Abstract
This paper presents research results on forest decline in Serbia. The results were obtained through monitoring defoliation of 34 tree species at 130 sample plots during the period from 2004 to 2018. This research aimed to determine whether the occurrence of defoliation and tree mortality were caused by drought. Defoliation was assessed in 5% steps according to the International Co-operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests (ICP Forests) methodology. All the trees recorded as dead were singled out, and annual mortality rates were calculated. To determine changes in air temperature and precipitation regimes during the study period, we processed and analysed climatic data related to air temperature and precipitation throughout the year and in the growing season at 28 main weather stations in Serbia. Tree mortality patterns were established by classifying trees into three groups. The first group of trees exhibited a gradual increase in defoliation during the last few years of monitoring, with dying as the final outcome. The second group was characterised by sudden death of trees. The third group of trees reached a higher degree of defoliation immediately after the first monitoring year, and the trees died after several years. Tree mortality rates were compared between years using the Standardised Precipitation Evaporation Index (SPI) and the Standardised Precipitation Evapotranspiration Index (SPEI), the most common methods used to monitor drought. The most intensive forest decline was recorded during the period from 2013 to 2016, when the largest percentage of the total number of all trees died. According to the annual mortality rates calculated for the three observation periods (2004–2008, 2009–2013, and 2014–2018) the highest forest decline rate was recorded in the period from 2014 to 2018, with no statistically significant difference between broadleaved and coniferous tree species. As the sample of coniferous species was small, the number of sample plots should be increased in order to achieve better systematic forest condition monitoring in Serbia. The analysis of the relationship between defoliation and climatic parameters proved the correlation between them. It was noted that the forest decline in Serbia was preceded by an extremely dry period with high temperatures from 2011 to 2013, supporting the hypothesis that it was caused by drought. We therefore conclude that these unfavourable climatic conditions had serious and long-term consequences on forest ecosystems in Serbia.
1. Introduction
Forest ecosystems and forest vitality are directly affected by rising mean annual temperatures, changing precipitation dynamics and quantity, and extreme weather events of increasing and varying frequency and timing [1]. The impact of the changing climate on forests results from the complex interaction of meteorological factors and soil [2]. One of the effects of global warming is increasing risk of drought, the stress of which has a negative effect on forests [3]. The effects of prolonged and intense drought affect all parts of the environment. Droughts develop slowly, and often go unnoticed until they become visible to the naked eye. The slow manifestation and long duration of droughts often make their quantification very difficult [4]. Their main characteristics, such as onset, duration, and severity, are not easily or quickly detectable [5]. Drought should not be confused with aridity, which represents a permanent trait of a dry climate. Droughts affect all the components of the water cycle, resulting in a deficit of soil moisture and a decrease in the levels of groundwaters, brooks, and rivers. As they study precipitation and temperature as meteorological input variables, our investigations deal with meteorological drought and its influence on forest decline. Meteorological drought is the primary cause of drought. Other types of droughts (agricultural, hydrological, groundwater, and socioeconomic) describe the secondary effects of droughts on certain ecological and economic components [6].
Droughts and drought periods are not exclusively related to arid areas, such as the Mediterranean region; they can occur in areas that, while according to their climate characteristics are not considered at risk, can be affected by prolonged droughts (e.g., Central and Western Europe). Numerous studies have focused on drought periods occurring in Europe, regardless of the usual climatic conditions prevailing in these parts of the continent [5,7,8,9,10,11,12,13]. Furthermore, many authors have indicated that drought and drought periods can affect various species of trees and types of forests in general [1,3,10,14,15,16,17,18,19,20]. Drought events (heat followed by a lack of precipitation) increase tree mortality, which in turn disturbs the overall functioning of forest ecosystems. Therefore, tree mortality data are considered a prerequisite for a more comprehensive understanding of the complex interactions between climate and forests [21,22,23,24,25,26,27,28]. Several drought periods were registered in Serbia in the last decade, with certain years characterised as extremely dry [29].
Climate impacts can trigger defoliation processes in various types of forests [14]. Defoliation of tree crowns is the most widely used parameter for the assessment of forest vitality [30,31,32,33]. The percentage of tree damage is determined based on the visual assessment of the lack of assimilation organs (i.e., percentage of defoliation). Defoliation may indicate various stress factors, and can be caused by numerous biotic, abiotic, and human factors which affect trees either individually or through their interaction. It essentially reveals the actual condition of forests and is considered to be an indicator of the balance between a tree and its environment [34]. Stand age has a significant impact on the occurrence of defoliation [35,36,37]. In line with this, large and old trees should show increasing mortality, while the mortality of young trees should show a decreasing rate [38]. Although tree mortality is a natural process, several studies have pointed to increasing mortality rates due to climate change [15,39].
Following its introduction as an indicator of forest condition (ICP Forests) in the early 1980s [40], defoliation has been used as a main indicator [41]. Tree defoliation assessed in 5% steps is a useful parameter in predicting year-to-year tree mortality [42]. It is defined as the loss of leaves in broadleaves or needles in conifers compared to a reference tree, i.e., a healthy tree without any defoliation symptoms in the immediate vicinity of the assessed tree or an imaginary tree with no loss of leaves/needles. Defoliation is assessed regardless of the cause of the loss of leaves or needles. As the assessment is subjective, it has to be repeated and verified in order to provide uniform and accurate results.
Defoliation may be caused by various stress factors, including weather conditions such as extreme air temperatures and precipitation as well as insect or fungal attacks, air pollution, acid rain, etc. Variations in defoliation at the annual level are completely reversible, and are associated with fluctuations in climatic factors [43]. They may be caused either by temporary impacts (e.g. defoliating insects) or inaccurate measurements. Therefore, results are typically focused on long-term trends. Furthermore, according to previous research studies based on defoliation monitoring, the variability in the number of sampled trees due to felling, removal of dead trees, and their replacement with new ones does not distort study results over the years [44]. Monitoring networks are an essential source of data needed to predict changes in ecosystems [45].
However, from the very beginning studies have emphasised that defoliation is not a good indicator of forest condition [46]. The same attitude can be noted in recent studies [47] and even in the latest findings [48]. Previous authors have suggested that defoliation is a more useful indicator when combined with other indicators [30]. However, a large number of researchers consider defoliation the best indicator of forest vitality, and use it in their research [32,33,49,50,51].
Led by claims that defoliation cannot be used as the main indicator of forest condition, we accessed the internal ICP Forests database on each individual tree in order to resolve this dilemma. This database is a kind of "health history form" that allowed us to trace the chronological chain of events in the recent or distant past of each tree and monitor the course of defoliation over the years. The long-term trend of monitoring the defoliation of individual trees allowed us to determine the reasons for their die-back and correlate them with the mass forest declines which in fact occurred in the territory of the Republic of Serbia [52]. Except for a few research studies [53,54,55,56], defoliation has not been described in detail as an indicator of forest condition in Serbia in previous research. Furthermore, the method of chronological monitoring and classifying each dead tree by groups relative to defoliation trends was applied in our study for the first time. We wanted to determine whether defoliation trends follow the trends of extended extreme drought and the way prolonged drought events affect defoliation. We aimed to study the differences in tree responses before, during, and after the drought. In order to address these issues, we conducted research at all permanent sample plots of the ICP Forests network in Serbia. We included all tree species present on sample plots, as our main goal was to investigate the impact of drought on the occurrence of defoliation and tree mortality as a final outcome. In addition, in order to study the differences in the response to drought of broadleaved and coniferous species and trees at different altitudes, these groups of species and sample plots were analysed separately.
2. Results
2.1. Forest Decline and Die-Back of Individual Trees in Serbia
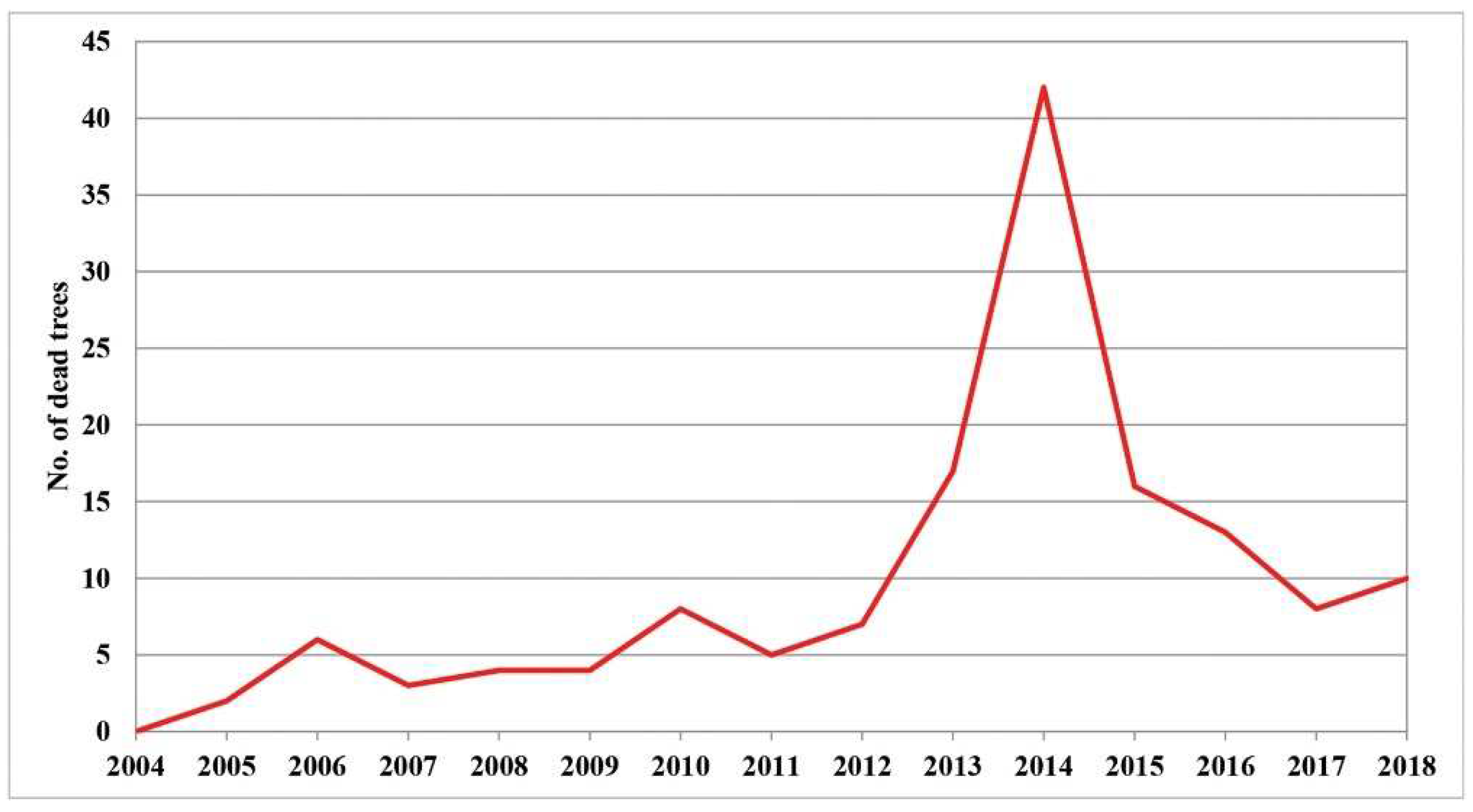
Increasing defoliation is one of the first symptoms of the die-back of individual trees. Therefore, it is very important to monitor its intensity in order to determine its causes. The largest number of dead trees with 100% defoliation was registered in 2014, which amounted to 29% of the total number of dead trees in the research period (2004–2018). This year was followed by 2013 with 11.7%, 2015 with 11%, and 2016 with 9%, while the remaining years had similar values, ranging from 2% to 7% of dead trees (Figure S1). The largest number of dead trees can be classified in the first group (the trees with a gradual increase in defoliation) and the second group of trees with ″sudden″ tree death. Compared to the number of trees that died in the whole fifteen-year research period (2004–2018), these two groups had the greatest number of trees in the period from 2013 to 2016. In only four years, 60.7% of the total number of all trees died. This statement is illustrated in the graph presented in Figure 1. It shows the trend of mortality of individual trees in the entire research period. A sharp increase in the number of dead trees can be seen in the period from 2013 to 2016, after which this number decreases. Such observations were made both in the immediate vicinity and further away from the sample plots, as noted by researchers in their field records.
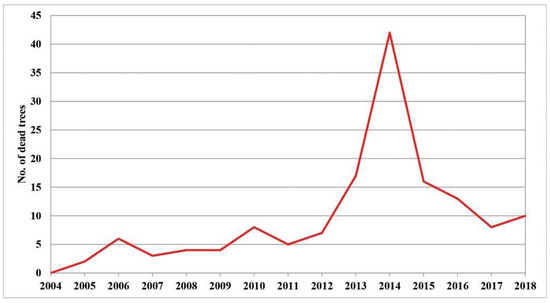
Figure 1.
Tree mortality in the Republic of Serbia (2004–2018) (data not shown).
Looking at localities with a large number of dead trees, it is evident that trees died either in the same year or in two consecutive years (Table S1). We registered the damage that may be related to climatic conditions where it deviated from the normal values in many parameters. Special attention was paid to damage from unknown causes, ie., damage with a cause that could not be determined with certainty during assessment (e.g., death of whole trees or die-back of their parts). According to the results of our analysis of the data on defoliation related to damage from unknown causes in the years when the damage significantly deviated from normal (i.e., 2011 to 2014), the percentage of trees of all species with damage from unknown causes amounted to 4.8% in 2011, while it was 5.6% in 2012 (the highest), 4.6% in 2013, and 4.4% in 2014. The percentage of this damage in the stated period was higher than the percentage of damage caused by human activity or abiotic factors (Figure S2). We rejected the impact of stand age on defoliation because the average numbers of dead trees did not differ significantly between different stand age categories (results not shown). The influence of biotic factors (insects and fungi) on tree die-back was rejected due to the high percentage of trees that died suddenly (Table S1, Group II). It amounted to 41% of the total number of dead trees. This was further indicated by trees whose defoliation increased in several consecutive years (Table S1, Group I), when unfavorable climatic conditions were at their peak and the number of these trees was 39%. Only one-fifth or 20% of dead trees (Table S1, Group III) had a higher percentage of defoliation over many years. Their defoliation was most commonly caused by fungi, which eventually resulted in the death of the tree.
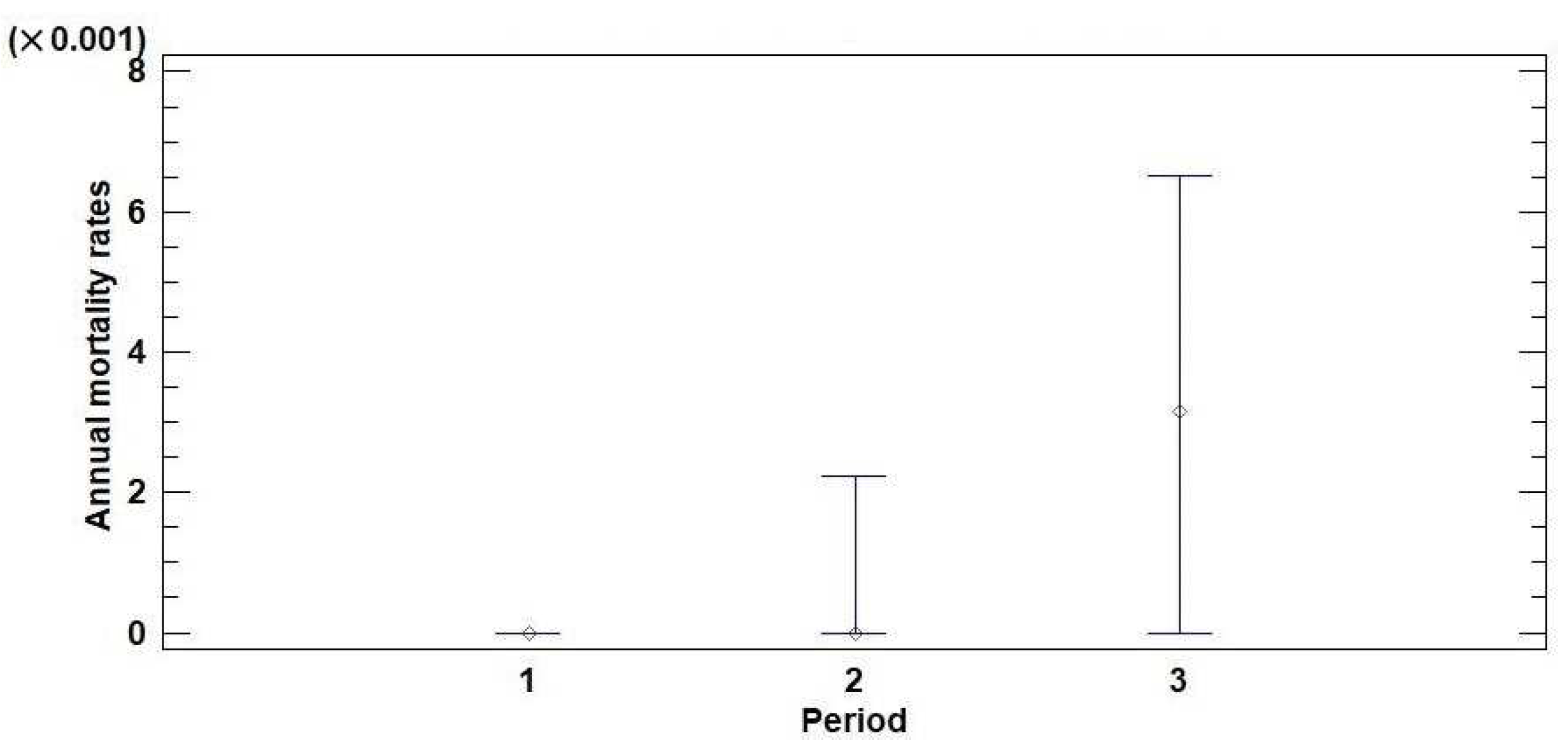
Based on the defoliation monitoring data (trees with defoliation of 100% were considered dead), annual mortality rates were calculated for the territory of Serbia in three observation periods (2004–2008, 2009–2013, and 2014–2018). The results of the descriptive and nonparametric statistics for the annual mortality rates of the monitored trees in three observation periods are presented in Table 1. The medians of the annual mortality rates were 0.000, 0.000, and 0.003 for the observation periods of 2004–2008, 2009–2013, and 2014–2018, respectively. According to the Kruskal–Wallis test (KWt), there is a statistically significant difference at the 95% confidence level (p = 0.00) between the medians that represent the three observation periods. The median plot (Figure 2) shows that the median of the annual mortality rate for the third observation period (2014–2018), which took place after the drought period from 2011 to 2013, was higher than the medians obtained for the previous two observation periods (2004–2008 and 2009–2013). The annual mortality rates did not differ significantly between coniferous and broadleaved trees or forests at different altitudes (Tables S2 and S3; Figures S3 and S4).

Table 1.
Descriptive and nonparametric statistics for the annual mortality rates of trees monitored in the territory of Serbia for three observation periods. M—median; MAD—median absolute deviation; MIN—minimum value; MAX—maximum value; KWt—Kruskal-Wallis test.
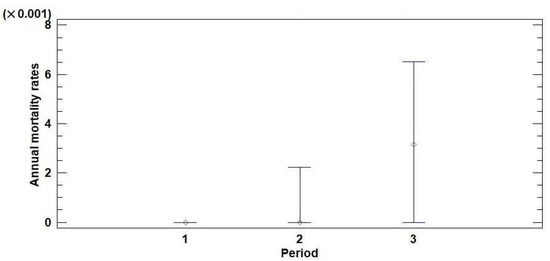
Figure 2.
Median plot with 95% confidence intervals for the annual mortality rates of trees monitored in the territory of Serbia in three observation periods: (1) 2004–2008, (2) 2009–2013, and (3) 2014–2018.
2.2. Extreme Climate Events and Forest Decline
Extreme climate events have undoubtedly affected forest ecosystems in the entire territory of Serbia (Tables S4–S7). Several extreme climate events were recorded in the research period [57]. They significantly contributed to the progressive increase of mortality (dying) of both individual trees and large forest areas in Serbia.
The analysis of the annual averages for the whole of Serbia shows that the period from 2011 to 2013 was continuously warm (Table S4). The mean annual air temperature in the growing season was the highest in 2012 (Table S5).
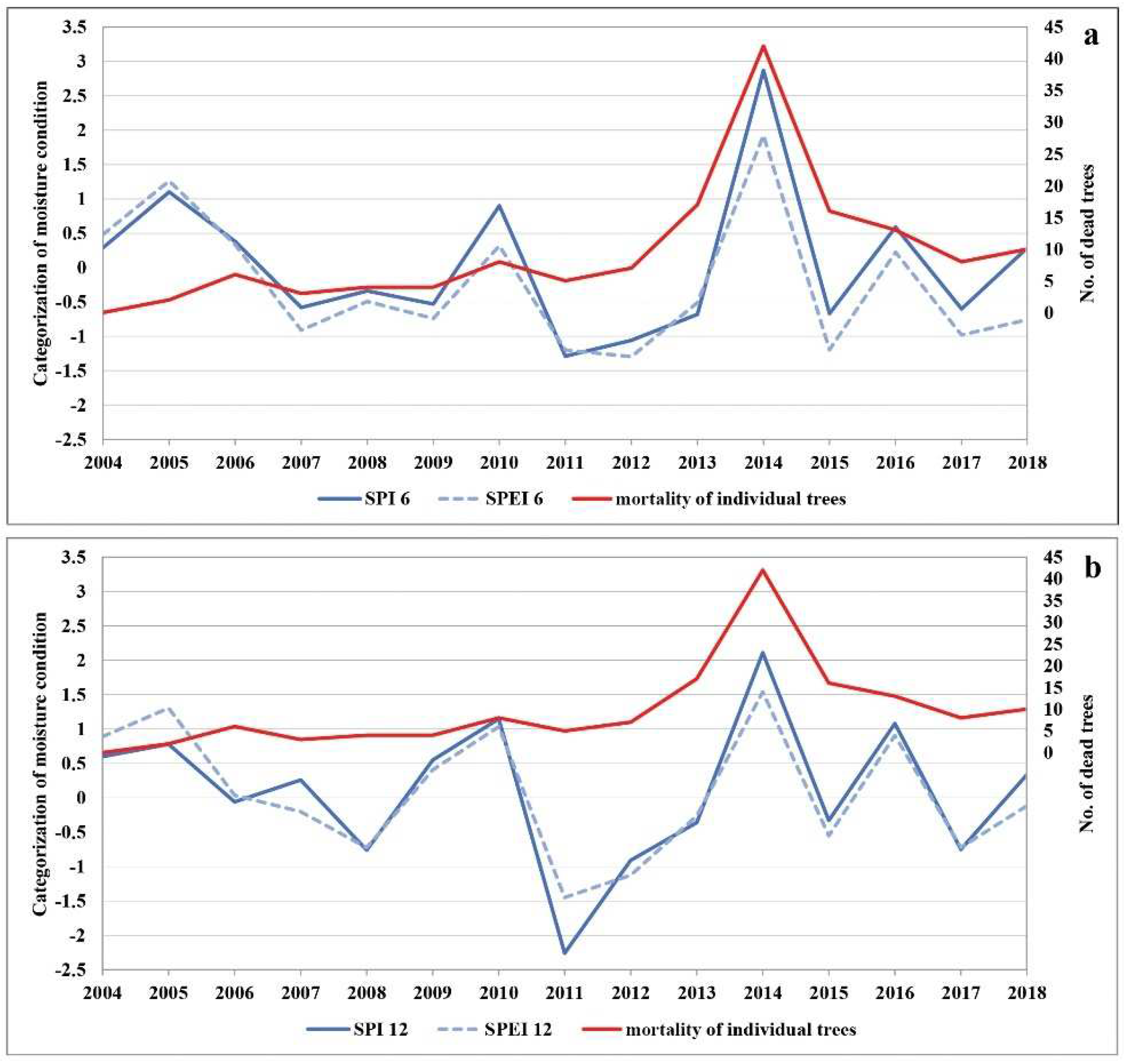
The lowest precipitation average in the research period was registered in 2011 (Table S6). This year was considered to be extremely dry, with the amount of precipitation below 500 mm, which is the limit for declaring drought [58]. The extremely dry 2011 was followed by extremely low and extremely high air temperatures in 2012, a year with the continuously lowest average amount of precipitation. While the following year, 2013, was dry again (especially in the growing season), it was followed by the highest amount of precipitation on record in 2014 (Tables S4–S7). Based on general characteristics related to the amount of precipitation during the growing season in 2011, the entire territory of Serbia was affected by severe and extreme drought (Table S7). Figure 3 shows the drought intensity based on the SPI and SPEI during the growing season (SPI-6 and SPEI-6, Figure 3a), i.e., from April to September 2004 to 2018, in the Republic of Serbia. Drought events of greater or lesser intensity occurred several times during the research period. However, the drought that lasted for two consecutive growing seasons (2011 and 2012) had long-term consequences for forest ecosystems. If we supplement this finding with the annual data on moisture conditions in the territory of Serbia (SPI-12 and SPEI-12), we can see that the lack of precipitation was even more intense outside the growing season (2011–2012) (Figure 3b). This is important to note because the drought began in the autumn of 2011, which according to SPI was categorised as the year with the most extreme drought (SPI ≤ −2). In no previous year had the drought period lasted as long or been as intense (three consecutive years, considering both the growing season and the whole year). In addition to the reduced amount of precipitation, the increased temperature significantly contributed to the severity of the dry period. The temperature had a strong impact on the intensity of drought during 2011 and 2012, as can be seen in Figure 3a,b. Figure 3a (SPEI-6) points to the significant influence of temperature that, together with the reduced amount of precipitation in the growing season, makes it the longest period of drought in the research period.
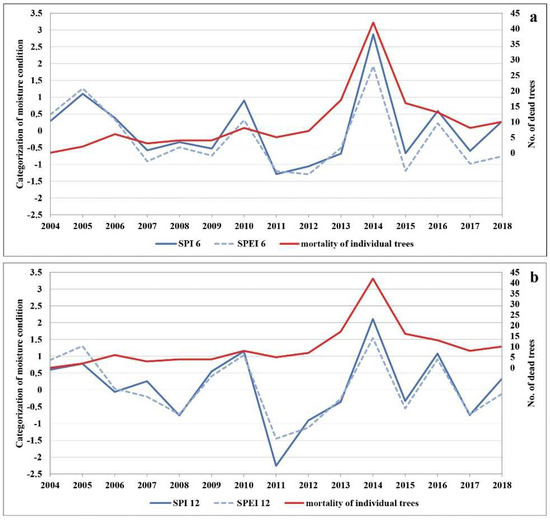
Figure 3.
The comparison of (a) SPI-6 and SPEI-6 and (b) SPI-12 and SPEI-12 with the number of dead trees.
Based on the above, we compared the SPI-6 and SPEI-6 as well as the SPI-12 and SPEI-12 with the number of dead trees in the research period (Figure 3a,b). Having in mind that reduced soil moisture disturbs the growth and development of plants, we compared the trend of defoliation with tree mortality in certain years. We found clear indications that the trend of increasing defoliation began with the onset of the drought period in 2011. It continued over the next two years (2012 and 2013) and reached its peak in 2014, when the largest number of dead trees was recorded. This is further confirmed by the three distinct groups of trees (Table S1) and by the correlation between the trees in Groups I and II and drought years (2011–2013).
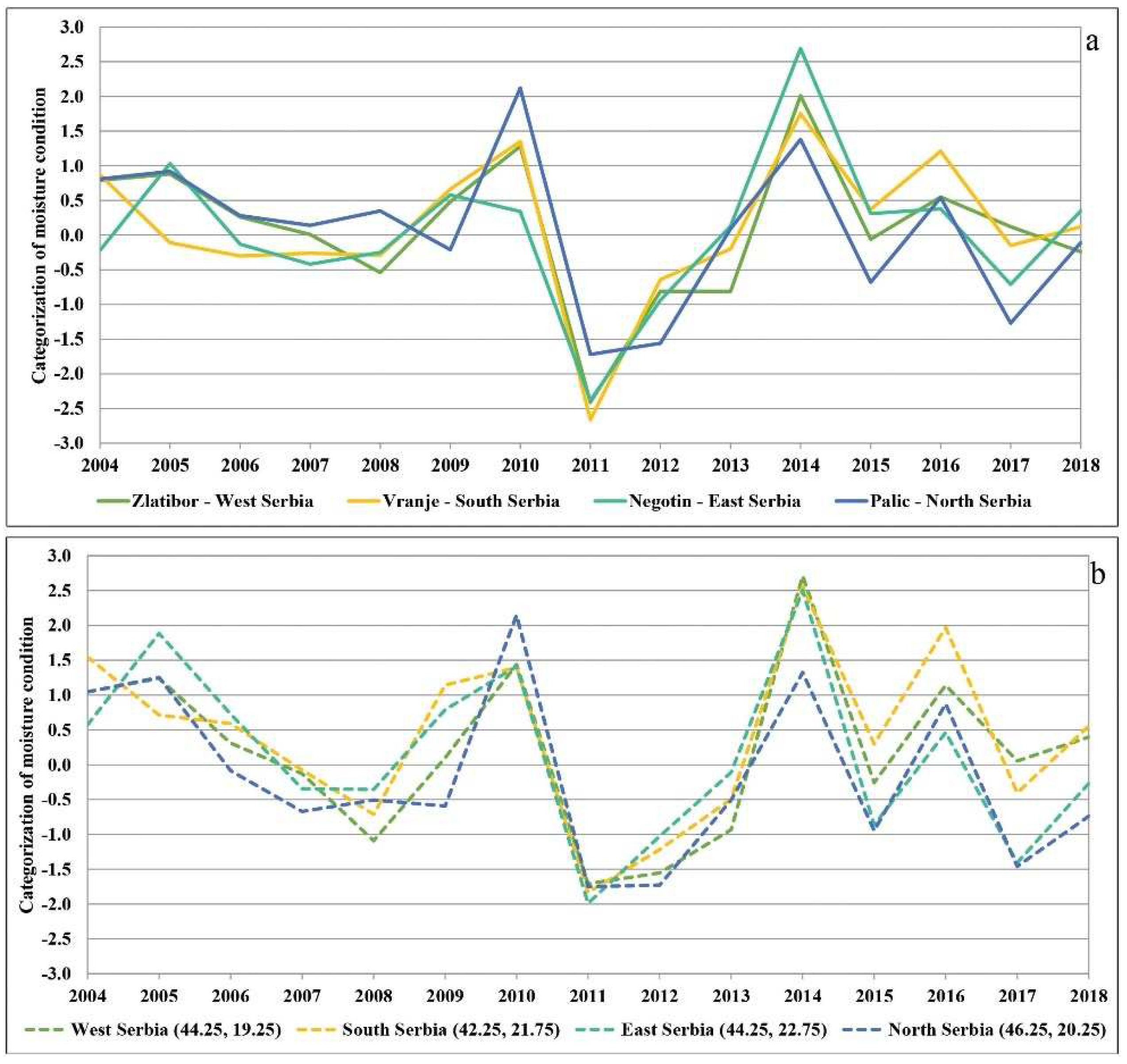
Because moisture and temperature conditions vary with altitude, we further analysed the conditions at individual localities based on the SPI and SPEI (Figure 4a,b). We decided to present the SPI and SPEI on a twelve-month basis, as the results indicated that the drought was present the whole year round and not just in the growing season. The SPI was calculated for the major weather stations, i.e., localities in the north (Palić, 102 m above sea level), west (Zlatibor, 1028 m above sea level), east (Negotin, 42 m above sea level), and south (Vranje, 432 m above sea level) in order to confirm the impact of drought regardless of altitude. The observations made at the main weather stations separately [57] revealed deviations in the amount of precipitation, which were conditioned by, among other things, the altitude. However, the amount of precipitation was typically far below the annual average at all major weather stations in 2011 and 2012, and according to the SPI criteria they all ranged from severe to extreme or even exceptional drought (Figure 4a). On the other hand, for the SPEI we calculated time series at a single grid cell according to coordinates in the north, west, east, and south of the country. Similar conditions were found to prevail at high and low altitudes. As can be seen in the Supplementary Materials Table S1, these conditions strongly contributed to mortality in the following years regardless of altitude and tree species.
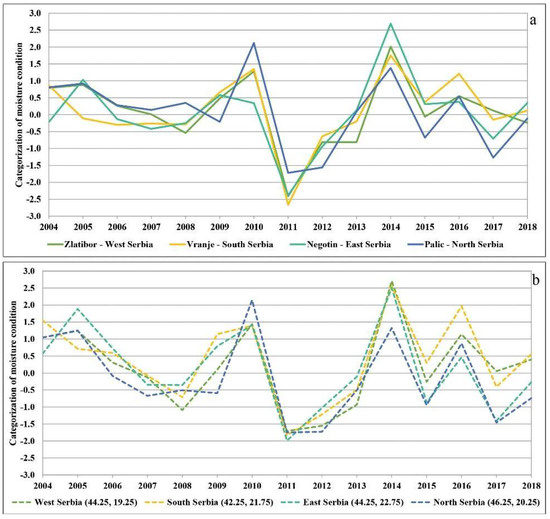
Figure 4.
(a) SPI-12 for the four major weather stations in the north, west, east, and south of Serbia; (b) SPEI-12 for time series in the north, west, east, and south of Serbia.
3. Discussion
Forest decline and die-back of individual trees are long-lasting processes and, in many cases they are not triggered at the same time as the unfavourable factor that disturbs the growth and development of a tree or the entire forest ecosystem. However, a large number of trees in Serbia died suddenly in the period from 2013 to 2016 (Table S1) without previous visible symptoms of defoliation and regardless of stand age, which stresses the severity, i.e., adverse effect of climate conditions that in this case imply extreme climate events in the form of prolonged drought.
According to “Srbijašume“, the State Enterprise dealing with forest management, the forest decline in this period has been the most massive forest decline during the monitoring period of forest conditions in the forestry sector of the Republic of Serbia. The long-lasting drought caused a massive forest decline in the whole territory of the Republic of Serbia, affecting an area of 13,885.00 ha. Die-back of individual trees was recorded in an area of 12,084.19 ha and die-back of groups of trees in an area of 1800.81 ha, while the deadwood volume amounted to 81,631.61 m3 [52].
The majority of broadleaved and coniferous trees that died in the period from 2012 to 2016 fell into the categories of intense defoliation and dead trees. As can be seen in Supplementary Tables S8 and S9, conifers showed intense defoliation in 2013 due to initial physiological weakening by drought followed by an attack of bark beetles. Unlike conifers, broadleaved trees had already been affected by intense defoliation in 2012. Seidling [35] similarly observed that certain tree species (conifers) reacted a year later than broadleaved trees, i.e., defoliation was detected a year later. This can be explained by the fact that defoliation is more noticeable in broadleaved trees than conifers, whose needles remain on the branches for a time after dying-back. Looking back at the climate framework, conifers show greater drought resistance than broadleaved trees, i.e., conifers are more resistant to the freezing and thawing cycle than broadleaved trees [59]. On the other hand, coniferous tree species are susceptible to attacks of secondary pests such as bark beetles, which attack physiologically weakened trees, and drought is assumed to be the crucial trigger of symptoms [60]. However, our statistical analysis showed that the highest forest decline was recorded in the period from 2014 to 2018, and annual mortality rates did not differ significantly between coniferous and broadleaved tree species. Although the number of main tree species on sample plots corresponded to the share of the same species in the forest cover on the territory of Serbia, the sample of coniferous tree species was small. Therefore, in order to achieve a better representation of these species, the number of sample plots should be increased. Air temperature and precipitation are the key factors in the growth and development of plants. For a plant to survive, these climate factors have to be at least at a certain minimum level, especially in the growing season [61,62]. Major parts of Serbia have a continental precipitation regime, with higher quantities in warmer part of the year [63]. Increasing amounts of precipitation enhance the growth of vegetation, while deficiency in precipitation over an extended period of time leads to drought as the most common cause of damage and die-back of individual trees and large forest areas [64]. Unfavourable climate, especially the lack of precipitation during the growing season, air temperatures above multi-annual averages (Tables S4–S7), and prolonged and frequent drought periods have had serious and long-term consequences on forest ecosystems in Serbia. Similar observations have been stated by domestic authors [53,54,55,65,66,67,68,69,70], whose research confirms the negative impacts of climate change and extreme climate events on the growth, development, and vitality of forest tree species and forest ecosystems as a whole.
In the last decade alone, Europe had several extremely hot and dry summers [71,72,73,74,75,76]. The data of the European Environment Agency (EEA) [77] showing drought periods and the areas affected throughout Europe can serve as a good indicator of the drought distribution. Thus, the Republic of Serbia had six periods without rainfall followed by high temperatures (i.e., drought). A study by Spinioni et al. [78] compiles a pan-European list of past drought events for the period from 1950 to 2012, with Europe divided into thirteen regions according to country borders and geographic and climatic characteristics. Regarding the Balkan countries (Albania, Bosnia and Herzegovina, Croatia, Montenegro, FYR Macedonia, Serbia, and Slovenia), the period from 2007 to 2009 was stated to be the longest drought period in terms of duration (number of months), while the period from 2011 to 2012 was the most severe in terms of drought and its effects, which coincides with our research results. These scenarios can be confirmed on websites that provide data on drought periods worldwide [79,80]. Several authors have analysed the relationship between tree mortality and drought based on tree mortality maps in Europe over a thirty-year period (1986–2016) [28]. These maps clearly show that 2012 and 2013 had the most intense drought and highest tree canopy mortality, which was the case in the wider area of Serbia as well [28,81]. Technical reports [82] based on data submitted by the countries participating in the ICP Forests Programme contain overviews of the state of European forests based on the monitoring of sample plots at the annual level. Other countries that were affected by drought in 2011 and 2012 (e.g., Croatia and Hungary) stated an increase in defoliation in this period and stressed climate conditions (i.e., drought) as the most obvious reasons for the increase. They warned that the damage could be much greater than was shown by research at the time. Furthermore, the report of the Intergovernmental Panel on Climate Change [83] highlighted the impact of extreme climate events such as heatwaves and droughts that significantly increase the exposure and vulnerability of certain ecosystems.
4. Conclusions
Based on our analysis of monitoring data on tree defoliation in Serbia over a fifteen-year period (2004–2018), it can be concluded that the damage caused by the registered extreme climate events occurred gradually and periodically after several consecutive dry years. However, it was of very high intensity and affected the entire territory of Serbia. It increased significantly in the period from 2013 to 2016. At first, defoliation was recorded as the impact of an unknown cause; however, the correlation with the number of dead and dying trees and climate characteristics in the research period revealed the causes. Due to extreme weather conditions, the tolerance threshold of certain tree species was exceeded, which eventually led to their gradual die-back and death regardless of stand age or the influence of biotic factors. The years preceding the ones with the most extensive tree mortality in Serbia (2013–2016) recorded mortality events of both individual trees and large forest complexes. However, there had not previously been such an intensive die-back with clear linking causes. The period from 2011 to 2013 showed the greatest stress on plants recorded during the period of tree vitality monitoring on sample plots. It should be noted that the occurrence of defoliation with ultimate die-back was recorded in the areas at both higher and lower altitudes. Although these higher-altitude areas are usually categorised as humid, they recorded a desert climate type in the above-stated years with extreme climate events. Furthermore, die-back was detected in both broadleaved and coniferous tree species, with the difference that defoliation was more easily observed in broadleaves. Our statistical analysis showed that the highest forest decline was recorded in the period from 2014 to 2018 and that annual mortality rates did not differ significantly between coniferous and broadleaved trees or among sites at different altitudes.
After many years of researching the impact of various factors on forest ecosystems, we have become aware of many advantages of the continuous monitoring method performed at a large number of sample plots. Monitoring of phenomena and processes over a long period and on a large number of specimens enables more precise identification of the real causes of forest decline. If symptoms found on selected trees can be diagnosed in their immediate surroundings, it is easier to draw conclusions on the causes of die-back. However, although the number of main tree species on sample plots coresponds to the share of the same species in forest cover on the territory of Serbia, for certain species (particularly conifers) the sample in the present study was small. Thus, to achieve better representation of these species and better forest condition monitoring, the number of sample plots should be increased.
Based on the results of this research, it can be concluded that unfavourable climate conditions, primarily the lack of precipitation, rising air temperatures, and increasingly frequent and long dry periods, had serious and long-term consequences on forest ecosystems in Serbia.
5. Materials and Methods
5.1. Study Area and Data Preparation
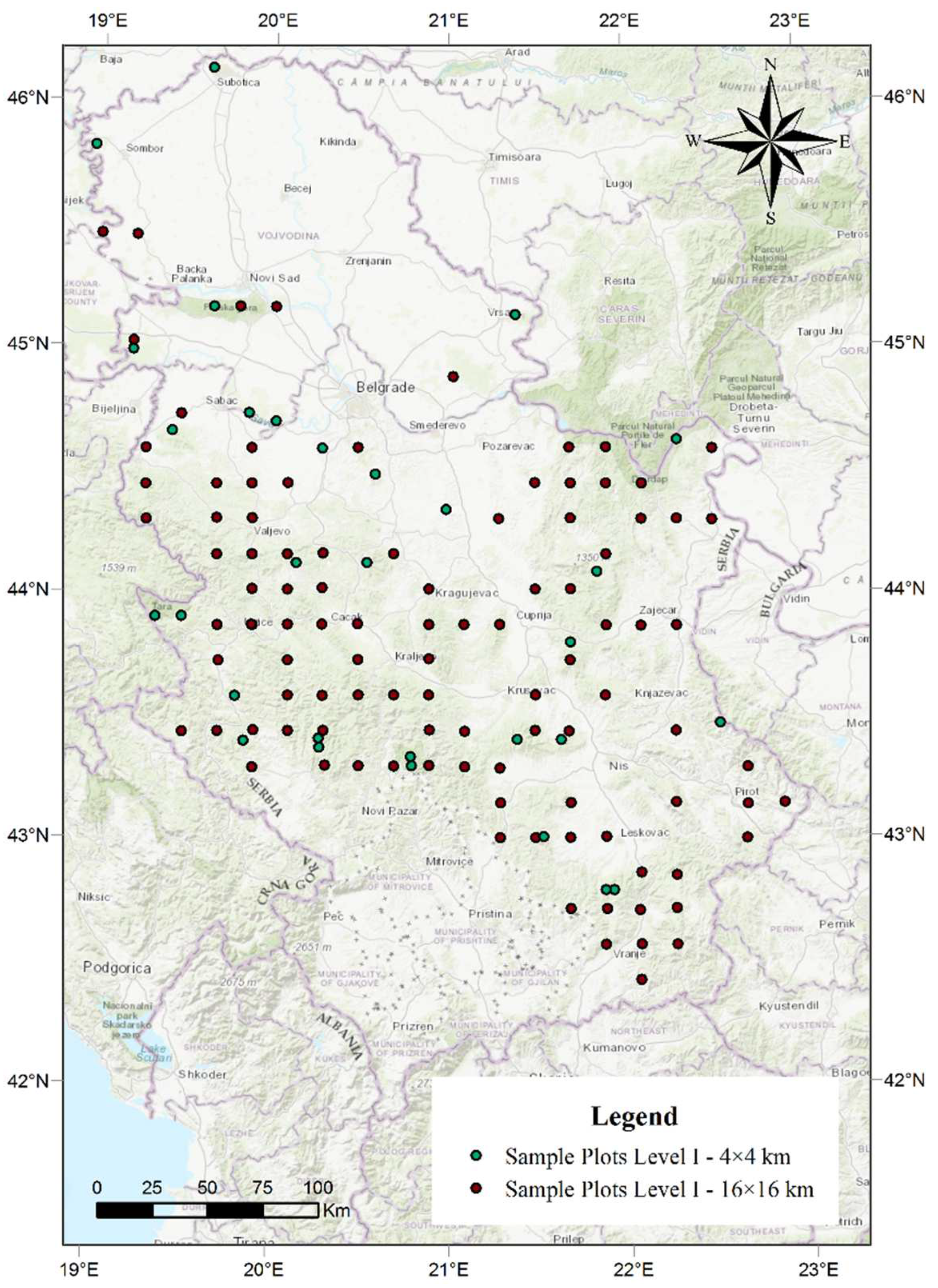
The total number of dead trees was determined based on the data collected in the territory of the Republic of Serbia within the International Cooperative Programme on Assessment and Monitoring of Air Pollution Effects on Forests (ICP Forests) [40]. The research was conducted over a period from 2004 to 2018 on all 130 sample plots established at the intersections of 16 × 16 km and 4 × 4 km (Figure 5). This network is located at 0 to 1600 m above sea level. The center of each sample plot was marked and six trees were selected in each cardinal direction, resulting in 24 trees in each sample plot [84]. The main criterion in the selection of trees for condition monitoring within the network of sample plots was the absence of any significant mechanical damage. Mechanical injuries make trees susceptible to attack by insects or fungi that can cause defoliation and eventual die-back, masking the primary cause. The selected trees had been monitored continuously following the establishment of each sample plot. Any change in the whole tree was detected and recorded and the cause of the damage was identified during the growing season, from the time leaves and needles are fully developed to the beginning of autumn senescence. Monitoring was focused on assimilation organs, as the damage caused by various factors can in most cases be observed on them. The assessment of defoliation included any kind of damage recorded on the examined trees. In most species, the most suitable time to perform observations is from early summer, when leaves or needles are fully formed, to late summer. Out of a total of 3800 trees monitored, the research encompassed an average of 2880 trees per year. The number of trees varied with various factors, such as regular felling, snow breakage, wind breakage, die-back, etc. Of the observed number of trees, the trees that were recorded as dead during the research period were selected and analysed in detail. The trees were then divided into three groups based on the changes in defoliation (Table S1).
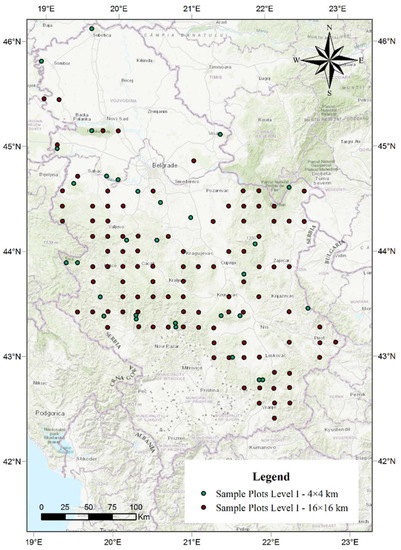
Figure 5.
The spatial arrangement of sample plots in the territory of the Republic of Serbia.
The main data on tree species, stand age, and altitude range at the investigated localities are presented in Supplementary Figure S5.
5.2. Defoliation
The principal method was based on the assessment of defoliation as the main parameter of the condition of forests. Defoliation was assessed in 5% steps, for instance 5 (>0–5%), 10 (>5–10%), etc. (Figure 6). The missing leaf mass was assessed compared to healthy trees growing in the same site and stand conditions and observed regardless of the cause of foliage loss. The method was as previously described in the literature [41].

Figure 6.
An illustration of defoliation assessment: (a) 10%; (b) 25%; (c) 65%.
During the research period (2004–2018), defoliation was assessed regardless of its cause. In order to understand the possible cause of death, we singled out all trees recorded as dead during the period (Table S1). The trees were classified into three different groups of trees whose death was caused by defoliation:
- I.
- The first group included trees with no defoliation (class 0) and slight defoliation (class 1) at the beginning of condition monitoring and during most of the years for which defoliation moved to higher classes of defoliation in the last few years, namely, classes 2 (moderate), 3 (severe), and 4 (dead).
- II.
- The second group included trees that died suddenly and moved from class 0 or class 1 to class 4.
- III.
- The third group included trees with higher classes of defoliation that occurred after the first year of monitoring, and which several years later led to their death.
5.3. Climate Characteristics
The non-reactive research method was used for the collection of data on climate characteristics during the research period [85]. The data on mean monthly air temperatures, extreme maximum and minimum air temperatures, and monthly precipitation amounts for the research period (2004–2018) were provided by the Republic Hydrometeorological Service of Serbia (RHSS) for 28 main meteorological stations in Serbia [57]. The data were used to calculate the mean monthly and annual values of the air temperature and precipitation amount for the growing period (April–September). Based on the arithmetical means of monthly values calculated for each year, annual and growing season values were obtained (Tables S4–S7).
According to the applied Köppen and Köppen–Geiger climate classification systems [86,87], Mihajlović, J. [88] distinguished two types of climate in Serbia, namely, temperate (S) and cold (D). A warm temperate rainy (S) climate is present in different variants, with dominant Sfb and Cfa classes, while a cold or boreal snow forest (D) climate is represented by the Dfb, Dfc, and Dfa classes [89].
5.4. Drought Index Quantification
In order to quantify the precipitation deficit for different time intervals, the Standardised Precipitation Index (SPI) was calculated according to McKee et al. [90]. When determining the SPI with precipitation as the only input parameter, we used the precipitation totals from 28 main weather stations of the Republic Hydrometeorological Service of Serbia in the period from 2004 to 2018 [57] to calculate the time series of previous droughts and assess their severity. We calculated the SPI at the semi-annual level (SPI-6) for the growing season (April–September) and the SPI at the annual level (SPI-12). We calculated SPI-6 in the growing season to provide a better representation of the amount of precipitation at the time plants need it most for their growth and development. The results of the SPI for the drought periods were then compared and correlated with the tree defoliation.
We further calculated the Standardised Precipitation Evapotranspiration Index (SPEI) in order to prove the existence of the drought period; SPEI input data included temperature and precipitation [91]. The SPEI data were obtained from the global SPEI database [79] as part of the weather series for the region of Serbia (coordinates: upper left 42.25, 23.25, and lower right 46.25, 18.75). By using air temperature alongside precipitation data, the SPEI allows a broader view of the effects of drought and links them to defoliation. The SPEI was calculated at 6- and 12-month intervals (SPEI-6 and SPEI-12).
This study used the SPI and SPEI as the most common methods for monitoring drought [92,93,94]. The SPI was used to estimate precipitation deviations from the normal state, while the SPEI included the temperature component in addition to precipitation to obtain a clearer picture of the drought. According to the SPI, a drought event begins when its values are equal to or below −1.0 and ends when the values become positive [90], which is the case with the SPEI as well [91].
5.5. Tree Mortality
Based on the ICP Forests Manual [41], detailed attention was paid to tree mortality in a given year, as the total number of dead trees per plot at any time did not provide information on mortality rates. According to the methodology, dead trees are commonly included in the sample if they are standing. Such trees are categorised as severely defoliated. In our study, these trees were considered dead at the time when intense defoliation (99%) occurred. By doing this, we were able to determine the exact year of die-back of a particular tree. The exact year of mortality was identified, and the results are presented in Supplementary Table S1. The mortality and the number of dead trees per plot are two different issues. Tree mortality was determined according to the year when defoliation was found to be 100% and divided into three groups based on the progression of defoliation. We then determined the connection between the tree mortality rate and the SPI and the SPEI during periods of drought.
5.6. Statistical Analysis
For a total of 3800 trees belonging to 34 species at 130 sites in the Republic of Serbia, annual mortality rates were calculated for three observation periods (2004–2008, 2009–2013, and 2014–2018). These calculations used the data obtained from monitoring defoliation according to the ICP Forests methodology [41]; trees with defoliation of 100% were considered dead. According to Sheil et al. [95], the true annual mortality is defined by the equation m = 1 − (N1/N0)1/t, where N0 and N1 are population counts at the beginning and end of the measurement interval, t. As m is recommended as a standard quantity for comparing annual mortality rates in plant ecology [95], it was adopted as the annual mortality rate in this study. The variation in mortality rates was captured using the mortality rate of each of the 34 tree species analysed as a subpopulation. To calculate m, we used three five-year intervals, because the five-year interval is the most commonly used census interval length (as recommended by Lewis et al. [96]) and maximises intercensus and intersite comparability. Before performing the statistical analysis, data on annual mortality rates were tested for normality. As the assumption that these data were normally distributed had not been confirmed, the medians (M) were used for both intervals of observation. The median absolute deviation (MAD) was determined for each median, and the comparison and determination of the difference between the medians was carried out using the Kruskal–Wallis test (KWt). All statistical analyses were performed using Statgraphics software (2009; Statpoint Technologies, Inc., Warrenton, VA, USA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11101286/s1, Figure S1: Forest decline on sample plots on the territory of the Republic of Serbia in the period from 2004 to 2018.; Figure S2: The percentage of trees of all species with the damage on the sample plots (2011–2014).; Figure S3: Median plot with 95% confidence intervals for the annual mortality rates of broadleaved and conifer tree species monitored in the territory of Serbia in the period of 2014–2018.; Figure S4: Median plot with 95% confidence intervals for the annual mortality rates of trees monitored at different altitude ranges in the territory of Serbia in the period of 2014–2018.; Figure S5: Distribution of 130 Sample plots by tree species, altitude and stand age.; Table S1: Trends in defoliation on trees during the years of research with the final outcome of dying and division into groups.; Table S2: Descriptive and nonparametric statistics for the annual mortality rates of broadleaf and conifer tree species monitored in the territory of Serbia in the period of 2014–2018.; Table S3: Descriptive and nonparametric statistics for the annual mortality rates of trees monitored at different altitude ranges in the territory of Serbia in the period of 2014–2018.; Table S4: Mean annual air temperatures (°C) in Serbia in the period from 2004 to 2018.; Table S5: Mean annual air temperatures (°C) in the growing season in Serbia from 2004 to 2018.; Table S6: Mean precipitation sum (mm) in Serbia in the period from 2004 to 2018.; Table S7: Mean precipitation sum (mm) in the growing season (April–September) in Serbia in the period from 2004 to 2018.; Table S8: Comparative analysis of defoliation in the period 2004–2018—Conifers.; Table S9: Comparative analysis of defoliation in the period 2004–2018—Broadleaves.
Author Contributions
Conceptualization, G.Č.; Data curation, G.Č. and L.B.-B.; Formal analysis, G.Č., and F.J.; Investigation, G.Č., I.Đ., S.M., S.E., T.Ć.-M. and A.L.; Methodology, G.Č. and F.J.; Project administration, G.Č.; Resources, L.B.-B., I.Đ., S.M., S.E., T.Ć.-M. and A.L.; Validation, G.Č., F.J., L.B.-B., I.Đ., S.M., S.E., T.Ć.-M. and A.L.; Visualization, G.Č., F.J., L.B.-B., I.Đ., S.M., S.E., T.Ć.-M. and A.L.; Writing—original draft, G.Č.; Writing—review and editing, G.Č. and F.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Education, Science, and Technological Development (Agreement no. 451-03-68/2022-14/200027) and the Ministry of Agriculture, Forestry, and Water Management of the Republic of Serbia’s Forest Directorate within the project “Monitoring and Assessment of Air Pollution Impacts and its Effects on Forest Ecosystems in Republic of Serbia—Forest Condition Monitoring“ (Agreement no. 401-00-41/2022-10).
Data Availability Statement
All data are included in the manuscript.
Acknowledgments
We highly appreciate the work of all researchers who have participated in field research all these years.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bennett, A.; McDowell, N.; Allen, C.; Anderson-Teixeira, K. Larger trees suffer most during drought in forests worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, A.; Mejia-Chang, M.; Ogaya, R.; Voltas, J.; Dawson, T.; Penuelas, J. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Glob. Change Biol. 2015, 21, 1213–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boczoń, A.; Kowalska, A.; Dudzińska, M.; Wróbel, M. Drought in Polish forests in 2015. Pol. J. Environ. Stud. 2016, 25, 1857–1862. [Google Scholar] [CrossRef]
- Wilhite, D.A. Drought and Water Crises: Science, Technology, and Management Issues; Taylor and Francis: Boca Raton, FL, USA, 2005; 406p. [Google Scholar]
- Dai, A. Drought under global warming: A review. WIREs Clim. Change 2011, 2, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Spinoni, J.; Naumann, G.; Vogt, J.; Barbosa, P. Meteorological Droughts in Europe: Events and Impacts—Past Trends and Future Projections; EUR 27748 EN; Publications Office of the European Union: Luxembour, 2016. [Google Scholar] [CrossRef]
- Bradford, R.B. Drought Events in Europe. In Drought and Drought Mitigation in Europe. Adv. Nat. Technol. Hazard. Res. 2000, 14, 7–20. [Google Scholar] [CrossRef]
- Fink, A.H.; Brucher, T.; Kruger, A.; Leckebusch, G.; Pinto, J.; Ulbrich, U. The 2003 European summer heatwaves and drought–synoptic diagnosis and impacts. Weather 2004, 59, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Gil-Pelegrín, E.; Peguero-Pina, J.J.; Camarero, J.J.; Fernández-Cancio, A.; Navarro-Cerrillo, R. Drought and forest decline in the Iberian Peninsula: A simple explanation for a complex phenomenon? In Droughts: Causes, Effects and Predictions; Sánchez, J.M., Ed.; Nova Science Publishers: New York, NY, USA, 2008; pp. 27–68. [Google Scholar]
- Jentsch, A.; Beierkuhnlein, C. Research frontiers in climate change: Effects of extreme meteorological events on ecosystems. Comptes Rendus Geosci. 2008, 340, 621–628. [Google Scholar] [CrossRef]
- Bissolli, P.; Ziese, M.; Pietzsch, S.; Finger, P.; Friedrich, K.; Nitsche, H.; Obregón, A. Drought Conditions in Europe in the Spring of 2012; Report; Deutscher Wetterdienst (DWD): Offenbach, Germany, 2012; pp. 1–30. [Google Scholar]
- Spinoni, J.; Antofie, T.; Barbosa, P.; Bihari, Z.; Lakatos, M.; Szalai, S.; Szentimrey, T.; Vogt, J. An overview of drought events in the Carpathian Region in 1961–2010. Adv. Sci. Res. 2013, 10, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Stahl, K.; Kohn, I.; Blauhut, V.; Urquijo, J.; De Stefano, L.; Acácio, V.; Dias, S.; Stagge, J.; Tallaksen, L.; Kampragou, E.; et al. Impacts of European drought events: Insights from an international database of text-based reports. Nat. Hazard. Earth Sys. 2016, 16, 801–819. [Google Scholar] [CrossRef] [Green Version]
- De la Cruz, A.; Gil, P.; Fernández-Cancio, A.; Minaya, M.; Navarro-Cerrillo, R.; Sánchez-Salguero, R.; Grau, J.M. Defoliation triggered by climate induced effects in Spanish ICP Forests monitoring plots. For. Ecol. Manag. 2014, 331, 245–255. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Vilalta, J.; Lloret, F.; Breshears, D.D. Drought-induced forest decline: Causes, scope and implication. Biol. Lett. 2012, 8, 689–691. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Carrer, M.; Gutiérrez, E.; Alla, A.Q.; Andreu-Hayles, L.; Hevia, A.; Koutavas, A.; Martínez-Sancho, E.; Nola, P.; et al. Climate extremes and predicted warming threaten Mediterranean Holocene firs forests refugia. Proc. Natl. Acad. Sci. USA 2017, 114, E10142–E10150. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Shao, M.; Jia, X.; Wei, X. Relationship of climatic and forest factors to drought-and heat-induced tree mortality. PLoS ONE 2017, 12, e0169770. [Google Scholar] [CrossRef]
- Neumann, M.; Mues, V.; Moreno, A.; Hasenauer, H.; Seidl, R. Climate variability drives recent tree mortality in Europe. Glob. Change Biol. 2017, 23, 4788–4797. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Hartmann, H.; Schuldt, B.; Sanders, T.G.M.; Macinnis-Ng, C.; Boehmer, H.J.; Allen, C.D.; Bolte, A.; Crowther, T.W.; Hansen, M.C.; Medlyn, B.E.; et al. Monitoring global tree mortality patterns and trends. Report from the VW symposium ‘Crossing scales and disciplines to identify global trends of tree mortality as indicators of forest health’. New Phytol. 2018, 217, 984–987. [Google Scholar] [CrossRef] [Green Version]
- Senf, C.; Pflugmacher, D.; Zhiqiang, Y.; Sebald, J.; Knorn, J.; Neumann, M.; Hostert, P.; Seidl, R. Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nat. Commun. 2018, 9, 4978. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Anderegg, L.D.L.; Kerr, K.L.; Trugman, A.T. Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Glob. Change Biol. 2019, 25, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Caudullo, G.; Barredo, J.I. A georeferenced dataset of drought and heat-induced tree mortality in Europe. One Ecosyst. 2019, 4, e37753. [Google Scholar] [CrossRef]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Senf, C.; Buras, A.; Zang, C.S.; Rammig, A.; Seidl, R. Excess forest mortality is consistently linked to drought across Europe. Nat. Commun. 2020, 11, 6200. [Google Scholar] [CrossRef] [PubMed]
- Republic Hydrometeorological Service of Serbia (RHSS) Annual Climate Characteristics for the Territory of Serbia, Republic Hydrometeorological Service of Serbia, Belgrade. Available online: http://www.hidmet.gov.rs/eng/meteorologija/klimatologija_produkti.php (accessed on 31 March 2022).
- Pollastrini, M.; Feducci, M.; Bonal, D.; Fotelli, M.; Gessler, A.; Grossiord, G.; Guyot, V.; Jactel, H.; Nguyen, D.; Radoglou, K.; et al. Physiological significance of forest tree defoliation: Results from a survey in a mixed forest in Tuscany (central Italy). For. Ecol. Manag. 2016, 361, 170–178. [Google Scholar] [CrossRef]
- Sousa-Silva, R.; Verheyen, K.; Ponette, Q.; Bay, E.; Sioen, G.; Titeux, H.; Van de Peer, T.; Van Meerbeek, K.; Muys, B. Tree diversity mitigates defoliation after a drought-induced tipping point. Glob. Change Biol. 2018, 24, 4304–4315. [Google Scholar] [CrossRef]
- Gottardini, E.; Cristofolini, F.; Cristofori, A.; Pollastrini, M.; Camin, F.; Ferretti, M. A multi-proxy approach reveals common and species-specific features associated with tree defoliation in broadleaved species. For. Ecol. Manag. 2020, 467, 118151. [Google Scholar] [CrossRef]
- Ferretti, M.; Bacaro, G.; Brunialti, G.; Calderisi, M.; Croisé, L.; Frati, L.; Nicolas, M. Tree canopy defoliation can reveal growth decline in mid-latitude temperate forests. Ecol. Indic. 2021, 127, 107749. [Google Scholar] [CrossRef]
- Bussoti, F.; Pollastrini, M. Traditional and novel indicators of climate change impacts on European forest trees. Forests 2017, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Seidling, W. Signals of summer drought in crown condition data from the German Level I network. Eur. J. For. Res. 2007, 126, 529–544. [Google Scholar] [CrossRef]
- Fabiánek, P.; Hellebrandová, K.; Čapek, M. Monitoring of defoliation in forest standsof the Czech Republic and its comparison with results of defoliation monitoring in other European countries. J. For. Sci. 2012, 58, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Eickenscheidt, N.; Augustin, N.H.; Wellbrock, N. Spatio-temporal modelling of forest monitoring data: Modelling German tree defoliation data collected between 1989 and 2015 for trend estimation and survey grid examination using GAMMs. iForest 2019, 12, 338–348. [Google Scholar] [CrossRef]
- Etzold, S.; Ziemińska, K.; Rohner, B.; Bottero, A.; Bose, A.; Ruehr, N.K.; Zingg, A.; Rigling, A. One Century of Forest Monitoring Data in Switzerland Reveals Species- and Site-Specific Trends of Climate-Induced Tree Mortality. Front. Plant Sci. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, N.; Allen, C.D.; Anderson-Teixeira, K.; Brando, P.; Brienen, R.; Chambers, J.; Christoffersen, B.; Davies, S.; Doughty, C.; Duqueet, A.; et al. Drivers and mechanisms of tree mortality in moist tropical forests. New Phytol. 2018, 219, 851–869. [Google Scholar] [CrossRef] [Green Version]
- ICP Forests—International Co-operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests. Available online: http://icp-forests.net/ (accessed on 31 January 2022).
- Eichhorn, J.; Roskams, P.; Potočić, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletković, I.; Schröck, H.-W.; et al. Part IV Visual Assessment of Crown Condition and Damaging Agents. In ICP Forests Manual; Version 2020-3; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; pp. 5–54. Available online: https://storage.ning.com/topology/rest/1.0/file/get/9995547265?profile=original (accessed on 31 January 2022).
- Dobbertin, M.; Brang, P. Crown defoliation improves tree mortality models. For. Ecol. Manag. 2001, 141, 271–284. [Google Scholar] [CrossRef]
- Ferretti, M.; Nicolas, M.; Bacaro, G.; Brunialti, G.; Calderisi, M.; Croisé, L.; Frati, L.; Lanier, M.; Maccherini, S.; Santi, E.; et al. Plot-scale modelling to detect size, extent, and correlates of changes in tree defoliation in French high forests. For. Ecol. Manag. 2014, 311, 56–69. [Google Scholar] [CrossRef]
- Lorenz, M.; Fischer, R.; Becher, G.; Mues, V.; Granke, O.; Braslavskaya, T.; Bobrinsky, A.; Clarke, N.; Lachmanová, Z.; Lukina, N.; et al. Work Report, Institute for World Forestry. Forest Condition in Europe. Technical Report of ICP Forests. 2009. Available online: https://www.icp-forests.org/pdf/TR2009.pdf (accessed on 31 January 2022).
- Nicolas, M.; Jolivet, C.; Jonard, M. How monitoring networks contribute to the understanding and to the management of soil and forest ecosystems? Rev. For. Fr. 2014, 66, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Innes, J.L. Forest health surveys—A critique. Environ Pollut. 1988, 54, 1–15. [Google Scholar] [CrossRef]
- Johnson, J.; Jacob, M. Monitoring the effects of air pollution on forest condition in Europe: Is crown defoliation an adequate indicator? iForest 2010, 3, 86–88. [Google Scholar] [CrossRef] [Green Version]
- Cherubini, P.; Battipaglia, G.; Innes, J.L. Tree Vitality and Forest Health: Can Tree-Ring Stable Isotopes Be Used as Indicators? Curr. For. Rep. 2021, 7, 69–80. [Google Scholar] [CrossRef]
- De Vries, W.; Klap, J.M.; Erisman, J.W. Effects of environmental stress on forest crown condition in Europe. Part I: Hypotheses and approach to the study. Water Air Soil Pollut. 2000, 119, 317–333. [Google Scholar] [CrossRef]
- De Marco, A.; Proietti, C.; Cionni, I.; Fischer, R.; Screpanti, A.; Vitale, M. Future impacts of nitrogen deposition and climate change scenarios on forest crown defoliation. Environ. Pollut. 2014, 194, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ognjenović, M.; Seletković, I.; Potočić, N.; Marušić, M.; Tadić, M.P.; Jonard, M.; Rautio, P.; Timmermann, V.; Lovreškov, L.; Ugarković, D. Defoliation Change of European Beech (Fagus sylvatica L.) Depends on Previous Year Drought. Plants 2022, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Jančić, G. Sušenje šuma—Uzroci sušenja i mere sanacije. Revija Šume 2013, 120, 10–11. Available online: https://srbijasume.rs/ssume/wp-content/uploads/2021/02/Sume_120.pdf (accessed on 31 January 2022).
- Češljar, G.; Nevenić, R.; Bilibajkić, S.; Stefanović, T.; Gagić Serdar, R.; Đorđević, I.; Poduška, Z. Viability of trees on bio-indicator plots Level 1 in Republic of Serbia in 2013. Sustain. For. 2013, 67/68, 69–78. [Google Scholar]
- Češljar, G.; Gagić Serdar, R.; Đorđević, I.; Poduška, Z.; Stefanović, T.; Bilibajkić, S.; Nevenić, R. Analysis of types of damages at the sample plots of Level 1 in 2013 at the territory of the Republic of Serbia. Sustain. For. 2014, 69/70, 63–71. [Google Scholar] [CrossRef]
- Češljar, G.; Đorđević, I.; Brašanac-Bosanac, L.; Eremija, S.; Mitrović, S.; Ćirković-Mitrović, T.; Lučić, A. Determination of forest decline due to the action of dominant stress factor through monitoring of defoliation—Case study of Maljen, Serbia. Agric. For. 2021, 67, 211–226. [Google Scholar] [CrossRef]
- Drekić, M.; Poljaković-Pajnik, L.; Orlović, S.; Kovačević, B.; Vasić, V.; Pilipović, A. Results of multiannual monitoring of tree crown condition. Poplar 2014, 193/194, 23–35. [Google Scholar]
- Republic Hydrometeorological Service of Serbia (RHSS). Meteorological Yearbook—Climatological Data. Republic Hydrometeorological Service of Serbia, Belgrade. Available online: http://www.hidmet.gov.rs/latin/meteorologija/klimatologija_godisnjaci.php (accessed on 31 March 2022).
- Rakićević, T. Klimatsko rejoniranje SR Srbije. In Zbornik Radova Geografskog Instituta “Jovan Cvijić”; SANU: Beograd, Srbija, 1980; Knjiga 27; pp. 29–42. [Google Scholar]
- Augusto, L.; Davies, T.J.; Delzon, S.; De Schrijver, A. The enigma of the rise of angiosperms: Can we untie the knot? Ecol. Lett. 2014, 17, 1326–1338. [Google Scholar] [CrossRef]
- Hentschel, R.; Rosner, S.; Kayler, Z.E.; Andreassen, K.; Børja, I.; Solberg, S.; Tveito, O.E.; Priesack, E.P.; Gess-ler, A. Norway spruce physiological and anatomical predisposition to dieback. For. Ecol. Manag. 2014, 322, 27–36. [Google Scholar] [CrossRef]
- Hacket-Pain, A.J.; Cavin, L.; Friend, A.D.; Jump, A.S. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur. J. For. Res. 2016, 135, 897–909. [Google Scholar] [CrossRef] [Green Version]
- Devi, N.M.; Kukarskih, V.V.; Galimova, A.A.; Mazepa, V.S.; Grigoriev, A.A. Climate change evidence in tree growth and stand productivity at the upper treeline ecotone in the Polar Ural Mountains. For. Ecosyst. 2020, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Republic Hydrometeorological Service of Serbia (RHSS). Basic Climate Characteristics for the Territory of Serbia. Republic Hydrometeorological Service of Serbia, Belgrade. Available online: https://www.hidmet.gov.rs/eng/meteorologija/klimatologija_srbije.php (accessed on 1 May 2022).
- Gazol, A.; Camarero, J.J. Compound climate events increase tree drought mortality across European forests. Sci. Total Environ. 2022, 816, 151604. [Google Scholar] [CrossRef] [PubMed]
- Popović, T.; Radulović, E.; Jovanović, M. Koliko nam se menja klima, kakva će biti naša buduća klima? EnE05—Konf. Zivotn. Sred. Ka Evropi. Beogr. 2005, 212–218. Available online: http://www.sepa.gov.rs/download/5_web.pdf (accessed on 10 January 2022).
- Vukovic, A.; Vujadinovic, M.; Rendulic, S.; Djurdjevic, V.; Ruml, M.; Babic, V.; Popovic, D. Global warming impact on climate change in Serbia for the period 1961–2100. Therm. Sci. 2018, 22, 2267–2280. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Orlović, S.; Zlatković, M.; Kostić, S.; Vasić, V.; Miletić, B.; Kesić, L.; Matović, B.; Božanić, D.; Pavlović, L.; et al. Climate change within Serbian forests: Current state and future perspectives. Poplar 2021, 208, 39–56. [Google Scholar] [CrossRef]
- Stanković, Z.; Govedar, Z.; Kapović, M.; Hrkić, Z. Climate Change Impact on Forest Vegetation in Republic of Srpska. In Proceedings of the International Scientific Conference “Forest Ecosystems and Climate Changes”, Belgrade, Serbia, 9–10 March 2010; Institute of Forestry: Belgrade, Serbia, 2010; Volume 1, pp. 21–25. Available online: http://www.forest.org.rs/pdf/konferencije/PROCEEDINGS-Vol1-FOREST-ECOSYSTEMSAND-CLIMATE-CHANGES.pdf (accessed on 10 January 2022).
- Brašanac-Bosanac, L.; Filipović, D.; Ćirković-Mitrović, T. Measurements for the adaptation of forest ecosystems on negative impacts of climate change in Serbia. Fresen. Environ. Bull. Ger. 2011, 20, 2653–2660. [Google Scholar]
- Ćirković-Mitrović, T.; Popović, V.; Brašanac-Bosanac, L.; Rakonjac, L.; Lučić, A. The impact of climate elements on the diameter increment of Austrian pine (Pinus nigra Arn.) in Serbia. Arch. Biol. Sci. 2013, 65, 161–170. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Barriopedro, D.; Fischer, E.M.; Luterbacher, J.; Trigo, R.M.; García-Herrera, R. The hot summer of 2010: Redrawing the temperature record map of Europe. Science 2011, 332, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Hanel, M.; Rakovec, O.; Markonis, Y.; Máca, P.; Samaniego, L.; Kyselý, J.; Kumar, R. Revisiting the recent European droughts from a long-term perspective. Sci. Rep. 2018, 8, 9499. [Google Scholar] [CrossRef] [PubMed]
- Hari, V.; Rakovec, O.; Markonis, Y.; Hanel, M.; Kumar, R. Increased future occurrences of the exceptional 2018–2019 Central European drought under global warming. Sci. Rep. 2020, 10, 12207. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, B.; Guo, L.; Huang, L.; Chen, D. Similarities and differences in the mechanisms causing the European summer heatwaves in 2003, 2010, and 2018. Earth’s Future 2020, 7, e2019EF001386. [Google Scholar] [CrossRef] [Green Version]
- Büntgen, U.; Urban, O.; Krusic, P.J.; Rybníček, M.; Kolář, T.; Kyncl, T.; Ač, A.; Koňasová, E.; Čáslavský, J.; Esper, J.; et al. Recent European drought extremes beyond Common Era background variability. Nat. Geosci. 2021, 14, 190–196. [Google Scholar] [CrossRef]
- European Environment Agency—EEA. Available online: https://www.eea.europa.eu/data-and-maps/figures/main-drought-events-in-europe (accessed on 10 January 2022).
- Spinonia, J.; Naumannb, G.; Vogta, J.V.; Barbosaa, P. The biggest drought events in Europe from 1950 to 2012. J. Hydrol Reg. Stud. 2015, 3, 509–524. [Google Scholar] [CrossRef]
- Standardized Precipitation Evapotranspiration Index (SPEI) Database. Available online: http://sac.csic.es/spei/database.html (accessed on 31 January 2022).
- Global Integrated Drought Monitoring and Prediction System (GIDMaPS). Available online: http://drought.eng.uci.edu/ (accessed on 31 January 2022).
- Senf, C.; Seidl, R. Mapping the forest disturbance regimes of Europe. Nat. Sustain. 2020, 4, 63–70. [Google Scholar] [CrossRef]
- Technical Report of ICP Forests 2013: Forest Condition in Europe. Available online: https://www.icp-forests.org/pdf/TR2013.pdf (accessed on 31 January 2022).
- Intergovernmental Panel on Climate Change. Climate Change—IPCC 2014, Synthesis Report, Summary for Policymakers. Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/AR5_SYR_FINAL_SPM.pdf (accessed on 31 January 2022).
- Ferretti, M.; Fischer, R.; Mues, V.; Granke, O.; Lorenz, M.; Seidling, W.; Nicolas, M. 2020: Part II: Basic Design Principles for the ICP Forests Monitoring Networks. Version 2020-2. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-Ordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; pp. 33 + Annex. Available online: http://icp-forests.net/page/icp-forests-manual (accessed on 31 March 2022).
- Neuman, W.L. Social Research Methods: Qualitative and Quantitative Approaches, 6th ed.; Part one; Pearson Inc.: London, UK, 2006; pp. 1–79. [Google Scholar]
- Köppen, W.P. Klassification der Klimate nach Temperatur, Niederschlag und Jahreslauf. Petermanns Geog. Mitt. 1918, 64, 243–248. [Google Scholar]
- Koppen, W.P.; Koppen, W. Das geographisca system der klimate. In Handbuch der Klimatologie; Koppen, W., Geiger, G.C., Eds.; Gebr. Borntraeger: Berlin, Germany, 1936; pp. 1–44. [Google Scholar]
- Mihajlović, J. Application of Recent Climate Classifications for the Climate Regionalization of Serbia. PhD Thesis, University of Belgrade, Faculty of Geography, Belgrade, Serbia, 2018; pp. 1–368. Available online: https://nardus.mpn.gov.rs/handle/123456789/10657 (accessed on 30 April 2022).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Koppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- McKee, T.B.; Doesken, N.J.; Kleist, J. The relationship of drought frequency and duration to time scales. Proceedings of the Eighth Conference on Applied Climatology. Boston MA. Am. Meteorol. Soc. 1993, 17, 179–184. [Google Scholar]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multi-scalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index—SPEI. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef] [Green Version]
- Tirivarombo, S.; Osupile, D.; Eliasson, P. Drought Monitoring and Analysis: Standardised Precipitation Evapotranspiration Index (SPEI) and Standardised Precipitation Index (SPI). Phys. Chem. Earth Parts A/B/C 2018, 106, 1–10. [Google Scholar] [CrossRef]
- Tefera, A.S.; Ayoade, J.O.; Bello, N.J. Comparative analyses of SPI and SPEI as drought assessment tools in Tigray Region, Northern Ethiopia. SN Appl. Sci. 2019, 1, 1265. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.; Fang, S.; Wang, L.; Yang, W. Comparative analysis of drought indicated by the SPI and SPEI at various timescales in Inner Mongolia, China. Water 2020, 12, 1925. [Google Scholar] [CrossRef]
- Sheil, D.; Burslem, D.F.R.P.; Alder, D. The interpretation and misinterpretation of mortality rate measures. J. Ecol. 1995, 83, 331–333. [Google Scholar] [CrossRef]
- Lewis, S.L.; Phillips, O.L.; Sheil, D.; Vinceti, B.; Baker, T.R.; Brown, S.; Graham, A.W.; Higuchi, N.; Hilbert, D.W.; Laurance, W.F.; et al. Tropical forest tree mortality, recruiting and turnover rates: Calculation, interpretation and comparison when census intervals vary. J. Ecol. 2004, 92, 929–944. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).