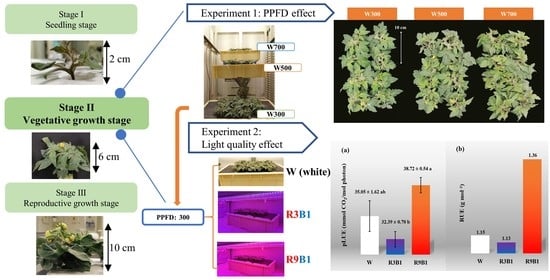
Optimization of Photosynthetic Photon Flux Density and Light Quality for Increasing Radiation-Use Efficiency in Dwarf Tomato under LED Light at the Vegetative Growth Stage
Abstract
:1. Introduction
2. Results
2.1. Experiment 1: Photosynthetic Photon Flux Density (PPFD) Effect
2.1.1. Growth Characteristics
2.1.2. Light Interception and Radiation-Use Efficiency (RUE)
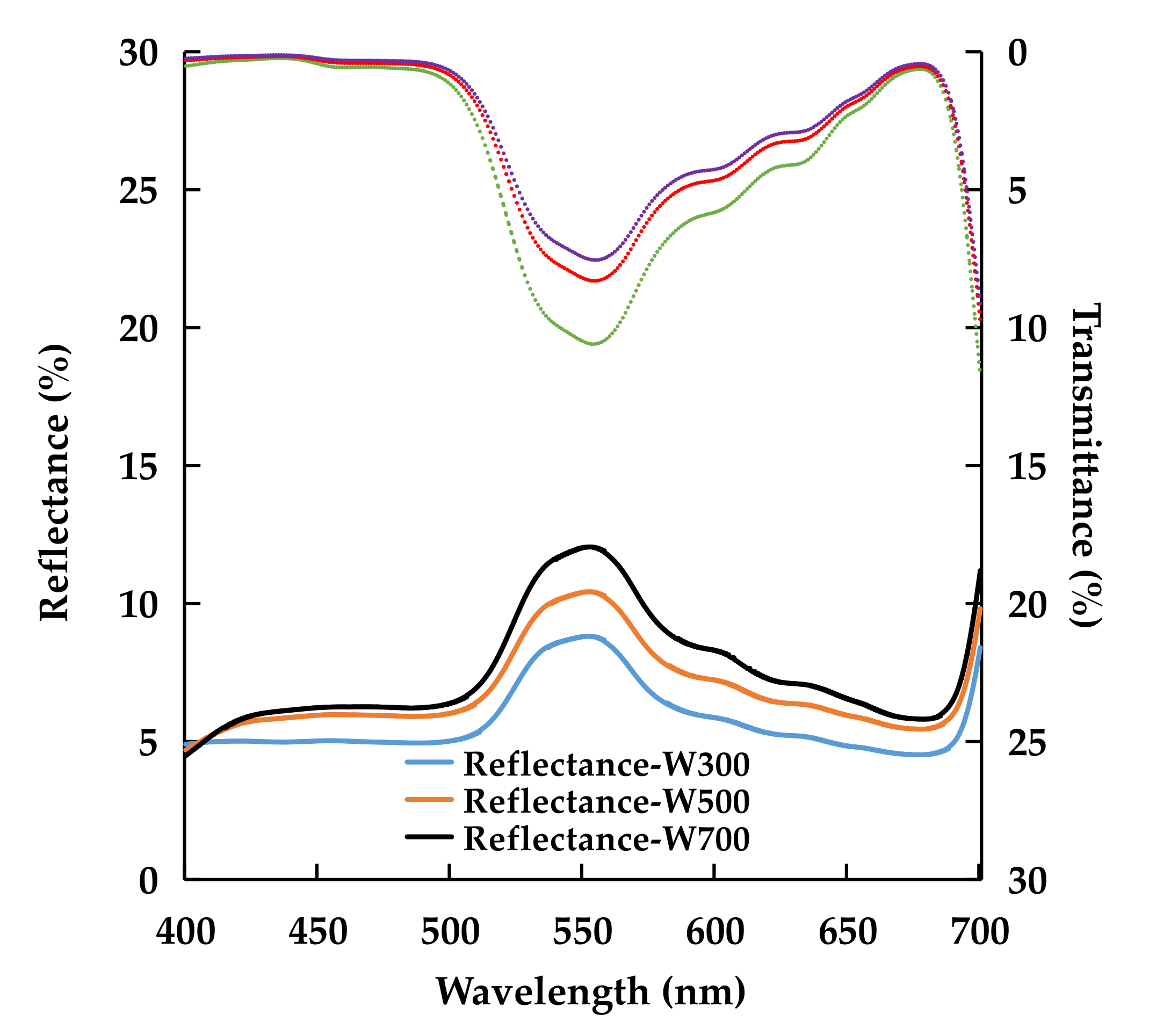
2.1.3. Leaf Optical Properties and Chlorophyll Concentration
2.1.4. Net Photosynthetic Rate (Pn) and Light Response
2.2. Experiment 2: Light Quality Effect
2.2.1. Growth Characteristics
2.2.2. Light Interception and RUE
2.2.3. Leaf Optical Properties and Chlorophyll Concentration
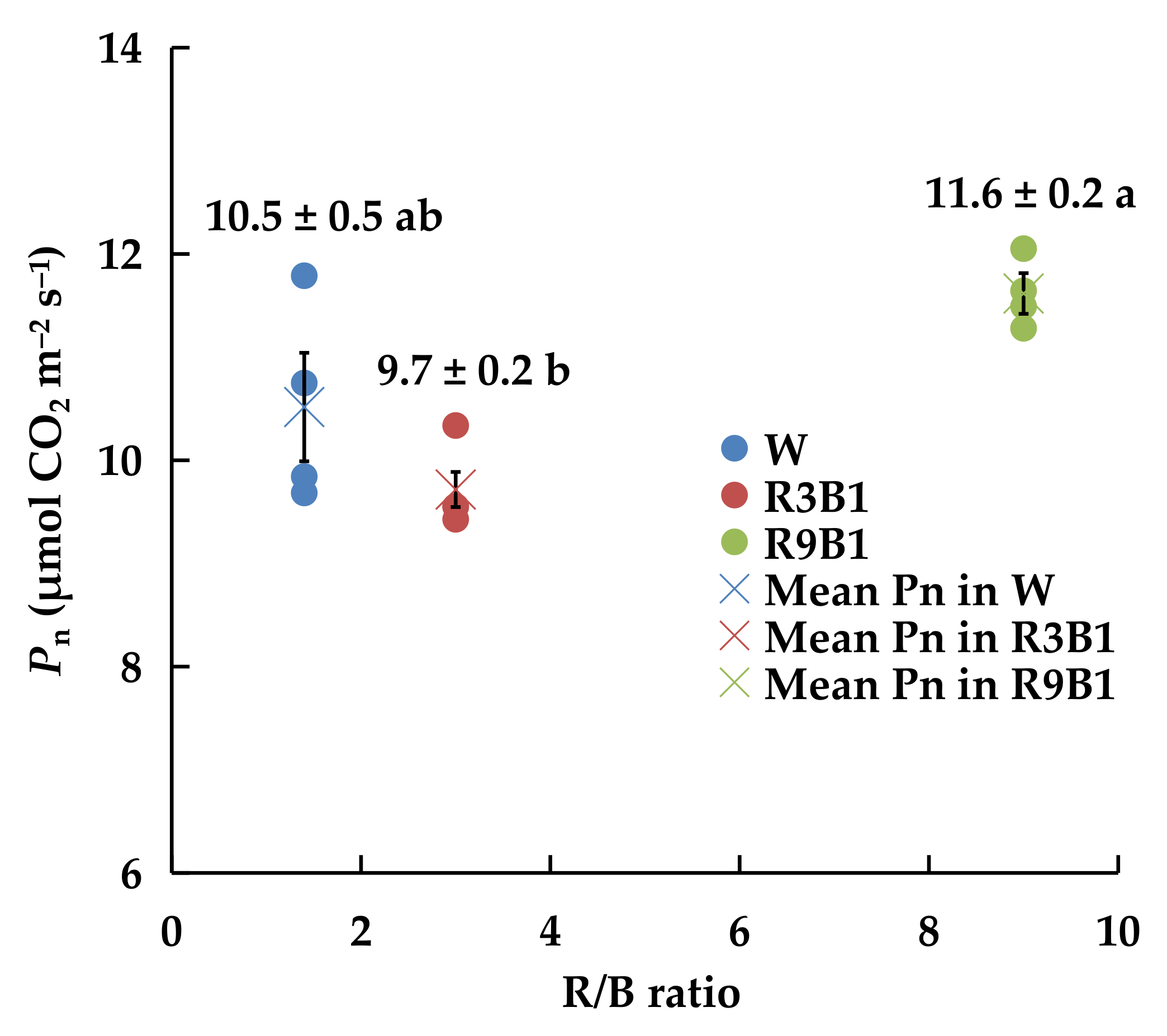
2.2.4. Pn and Light Response
3. Discussion
3.1. Experiment 1: PPFD Effect
3.2. Experiment 2: Light Quality Effect
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Experimental Conditions
4.2.1. Experiment 1: PPFD Effect
4.2.2. Experiment 2: Light Quality Effect
4.3. Growth Measurement
4.4. RUE of Canopy
4.5. Leaf Optical Properties and Chlorophyll Concentration
4.6. Gas Exchange
4.6.1. Leaf Pn
4.6.2. Light Response Curves
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozai, T.; Niu, G. Overview and concept of closed plant production system (CPPS). In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 3–6. [Google Scholar] [CrossRef]
- Meissner, R.; Jacobson, Y.; Melamed, S.; Levyatuv, S.; Shalev, G.; Ashri, A.; Elkind, Y.; Levy, A. A new model system for tomato genetics. Plant J. 1997, 12, 1465–1472. [Google Scholar] [CrossRef]
- Kato, K.; Maruyama, S.; Hirai, T.; Hiwasa-Tanase, K.; Mizoguchi, T.; Goto, E.; Ezura, H. A trial of production of the plant-derived high-value protein in a plant factory: Photosynthetic photon fluxes affect the accumulation of recombinant miraculin in transgenic tomato fruits. Plant Signal. Behav. 2011, 6, 1172–1179. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.J.; Uchii, S.; Watanabe, S.; Ezura, H. A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol. 2006, 47, 426–431. [Google Scholar] [CrossRef]
- Ohyama, K.; Kozai, T.; Kubota, C.; Chun, C.; Hasegawa, T.; Yokoi, S.; Nishimura, M. Coefficient of performance for cooling of a home-use air conditioner installed in a closed-type transplant production system. J. Soc. High Technol. Agric. 2002, 14, 141–146. [Google Scholar] [CrossRef]
- Graamans, L.; Baeza, E.; Van Den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant factories versus greenhouses: Comparison of resource use efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Goto, E. Production of pharmaceutical materials using genetically modified plants grown under artificial lighting. Acta Hortic. 2011, 907, 45–52. [Google Scholar] [CrossRef]
- Williams, W.A.; Loomis, R.S.; Lepley, C.R. Vegetative growth of corn as affected by population density. I. Productivity in relation to interception of solar radiation1. Crop Sci. 1965, 5, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Shibles, R.; Weber, C. Interception of solar radiation and dry matter production by various soybean planting patterns 1. Crop Sci. 1966, 6, 55–59. [Google Scholar] [CrossRef]
- Jayalath, T.C.; van Iersel, M.W. Canopy size and light use efficiency explain growth differences between lettuce and mizuna in vertical farms. Plants 2021, 10, 704. [Google Scholar] [CrossRef]
- Knapp, A.K.; Smith, W.K. Stomatal and photosynthetic responses to variable sunlight. Physiol. Plant. 1990, 78, 160–165. [Google Scholar] [CrossRef]
- O’Carrigan, A.; Hinde, E.; Lu, N.; Xu, X.; Duan, H.; Huang, G.; Mak, M.; Bellotti, B.; Chen, Z. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 2014, 98, 65–73. [Google Scholar] [CrossRef]
- Onoda, Y.; Saluñga, J.B.; Akutsu, K.; Aiba, S.i.; Yahara, T.; Anten, N.P. Trade-off between light interception efficiency and light use efficiency: Implications for species coexistence in one-sided light competition. J. Ecol. 2014, 102, 167–175. [Google Scholar] [CrossRef]
- He, W.; Huang, Z.; Li, J.; Su, W.; Gan, L.; Xu, Z. Effect of different light intensities on the photosynthate distribution in cherry tomato seedlings. J. Hortic. Sci. Biotechnol. 2019, 94, 611–619. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Xiu, W.; Chen, B.; Jie, G. Effects of low light on agronomic and physiological characteristics of rice including grain yield and quality. Rice Sci. 2014, 21, 243–251. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth of impatiens, petunia, salvia, and tomato seedlings under blue, green, and red light-emitting diodes. HortScience 2014, 49, 734–740. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, Y.; Wang, L.; Guo, W. Red and blue wavelengths affect the morphology, energy use efficiency and nutritional content of lettuce (Lactuca sativa L.). Sci. Rep. 2021, 11, 8374. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.; Brown, C. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi-Kaneko, K.; Matsuda, R.; Goto, E.; Fujiwara, K.; Kurata, K. Growth of rice plants under red light with or without supplemental blue light. J. Soil Sci. Plant Nutr. 2006, 52, 444–452. [Google Scholar] [CrossRef]
- Ouzounis, T.; Heuvelink, E.; Ji, Y.; Schouten, H.; Visser, R.; Marcelis, L. Blue and red LED lighting effects on plant biomass, stomatal conductance, and metabolite content in nine tomato genotypes. Acta Hortic. 2016, 1134, 251–258. [Google Scholar] [CrossRef]
- Goto, E. Effects of light quality on growth of crop plants under artificial lighting. Environ. Control Biol. 2003, 41, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ Cult. 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Son, K.-H. Effects of white LED lighting with specific shorter blue and/or green wavelength on the growth and quality of two lettuce cultivars in a vertical farming system. Agronomy 2021, 11, 2111. [Google Scholar] [CrossRef]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ito, Y.; Ichimura, T.; Shinohara, Y. Effects of supplemental lighting with light-emitting diodes (LEDs) on tomato yield and quality of single-truss tomato plants grown at high planting density. Environ. Control Biol. 2012, 50, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Cope, K.R.; Bugbee, B. Spectral effects of three types of white light-emitting diodes on plant growth and development: Absolute versus relative amounts of blue light. HortScience 2013, 48, 504–509. [Google Scholar] [CrossRef]
- Dong, C.; Fu, Y.; Liu, G.; Liu, H. Growth, photosynthetic characteristics, antioxidant capacity and biomass yield and quality of wheat (Triticum aestivum L.) exposed to LED light sources with different spectra combinations. J. Agron. Crop Sci. 2014, 200, 219–230. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Martínez-Estrada, E.; Caamal-Velázquez, J.H.; Morales-Ramos, V. Effect of LED light quality on in vitro shoot proliferation and growth of vanilla (Vanilla planifolia Andrews). Afr. J. Biotechnol. 2016, 15, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-H.; Wheeler, R.M.; Sager, J.C.; Yorio, N.C.; Goins, G.D. Light-emitting diodes as an illumination source for plants: A review of research at Kennedy Space Center. Habitation 2005, 10, 71–78. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Monte, J.A.; Carvalho, D.F.D.; Medici, L.O.; da Silva, L.D.; Pimentel, C. Growth analysis and yield of tomato crop under different irrigation depths. Rev. Bras. Eng. Agric. Ambient. 2013, 17, 926–931. [Google Scholar] [CrossRef] [Green Version]
- Dieleman, J.A.; Heuvelink, E. Factors affecting the number of leaves preceding the first inflorescence in the tomato. J. Hortic. Sci. 1992, 67, 1–10. [Google Scholar] [CrossRef]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012, 956, 261–266. [Google Scholar] [CrossRef]
- Gommers, C.M.; Visser, E.J.; St Onge, K.R.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Runkle, E.S. Far-red radiation and photosynthetic photon flux density independently regulate seedling growth but interactively regulate flowering. Environ. Exp. Bot. 2018, 155, 206–216. [Google Scholar] [CrossRef]
- Feng, L.; Raza, M.A.; Li, Z.; Chen, Y.; Khalid, M.H.B.; Du, J.; Liu, W.; Wu, X.; Song, C.; Yu, L. The influence of light intensity and leaf movement on photosynthesis characteristics and carbon balance of soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Jones-Baumgardt, C.; Llewellyn, D.; Ying, Q.; Zheng, Y. Intensity of sole-source light-emitting diodes affects growth, yield, and quality of Brassicaceae microgreens. HortScience 2019, 54, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Li, S.; Zhang, M.; Jiang, S.; Xiao, Y. Light intensity affects growth, photosynthetic capability, and total flavonoid accumulation of Anoectochilus plants. HortScience 2010, 45, 863–867. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Liu, X.; Xu, Z.; Jiao, X. Effects of light intensity on leaf microstructure and growth of rape seedlings cultivated under a combination of red and blue LEDs. J. Integr. Agric. 2017, 16, 97–105. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Matsuda, R.; Yamano, T.; Murakami, K.; Fujiwara, K. Effects of spectral distribution and photosynthetic photon flux density for overnight LED light irradiation on tomato seedling growth and leaf injury. Sci. Hortic. 2016, 198, 363–369. [Google Scholar] [CrossRef]
- Larsen, D.H.; Woltering, E.J.; Nicole, C.; Marcelis, L.F. Response of basil growth and morphology to light intensity and spectrum in a vertical farm. Front. Plant Sci. 2020, 11, 597906. [Google Scholar] [CrossRef]
- Lee, D.W.; Graham, R. Leaf optical properties of rainforest sun and extreme shade plants. Am. J. Bot. 1986, 73, 1100–1108. [Google Scholar] [CrossRef]
- Araus, J.L.; Hogan, K.P. Leaf structure and patterns of photoinhibition in two neotropical palms in clearings and forest understory during the dry season. Am. J. Bot. 1994, 81, 726–738. [Google Scholar] [CrossRef]
- Sanches, M.C.; Válio, I.F.M. Leaf optical properties of two liana species Canavalia parviflora Benth. and Gouania virgata Reissk in different light conditions. Rev. Bras. Bot. 2006, 29, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Tanner, V.; Eller, B. Epidermis structure and its significance for the optical properties of leaves of the Mesembryanthemaceae. J. Plant Physiol. 1986, 125, 285–294. [Google Scholar] [CrossRef]
- Li, T.; Heuvelink, E.; Dueck, T.A.; Janse, J.; Gort, G.; Marcelis, L.F.M. Enhancement of crop photosynthesis by diffuse light: Quantifying the contributing factors. Ann. Bot. 2014, 114, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Barreiro, R.; Guiamét, J.J.; Beltrano, J.; Montaldi, E.R. Regulation of the photosynthetic capacity of primary bean leaves by the red: Far-red ratio and photosynthetic photon flux density of incident light. Physiol. Plant. 1992, 85, 97–101. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, L.; Shi, A.; Zhang, Q. Photosynthetic responses of four Hosta cultivars to shade treatments. Photosynthetica 2004, 42, 213–218. [Google Scholar] [CrossRef]
- Tateno, M.; Taneda, H. Photosynthetically versatile thin shade leaves: A paradox of irradiance-response curves. Photosynthetica 2007, 45, 299–302. [Google Scholar] [CrossRef]
- Karpinski, S.; Escobar, C.; Karpinska, B.; Creissen, G.; Mullineaux, P.M. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 1997, 9, 627–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpinski, S.; Reynolds, H.; Karpinska, B.; Wingsle, G.; Creissen, G.; Mullineaux, P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 1999, 284, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Smith, H. Light quality, photoperception, and plant strategy. Annu. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Mazzella, M.; Alconada Magliano, T.; Casal, J. Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ. 1997, 20, 261–267. [Google Scholar] [CrossRef]
- Liu, X.; Chang, T.; Guo, S.; Xu, Z.; Li, J. Effect of different light quality of LED on growth and photosynthetic character in cherry tomato seedling. Acta Hortic. 2011, 907, 325–330. [Google Scholar] [CrossRef]
- Bugbee, B. Toward an optimal spectral quality for plant growth and development: The importance of radiation capture. Acta Hortic. 2016, 1134, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: Interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef]
- Kong, Y.; Nemali, K. Blue and far-red light affect area and number of individual leaves to influence vegetative growth and pigment synthesis in lettuce. Front. Plant Sci. 2021, 12, 667407. [Google Scholar] [CrossRef]
- Nishio, J. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2000, 23, 539–548. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, V.; Tripathy, B.C. Photoregulation of the greening process of wheat seedlings grown in red light. Plant Mol. Biol. 2005, 59, 269–287. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red: Blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef] [PubMed]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. ASABE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Stutte, G.W. Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Liu, N.; Ji, F.; Xu, L.; He, D. Effects of LED light quality on the growth of pepper seedling in plant factory. Int. J. Agric. Biol. Eng. 2019, 12, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Rihan, H.Z.; Aldarkazali, M.; Mohamed, S.J.; McMulkin, N.B.; Jbara, M.H.; Fuller, M.P. A novel new light recipe significantly increases the growth and yield of sweet basil (Ocimum basilicum) grown in a plant factory system. Agronomy 2020, 10, 934. [Google Scholar] [CrossRef]
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Continuous lighting and high daily light integral enhance yield and quality of mass-produced nasturtium (Tropaeolum majus L.) in plant factories. Plants 2021, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F. Adding far-red to red-blue light-emitting diode light promotes yield of lettuce at different planting densities. Front. Plant Sci. 2020, 11, 609977. [Google Scholar] [CrossRef]
- Legendre, R.; van Iersel, M.W. Supplemental far-red light stimulates lettuce growth: Disentangling morphological and physiological effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, S.; Ishigami, Y.; Hikosaka, S.; Goto, E. Estimation of lighting energy consumption required for red leaf lettuce production under different blue/red ratios and light intensity conditions in a plant factory with artificial lighting. J. SHITA 2017, 29, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Bauerle, W.L.; McCullough, C.; Iversen, M.; Hazlett, M. Leaf age and position effects on quantum yield and photosynthetic capacity in hemp crowns. Plants 2020, 9, 271. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, K.; Kuwabara, Y.; Motobayashi, T. Photosynthetic light-use efficiency of rice leaves under fluctuating incident light. Agrosyst. Geosci. Environ. 2020, 3, e20030. [Google Scholar] [CrossRef]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]




| Treatment | Stem Length (cm) | Leaf Number | Total Fresh Weight (g) | Total Dry Weight (g) | Dry Matter Ratio (%) | Leaf Area (cm2) | Specific Leaf Area (cm2 g−1) |
|---|---|---|---|---|---|---|---|
| 0 DAT | 2.4 ± 0.1 | 3.5 ± 0.2 | 0.50 ± 0.03 | 0.05 ± 0.00 | 9.81 ± 0.20 | ||
| W300 | 3.4 ± 0.2 | 6.6 ± 0.2 | 5.69 ± 0.54 c | 0.50 ± 0.04 c | 8.81 ± 0.15 | 73.46 ± 4.18 b | 257.60 ± 14.92 a |
| W500 | 3.8 ± 0.1 | 6.6 ± 0.2 | 7.71 ± 0.60 b | 0.73 ± 0.06 b | 9.55 ± 0.15 | 83.66 ± 2.33 b | 199.68 ± 20.14 b |
| W700 | 3.8 ± 0.2 | 6.4 ± 0.2 | 9.55 ± 0.33 a | 0.92 ± 0.05 a | 9.57 ± 0.30 | 95.50 ± 2.01 a | 167.96 ± 9.10 b |
| Treatment | Integrated PPFD Received by the Plant during the Period (mol) | Daily Average Intercepted PPFD Proportion during the Period | ΔW (g) | RUE (g mol−1) |
|---|---|---|---|---|
| W300 | 0.30 | 0.89 | 0.34 | 1.15 |
| W500 | 0.49 | 0.88 | 0.56 | 1.14 |
| W700 | 0.66 | 0.87 | 0.62 | 0.94 |
| Treatment | pLUE (mmol CO2/mol Photon) |
|---|---|
| W300 | 40.82 ± 0.57 a |
| W500 | 39.38 ± 0.36 a |
| W700 | 34.31 ± 0.67 b |
| Treatment | Transmittance (%) | Reflectance (%) | ||||
|---|---|---|---|---|---|---|
| 400–499 nm (Blue) | 500–599 nm (Green) | 600–700 nm (Red) | 400–499 nm (Blue) | 500–599 nm (Green) | 600–700 nm (Red) | |
| W300 | 0.5 ± 0.1 | 7.3 ± 0.7 a | 3.4 ± 0.4 | 5.0 ± 0.1b | 7.1 ± 0.3 b | 5.2 ± 0.1 b |
| W500 | 0.3 ± 0.1 | 5.6 ± 0.5 ab | 2.7 ± 0.4 | 5.8 ± 0.1 a | 8.5 ± 0.2 a | 6.3 ± 0.1 a |
| W700 | 0.3 ± 0.0 | 5.1 ± 0.5 b | 2.5 ± 0.3 | 6.0 ± 0.3 a | 9.7 ± 0.5 a | 6.9 ± 0.3 a |
| Treatment | Chlorophyll a Conc. (mg g−1 DW) | Chlorophyll b Conc. (mg g−1 DW) | Chlorophyll a + b Conc. (mg g−1 DW) | Chlorophyll a/b |
|---|---|---|---|---|
| W300 | 2.07 ± 0.20 a | 0.59 ± 0.04 a | 2.66 ± 0.24 a | 3.52 ± 0.21 |
| W500 | 1.67 ± 0.06 ab | 0.46 ± 0.02 b | 2.13 ± 0.08 a | 3.61 ± 0.07 |
| W700 | 1.25 ± 0.02 b | 0.34 ± 0.00 c | 1.59 ± 0.02 b | 3.66 ± 0.04 |
| Treatment | Stem Length (cm) | Leaf Number | Total Fresh Weight (g) | Total Dry Weight (g) | Dry Matter Ratio (%) | Leaf Area (cm2) | Specific Leaf Area (cm2 g−1) |
|---|---|---|---|---|---|---|---|
| 0 DAT | 3.5 ± 0.3 | 3.4 ± 0.1 | 0.55 ± 0.03 | 0.06 ± 0.00 | 10.92 ± 0.41 | - | - |
| W | 4.7 ± 0.1 a | 6.4 ± 0.2 | 7.93 ± 0.46 | 0.66 ± 0.04 ab | 8.34 ± 0.12 b | 130.60 ± 4.12 | 313.59 ± 6.91 a |
| R3B1 | 4.4 ± 0.0 b | 6.4 ± 0.2 | 7.10 ± 0.39 | 0.59 ± 0.03 b | 8.33 ± 0.21 b | 115.13 ± 5.91 | 308.03 ± 6.07 a |
| R9B1 | 4.8 ± 0.1 a | 6.0 ± 0.0 | 7.90 ± 0.27 | 0.71 ± 0.03 a | 9.00 ± 0.52 a | 130.36 ± 4.79 | 281.91 ± 4.51 b |
| Treatment | Integrated PPFD Received by the Plant during the Period (mol) | Daily Average Intercepted PPFD Proportion during the Period | ΔW (g) | RUE (g mol−1) |
|---|---|---|---|---|
| W | 0.43 | 0.92 | 0.50 | 1.15 |
| R3B1 | 0.40 | 0.92 | 0.46 | 1.13 |
| R9B1 | 0.42 | 0.92 | 0.56 | 1.36 |
| Treatment | pLUE (mmol CO2/mol Photon) |
|---|---|
| W | 35.05 ± 1.62 ab |
| R3B1 | 32.39 ± 0.70 b |
| R9B1 | 38.72 ± 0.54 a |
| Treatment | Reflectance (%) | ||
|---|---|---|---|
| 400–499 nm (Blue) | 500–599 nm (Green) | 600–700 nm (Red) | |
| W | 5.1 ± 0.3 | 7.4 ± 0.2 b | 5.3 ± 0.1 |
| R3B1 | 5.6 ± 0.1 | 7.8 ± 0.2 ab | 5.7 ± 0.2 |
| R9B1 | 5.3 ± 0.1 | 8.4 ± 0.3 a | 5.7 ± 0.2 |
| Treatment | Chlorophyll a Conc. (mg g−1 DW) | Chlorophyll b Conc. (mg g−1 DW) | Chlorophyll a + b Conc. (mg g−1 DW) | Chlorophyll a/b |
|---|---|---|---|---|
| W | 2.29 ± 0.12 a | 0.73 ± 0.05 a | 3.01 ± 0.15 a | 3.15 ± 0.06 |
| R3B1 | 1.96 ± 0.06 ab | 0.61 ± 0.02 ab | 2.57 ± 0.07 ab | 3.22 ± 0.02 |
| R9B1 | 1.69 ± 0.05 b | 0.52 ± 0.02 b | 2.21 ± 0.06 b | 3.24 ± 0.04 |
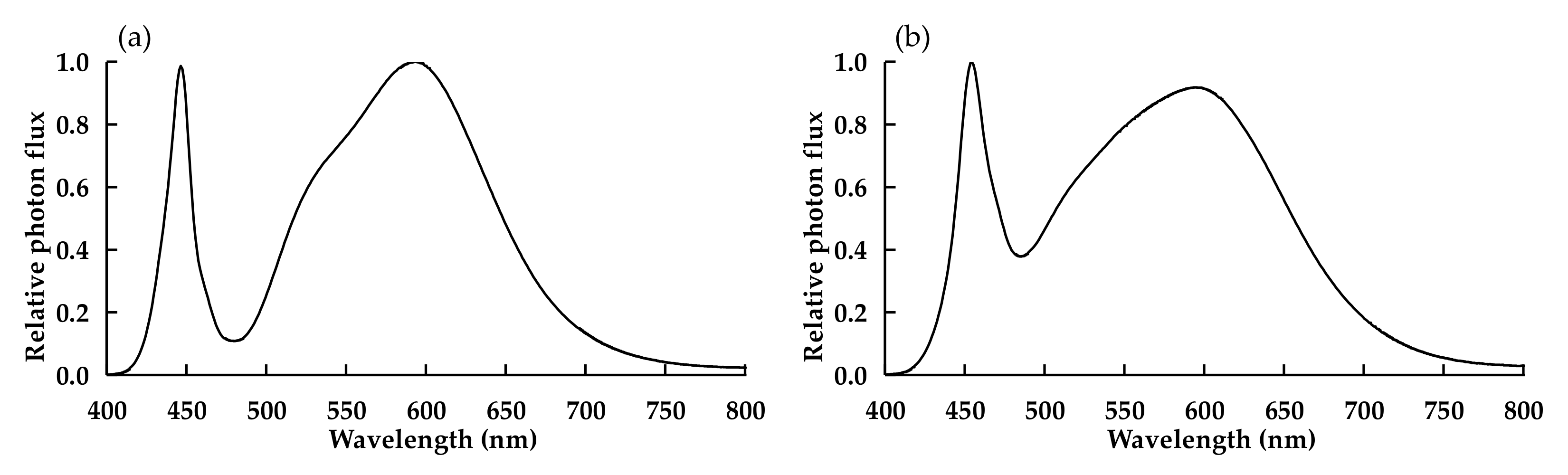
| W in Experiment 1 | W in Experiment 2 | R3B1 | R9B1 | |
|---|---|---|---|---|
| % Blue (400–499 nm) | 17.1 | 21.5 | 24.5 | 9.9 |
| % Green (500–599 nm) | 46.7 | 42.9 | 0.4 | 0.4 |
| % Red (600–699 nm) | 32.9 | 31.5 | 74.7 | 89.0 |
| % Far-red (700–800 nm) | 3.3 | 4.0 | 0.4 | 0.7 |
| R/B ratio | 1.9 | 1.5 | 3.0 | 9.0 |
| PSS | 0.85 | 0.84 | 0.87 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, X.; Yoshida, H.; Hikosaka, S.; Goto, E. Optimization of Photosynthetic Photon Flux Density and Light Quality for Increasing Radiation-Use Efficiency in Dwarf Tomato under LED Light at the Vegetative Growth Stage. Plants 2022, 11, 121. https://doi.org/10.3390/plants11010121
Ke X, Yoshida H, Hikosaka S, Goto E. Optimization of Photosynthetic Photon Flux Density and Light Quality for Increasing Radiation-Use Efficiency in Dwarf Tomato under LED Light at the Vegetative Growth Stage. Plants. 2022; 11(1):121. https://doi.org/10.3390/plants11010121
Chicago/Turabian StyleKe, Xinglin, Hideo Yoshida, Shoko Hikosaka, and Eiji Goto. 2022. "Optimization of Photosynthetic Photon Flux Density and Light Quality for Increasing Radiation-Use Efficiency in Dwarf Tomato under LED Light at the Vegetative Growth Stage" Plants 11, no. 1: 121. https://doi.org/10.3390/plants11010121
APA StyleKe, X., Yoshida, H., Hikosaka, S., & Goto, E. (2022). Optimization of Photosynthetic Photon Flux Density and Light Quality for Increasing Radiation-Use Efficiency in Dwarf Tomato under LED Light at the Vegetative Growth Stage. Plants, 11(1), 121. https://doi.org/10.3390/plants11010121