacre, D., Ed.; Springer: New York, NY, USA, 2011; Volume 213. [Google Scholar] [CrossRef] [Green Version]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments-an emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Mitrović, M.; Stevanović, B.; Pavlović, P. Assessment of the phytoremediation potential and an adaptive response of Festuca rubra L. sown on fly ash deposits: Native grass has a pivotal role in ecorestoration management. Ecol. Eng. 2016, 93, 250–261. [Google Scholar] [CrossRef]
- Wang, J.J.; Harrell, D.L.; Henderson, R.E.; Bell, P.F. Comparison of soil-test extractants for phosphorus, potassium, calcium, magnesium, sodium, zinc, copper, manganese, and iron in Louisiana soils. Commun. Soil Sci. Plant Anal. 2004, 35, 145–160. [Google Scholar] [CrossRef]
- Massas, I.; Kalivas, D.; Ehaliotis, C.; Gasparatos, D. Total and available heavy metal concentrations in soils of the Thriassio plain (Greece) and assessment of soil pollution indexes. Environ. Monit. Ass. 2013, 185, 6751–6766. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching behavior of elements from coal combustion fly ash: An overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants; CRC Press LLC: Boca Raton, FL, USA, 2001; p. 413. [Google Scholar]
- De Groot, G.J.; Wijkstra, J.; Hoede, D.; van der Sloot, H.A. Leaching characteristics of selected elements from coal fly ash as function of the acidity of the contact solution and the liquid/solid ratio. In Environmental Aspects of Stabilization and Solidification of Hazardous and Radioactive Wastes; Cote, P.L., Gilliam, T.M., Eds.; American Society fof Testing and Materials: West Conshohocken, PA, USA, 1989; pp. 170–183. [Google Scholar]
- Panda, D.; Mandal, L.; Barik, J. Phytoremediation potential of naturally growing weed plants grown on fly ash-amended soil for restoration of fly ash deposit. Int. J. Phytoremediation 2020, 22, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Bhattacharya, T.; Chakraborty, S. Metal phytoremediation potential of naturally growing plants on fly ash dumpsite of Patratu thermal power station, Jharkhand, India. Int. J. Phytoremediation 2016, 18, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Krgović, R.; Trifković, J.; Milojković-Opsenica, D.; Manojlović, D.; Marković, M.; Mutić, J. Phytoextraction of metals by Erigeron canadensis L. from fly ash landfill of power plant “Kolubara”. Environ. Sci. Pollut. Res. 2015, 22, 10506–10515. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C.; Singh, N.; Singh, R.P.; Singh, D.P. Rhizomediation potential of spontaneous grown Typha latifolia on fly ash basins: Study from the field. Ecol. Eng. 2014, 71, 722–727. [Google Scholar] [CrossRef]
- Pandey, V.C. Phytoremediation of heavy metals from fly ash pond by Azolla caroliniana. Ecotoxicol. Environ. Saf. 2012, 82, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Srivastava, S.; Mishra, S.; Dixit, B.; Kumar, A.; Tripathi, R.D. Screening of native plants and algae growing on fly-ash affected areas near National Thermal Power Corporation, Tanda, Uttar Pradesh, India for accumulation of toxic heavy metals. J. Hazard. Mater. 2008, 158, 359–365. [Google Scholar] [CrossRef]
- Maiti, S.K.; Nandhini, S. Bioavailability of metals in fly ash and their bioaccumulation in naturally occurring vegetation: A pilot scale study. Environ. Monit. Ass. 2006, 116, 263–273. [Google Scholar] [CrossRef]
- Pandey, V.C.; Mishra, T. Assessment of Ziziphus mauritiana grown on fly ash dumps: Prospects for phytoremediation but concerns with the use of edible fruit. Int. J. Phytoremediation 2018, 20, 1250–1256. [Google Scholar] [CrossRef]
- Jambhulkar, H.P.; Juwarkar, A.A. Assessment of bioaccumulation of heavy metals by different plant species grown on fly ash dump. Ecotoxicol. Environ. Saf. 2009, 72, 1122–1128. [Google Scholar] [CrossRef]
- Cheung, K.C.; Wong, J.P.K.; Zhang, Z.Q.; Wong, J.W.C.; Wong, M.H. Revegetation of lagoon ash using the legume species Acacia auriculiformis and Leucaena leucocephala. Environ. Pollut. 2000, 109, 75–82. [Google Scholar] [CrossRef]
- Vajpayee, P.; Rai, U.; Choudhary, S.; Tripathi, R.D.; Singh, S.N. Management of fly ash landfills with Cassia surattensis Burm: A case study. Bull. Environ. Contam. Toxicol. 2000, 65, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Dellantonio, A.; Fitz, J.W.; Custovac, H.; Repmann, F.; Schneider, U.B.; Grünewald, H.; Gruber, V.; Zgorelec, Z.; Zerem, N.; Carter, C.; et al. Environmental risk of farmed and barren alkaline coal ash landfills in Tuzla, Bosnia and Herzegovina. Environ. Pollut. 2008, 153, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Yang, Y.; An, S.; Zhu, Z. Effects of different vegetation restoration measures on soil aggregate stability and erodibility on the Loess Plateau, China. Catena 2020, 185, 104294. [Google Scholar] [CrossRef]
- Alday, J.G.; Marrs, R.H.; Martinez-Ruiz, C. Soil and vegetation development during early succession on restored coal wastes: A six-year permanent plot study. Plant Soil. 2012, 353, 305–320. [Google Scholar] [CrossRef]
- Gonsoulin, G.J. A Study of Plant Succession on Three TVA Fly Ash Pits in Middle Tennessee. Castanea 1975, 40, 45–56. Available online: https://www.jstor.org/stable/pdf/4032839.pdf (accessed on 21 August 2021).
- Ram, L.C.; Jha, S.K.; Tripathi, R.C.; Mesto, R.E.; Selvi, V.A. Remediation of Fly Ash Landfills Through Plantation. Remed. J. 2008, 18, 71–9056. [Google Scholar] [CrossRef]
- Frouz, J.; Prach, K.; Pižl, V.; Háněl, L.; Starý, J.; Tajovský, K.; Materna, J.; Balík, V.; Kalčík, J.; Řehounková, K. Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. Eurasian J. Soil Biol. 2008, 44, 109–121. [Google Scholar] [CrossRef]
- Rippon, J.E.; Wood, M.J. Microbal aspects of pulverized fuel ash. In The Ecology of Resource Degradation and Renewal; Chadwick, M.J., Goodman, G.T., Eds.; Blackwell Scientific Publications: Oxford, UK, 1975; pp. 331–349. [Google Scholar]
- Helingerová, M.; Frouz, J.; Šantrüčková, H. Microbal activity in reclaimed and unreclaimed post-mining sites near Sokolov (Czech Republic). Ecol. Eng. 2010, 36, 768–776. [Google Scholar] [CrossRef]
- Machulla, G.; Zikeli, S.; Kastler, M.; Jahn, R. Microbal biomass and respiration in soils derived from lignite ashes: A profile study. J. Plant. Nutr. Soil Sci. 2004, 167, 449–456. [Google Scholar] [CrossRef]
- Filcheva, E.; Noustorova, M.; Gentcheva-Konstadiniva, V.S.; Haigh, J.M. Organic accumulation and microbal action in surface coal-mine spoils, Pernik, Bulgaria. Ecol. Eng. 2000, 15, 1–15. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rai, U.N.; Tripathi, R.M.; Inouhe, M. Impacts of fly ash on soil and plant responses. J. Plant Res. 2002, 115, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Bendfeldt, E.S.; Burger, J.A.; Daniels, W.L. Quality of amended mine soils after sixteen years. Soil Sci. Soc. Am. J. 2001, 65, 1736–1744. [Google Scholar] [CrossRef]
- Jones, L.H.; Lewis, A.V. Weathering of fly-ash. Nature 1960, 185, 404–405. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. Int. 2021, 28, 30528–30550. [Google Scholar] [CrossRef]
- Samuel, A.D.; Bungau, S.; Tit, D.M.; Melinte (Frunzulica), C.E.; Purza, L.; Badea, G.E. Effects of Long Term Application of Organic and Mineral Fertilizers on Soil Enzymes. Rev. Chim. 2018, 69, 2608–2612. [Google Scholar] [CrossRef]
- Uzarowicz, Ł.; Zagórski, Z.; Mendak, E.; Bartmiński, P.; Szara, E.; Kondras, M.; Oktaba, L.; Turek, A.; Rogoziński, R. Technogenic soils (Technosols) developed from fly ash and bottom ash from thermal power stations combusting bituminous coal and lignite. Part, I. Properties, classification, and indicators of early pedogenesis. Catena 2017, 157, 75–89. [Google Scholar] [CrossRef]
- Weber, J.; Strączyńska, S.; Kocowicz, A.; Gilewska, M.; Bogacz, A.; Gwiżdż, M.; Debicka, M. Properties of soil materials derived from fly ash 11 years after revegetation of post-mining excavation. Catena 2015, 133, 250–254. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, S.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Garousi, F. The essentiality of selenium for humans, animals, and plants, and the role of selenium in plant metabolism and physiology. Acta Univ. Sapientiae Aliment. 2017, 10, 75–90. [Google Scholar] [CrossRef] [Green Version]
- Alloway, B.J. Heavy Metals in Soil; Blackie and Son Ltd.: London, UK, 1990; p. 339. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Shannon, M.C.; Ajwa, H.; Draper, J.H.; Jordahl, J.; Licht, J. Phytoextraction and accumulation of boron and selenium by Poplar (Populus) hybrid clones. Int. J. Phytoremediation 1999, 1, 81–96. [Google Scholar] [CrossRef]
- Cornelis, G.; Johnson, C.A.; Gerven, T.V.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Davis, A.P.; Kim, K.W. Stabilization of available arsenic in highly contaminated mine tailings using iron. Environ. Sci. Technol. 2003, 37, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Djurdjević, L.; Mitrović, M.; Pavlović, P.; Gajić, G.; Kostić, O. Phenolic acids as bioindicators of fy ash deposit revegetation. Arch. Environ. Contam. Toxicol. 2006, 50, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mulligan, C.N. Effect of natural organic matter on arsenic release from soil and sediments into groundwater. Environ. Geochem. Health 2006, 28, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Waren, C.J.; Evans, L.J.; Sheard, R.W. Release of some trace elements from sluiced fly ash in acidic soils with particular reference to boron. Waste Manag. Res. 1993, 11, 3–15. [Google Scholar] [CrossRef]
- Iwashita, A.; Sakaguchi, Y.; Nakajima, T.; Takanashi, H.; Ohki, A.; Kambra, S. Leaching characteristics of boron and selenium for various coal fly ashes. Fuel 2005, 84, 479–485. [Google Scholar] [CrossRef]
- Aldmour, S.T.; Burke, I.T.; Bray, A.W.; Baker, D.L.; Ross, A.B.; Gill, F.L.; Cibin, G.; Ries, M.E.; Stewart, D.I. Abiotic reduction of Cr(VI) by humic acids derived from peat and lignite: Kinetics and removal mechanism. Environ. Sci. Pollut. Res. 2019, 26, 4717–4729. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Terzić, A.; Radojević, Z.; Miličić, L.J.; Pavlović, L.J.; Aćimović, Z. Leaching of the potentially toxic pollutants from composites based on waste raw material. Chem. Ind. Chem. Eng. Q. 2012, 18, 373–383. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; Garcia-Sanchez, A. Palygorskite as a feasible amendment to stabilize heavy metal polluted soils. Environ. Pollut. 2003, 125, 337–344. [Google Scholar] [CrossRef]
- Querol, X.; Umana, J.C.; Alastuey, A.; Ayora, C.; Lopez-Soler, A.; Plana, F. Extraction of soluble major and trace elements from fly ash in open and closed leaching systems. Fuel 2001, 80, 801–813. [Google Scholar] [CrossRef]
- Filipović-Trajković, R.; Ilić, S.Z.; Šunić, L.; Andjelković, S. The potential of different plant species for heavy metals accumulation and distribution. J. Food Agric. Environ. 2012, 10, 959–964. [Google Scholar]
- Robinson, B.H.; Green, S.R.; Chancerel, B.; Mills, T.M.; Clothier, B.E. Poplar for the phytomanagement of boron contaminated sites. Environ. Pollut. 2007, 150, 225–233. [Google Scholar] [CrossRef]
- Rees, R.; Robinson, B.H.; Menon, M.; Lehmann, E.; Günthardt-Goerg, M.S.; Schulin, R. Boron Accumulation and Toxicity in Hibrid Poplar (Populus nigra x euroamericana). Environ. Sci. Technol. 2011, 45, 10538–10543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.M. Responses of maize and sorghum to excess boron and salinity. Biol. Plant. 2003, 47, 313–316. [Google Scholar] [CrossRef]
- El-Motaium, R.; Hu, H.; Brown, P.H. The relative tolerance of six Prunus rootstocks to boron and salinity. J. Am. Soc. Hortic. Sci. 1994, 119, 1169–1175. [Google Scholar] [CrossRef]
- Ohrtman, M.K.; Shera, A.A.; Lairc, B.D. Quantifying soil salinity in areas invaded by Tamarix spp. J. Arid Environ. 2012, 85, 114–121. [Google Scholar] [CrossRef]
- Yermiyahu, U.; Ben-Gal, A.; Keren, R.; Redi, R.J. Combined effect of salinity and boron on plant growth and yield. Plant Soil 2008, 304, 73–87. [Google Scholar] [CrossRef]
- Hsiao, C.M.; Wang, H.P.; Wei, L.Y.; Chang, J.E.; Jou, J.C. Speciation of copper in the incineration fly ash of a municipal solid waste. J. Hazard. Mater. 2002, 91, 301–307. [Google Scholar] [CrossRef]
- Mitrović, M.; Jaić, S.; Kostić, O.; Gajić, G.; Karadžić, B.; Djurdjević, L.; Oberan, L.; Pavlović, D.; Pavlović, M.; Pavlović, P. Photosynthetic Efficiency of Four Woody Species Growing on Fly Ash Deposits of a Serbian “Nikola Tesla—A” Thermoelecric Plant. Pol. J. Environ. Stud. 2012, 21, 1339–1347. [Google Scholar]
- Brown, P.H. Ni. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 329–350. [Google Scholar]
- Singh, J.P.; Dahiya, D.J.; Narwal, R.P. Boron uptake and toxicity in wheat in relation to zinc supply. Fert. Res. 1990, 24, 105–110. [Google Scholar] [CrossRef]
- Mataruga, Z.; Jarić, S.; Marković, M.; Pavlović, M.; Pavlović, D.; Jakovljević, K.; Mitrović, M.; Pavlović, P. Evaluation of Salix alba, Juglans regia and Populus nigra as biomonitors of PTEs in the riparian soils of the Sava River. Environ. Monit. Ass. 2020, 192, 131. [Google Scholar] [CrossRef]
- Madejón, P.; Marañón, T.; Murillo, J.M.; Robinson, B. White poplar (Populus alba) as a biomonitor of trace elements in contaminated riparian forests. Environ. Pollut. 2004, 132, 145–155. [Google Scholar] [CrossRef]
- Kryvoruchko, I.S. Zn-use efficiency for optimization of symbiotic nitrogen fixation in chickpea (Cicer arietinum L.). Turk. J. Bot. 2017, 41, 423–441. [Google Scholar] [CrossRef]
- Wang, J.R.; Zhao, F.J.; Meharg, A.A.; Raab, A.; Feldmann, J.; McGrath, S.P. Mechanisms of arsenic hyperaccumulation in Pteris vittata: Arsenic species uptake kinetics and interaction with phosphate. Plant Physiol. 2002, 130, 1552–1561. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Akram, M.; Abbas, G.; Murtaza, B.; Shahid, M.; Shah, N.S.; Bibi, I.; Niazi, N.K. Arsenic tolerance and phytoremediation potential of Conocarpus erectus L. and Populus deltoides L. Int. J. Phytoremediation 2017, 19, 985–991. [Google Scholar] [CrossRef]
- Pokhrel, G.R.; Wang, K.T.; Zhuang, H.; Wu, Y.; Chen, W.; Lan, Y.; Zhu, X.; Li, Z.; Fu, F.; Yang, G. Effect of selenium in soil on the toxicity and uptake of arsenic in rice plant. Chemosphere 2020, 239, 124712. [Google Scholar] [CrossRef] [PubMed]
- Cervilla, L.M.; Blasco, B.; Rìos, J.J.; Romero, L.; Ruiz, J.M. Oxidative stress and antioxidants in tomato (Solanum lycopersicum) plants subjected to boron toxicity. Ann. Bot. 2007, 100, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, K.; Takano, J.; Omori, H.; Seki, M.; Shinozaki, K.; Fijiwara, T. Plants tolerant of high boron levels. Science 2007, 318, 1417. [Google Scholar] [CrossRef]
- Sutton, T.; Baumann, U.; Hayes, J.; Collins, N.C.; Shi, B.J.; Schnurbusch, T.; Hay, A.; Mayo, G.; Pallotta, M.; Tester, M.; et al. Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 2007, 318, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Pathak, B.; Fulekar, M.H. Heavy Metal Accumulation by Plant Species at Fly-Ash Dumpsites: Thermal Power Plant, Gandhinagar, Gujarat. Int. J. Plant Environ. 2019, 5, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.D.; Vajpayee, P.; Singh, N.; Rai, U.N.; Kumar, A.; Ali, M.B.; Kumar, B.; Yunis, M. Efficiency of various amendments for amelioration of fly-ash toxicity: Growth performance and metal composition of Casia siamea Lamk. Chemosphere 2004, 54, 1581–1588. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Shahsavari, M.; Rezaei, M. A general overview on manganese (Mn) importance for crops production. Aust. J. Basic Appl. Sci. 2011, 5, 1799–1803. [Google Scholar]
- Ellis, D.R.; Salt, D.E. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Zayed, A.M.; Lytle, C.M.; Terry, N. Accumulation and volatilization of different chemical species of selenium by plants. Planta 1998, 206, 284–292. [Google Scholar] [CrossRef]
- Chen, C.; Huang, D.; Liu, J. Functions and Toxicity of Nickel in Plants: Recent Advances and Future Prospects. Clean 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.-J.; Wood, B.A.; Raab, A.; McGrath, S.P.; Zhao, F.-J.; Feldmann, J. Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol. 2010, 152, 2211–2221. [Google Scholar] [CrossRef] [Green Version]
- Thoresby, P.; Thortnton, I. Heavy metals and arsenic in soil, pasture herbage and barely in some mineralised areas in Britain. In Trace Supstances in Environmental Health; Hemphill, D.D., Ed.; University of Missouri: Columbia, MO, USA, 1979; Volume 3, pp. 93–103. [Google Scholar]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- MP TENT 2004. Main Project for the Recultivation of the Ash and Slag Deposit Sites at TENT-A and TENT-B; Soil Institute: Belgrade, Serbia, 2004. (In Serbian) [Google Scholar]
- Dawalibi, V.; Monteverdi, M.C.; Moscatello, S.; Battistelli, A.; Valentini, R. Effect of salt and drought on growth, physiological and biochemical responses of two Tamarix species. iForest 2015, 8, 772–779. [Google Scholar] [CrossRef] [Green Version]
- Sabo, A.E. Robinia pseudoacacia Invasions and Control in North America and Europe. Restor. Reclam. Rev. 2000, 6, 1–9. Available online: https://hdl.handle.net/11299/59729 (accessed on 15 July 2021).
- Kozuharova, E.; Matkowski, A.; Wózniak, D.; Simeonova, R.; Naychov, Z.; Malainer, C.; Mocan, A.; Nabavi, S.M.; Atanasov, A.G. Amorpha fruticosa—A Noxious Invasive Alien Plant in Europe or a Medicinal Plant against Metabolic Disease? Front. Pharmacol. 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caudullo, G.; de Rigo, D. Populus alba in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; The Publications Office of the European Union: Luxembourg, 2016; pp. 134–135. [Google Scholar]
- Simakov, V.N. The use of phenylanthranilic acid in the determination of humus by Tyurin’s method. Pochvovedenie 1957, 8, 72–73. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchungen uber die chemische Bodenanalyse als Grundlage fur die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor-und Kaliumbestimmung. Kungliga Lantbrukshögskolans Annaler 1960, 26, 199–215. [Google Scholar]
- Kappen, H. Die Bodenazidität in ihrer Bedeutung für den Bodenfruchtabarkeitzustand sowie die Methoden ihrer Erkennung und der Bestimmung des Kalkbedarfes der sauren Böden. In Handbuch der Bodenlehre; Springer: Berlin/Heidelberg, Germany, 1931. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil. Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. USEPA Method 3052. Microwave assisted acid digestion of siliceous and organically based matrices. In Test Methods for Evaluating Solid Waste, SW 846; U.S. Environmental Protection Agency: Washington, DC, USA, 1996. [Google Scholar]
- Baker, A.J.M. Accumulators and excluders −strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
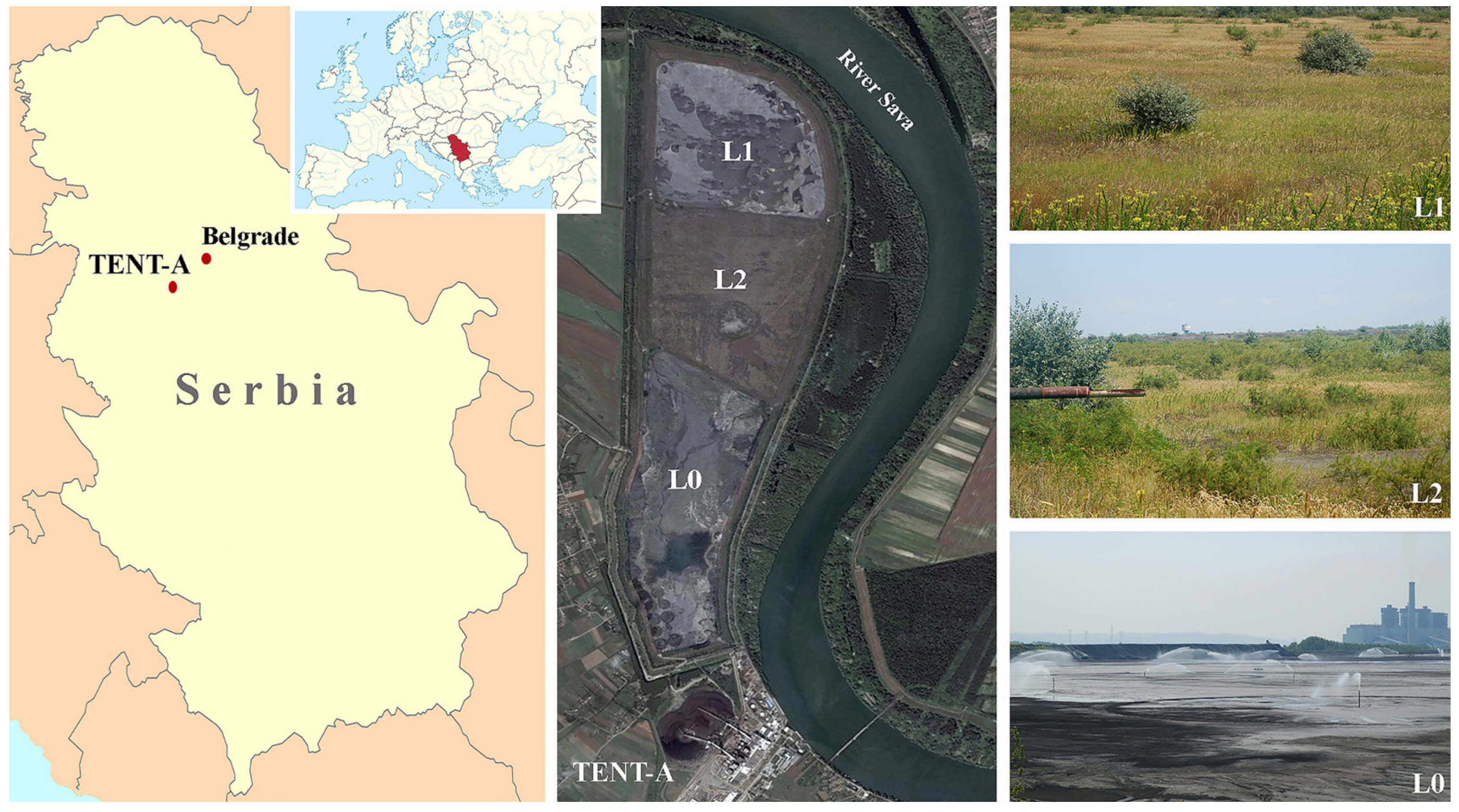
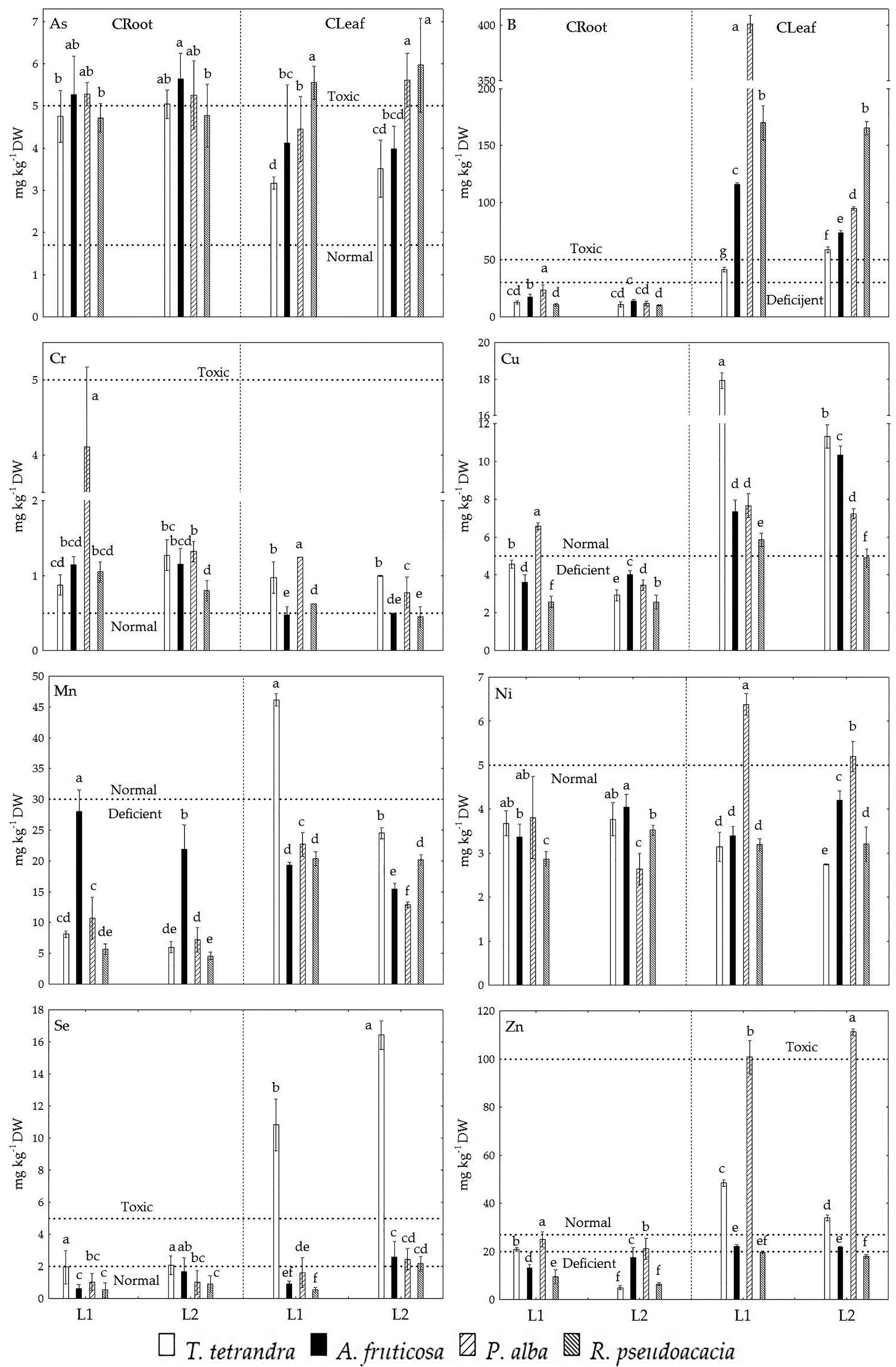
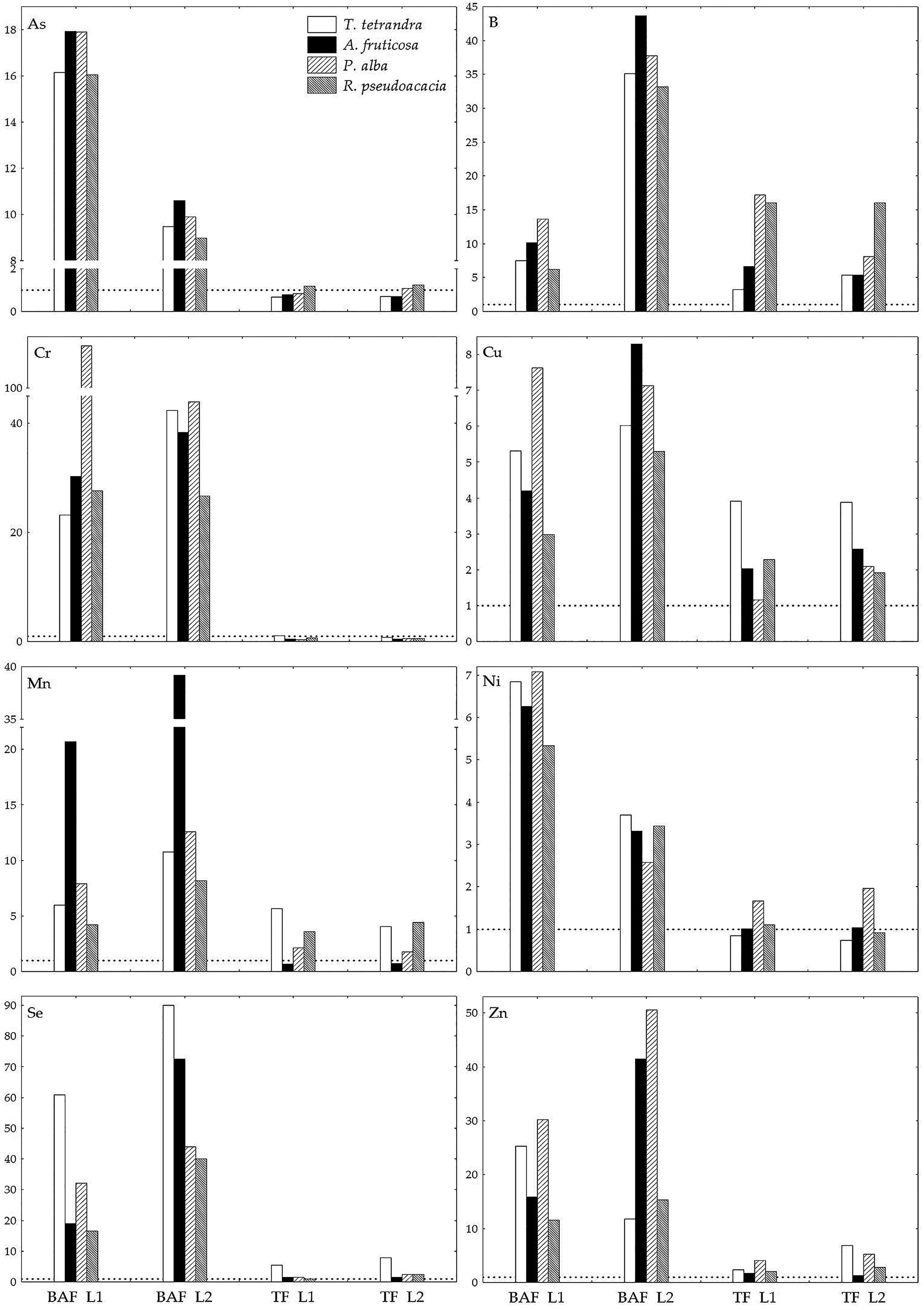
| Sand | Silt + Clay | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | 2.0–0.02 mm | <0.02 mm | pHH2O | pHKCl | EC | CEC | C | N | C/N | K2O | P2O5 | |
| % | % | dS m−1 | cmol kg−1 | % | % | mg/100 g | mg/100 g | |||||
| L1 | M (SD) | 83.33 (1.48) a | 16.67 (1.48) b | 7.95 (0.08) a | 7.25 (0.05) a | 0.27 (0.02) a | 47.13 (3.90) b | 1.85 (0.16) a | 0.05 (0.00) b | 37.0 | 44.40 (2.59) b | 19.12 (1.35) a |
| L2 | M (SD) | 71.60 (2.51) b | 28.40 (2.53) a | 7.72 (0.02) b | 6.74 (0.14) b | 0.19 (0.01) b | 67.02 (1.16) a | 1.13 (0.23) b | 0.11 (0.02) a | 10.27 | 52.50 (0.41) a | 10.01 (2.05) b |
| Site | As | B | Cr | Cu | Mn | Ni | Se | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CPT (mg kg−1) | L1 | M (SD) | 21.62 (2.53) a | 41.28 (4.33) a | 87.26 (9.79) a | 49.51 (5.32) a | 243.95 (11.0) a | 73.97 (8.31) a | 1.96 (0.11) a | 49.95 (6.57) a |
| L2 | M (SD) | 12.95 (0.53) b | 22.37 (1.21) b | 40.02 (4.84) b | 36.12 (2.39) b | 160.85 (9.01) b | 86.27 (13.61) a | 1.85 (0.51) a | 19.55 (2.51) b | |
| CDTPA (mg kg−1) | L1 | M (SD) | 0.29 (0.01) b | 1.71 (0.06) a | 0.04 (0.00) a | 0.86 (0.02) a | 1.36 (0.04) a | 0.54 (0.01) b | 0.03 (0.01) a | 0.82 (0.09) a |
| L2 | M (SD) | 0.53 (0.02) a | 0.31 (0.01) b | 0.03 (0.00) b | 0.48 (0.02) b | 0.56 (0.03) b | 1.02 (0.04) a | 0.02 (0.01) a | 0.42 (0.13) b | |
| AR (%) | L1 | 1.36 | 4.14 | 0.04 | 1.72 | 0.56 | 0.73 | 1.64 | 1.65 | |
| L2 | 4.12 | 1.40 | 0.06 | 1.34 | 0.35 | 1.18 | 1.25 | 2.14 | ||
| Typical range in fly ash (mg kg−1) [11] | 2–70 | 2–5000 | 3–900 | 10–2000 | 30–3000 | 10–3000 | 0.2–50 | 10–1000 | ||
| Average range in soil (mg kg−1) [45] | 4.4–8.4 | 22–40 | 47–51 | 13–23 | 270–525 | 13–26 | 0.25–0.34 | 45–60 | ||
| Critical range in soil (mg kg−1) [77] | 20–50 | - | 75–100 | 60–125 | 1500–3000 | 100 | 5–10 | 70–400 | ||
| Plants | Fly Ash (CDTPA) | |||||||
|---|---|---|---|---|---|---|---|---|
| As | B | Cr | Cu | Mn | Ni | Se | Zn | |
| CRoot | ||||||||
| T. tetrandra | 0.560 | 0.751 c | −0.834 b | 0.990 a | 0.954 a | 0.297 | −0.473 | 0.892 a |
| A. fruticosa | 0.447 | 0.857 b | −0.235 | −0.833 b | 0.843 b | 0.906 a | −0.594 | −0.841 b |
| P. alba | −0.117 | 0.954 a | 0.913 a | 0.994 a | 0.812 b | −0.887 a | 0.010 | 0.774 b |
| R. pseudoacacia | −0.001 | −0.306 | 0.905 a | −0.063 | 0.857 b | 0.980 a | −0.229 | 0.716 c |
| CLeaf | ||||||||
| T. tetrandra | 0.565 | −0.993 a | −0.193 | 0.997 a | 0.997 a | −0.884 a | −0.593 | 0.883 a |
| A. fruticosa | −0.143 | 0.998 a | −0.523 | −0.984 a | 0.983 a | 0.970 a | −0.611 | 0.704 c |
| P. alba | 0.877 a | 0.998 a | 0.955 a | 0.690 c | 0.986 a | −0.988 a | −0.157 | −0.744 c |
| R. pseudoacacia | 0.456 | 0.399 | 0.842 b | 0.950 a | 0.214 | 0.122 | −0.661 c | 0.774 b |
| CRoot | CLeaf | |||||||
|---|---|---|---|---|---|---|---|---|
| As | B | Cr | Cu | Mn | Ni | Se | Zn | |
| T. tetrandra | 0.638 c | −0.715 c | 0.317 | 0.994 a | 0.955 a | −0.082 | 0.103 | 0.997 a |
| A fruticosa | −0.365 | 0.868 b | 0.108 | 0.837 b | 0.830 b | 0.917 a | 0.949 a | −0.431 |
| P. alba | −0.203 | 0.960 a | 0.933 a | 0.705 c | 0.774 b | 0.882 a | −0.167 | −0.602 |
| R. pseudoacacia | 0.318 | 0.289 | 0.782 b | −0.058 | 0.170 | 0.095 | 0.593 | 0.875 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, O.; Gajić, G.; Jarić, S.; Vukov, T.; Matić, M.; Mitrović, M.; Pavlović, P. An Assessment of the Phytoremediation Potential of Planted and Spontaneously Colonized Woody Plant Species on Chronosequence Fly Ash Disposal Sites in Serbia—Case Study. Plants 2022, 11, 110. https://doi.org/10.3390/plants11010110
Kostić O, Gajić G, Jarić S, Vukov T, Matić M, Mitrović M, Pavlović P. An Assessment of the Phytoremediation Potential of Planted and Spontaneously Colonized Woody Plant Species on Chronosequence Fly Ash Disposal Sites in Serbia—Case Study. Plants. 2022; 11(1):110. https://doi.org/10.3390/plants11010110
Chicago/Turabian StyleKostić, Olga, Gordana Gajić, Snežana Jarić, Tanja Vukov, Marija Matić, Miroslava Mitrović, and Pavle Pavlović. 2022. "An Assessment of the Phytoremediation Potential of Planted and Spontaneously Colonized Woody Plant Species on Chronosequence Fly Ash Disposal Sites in Serbia—Case Study" Plants 11, no. 1: 110. https://doi.org/10.3390/plants11010110







