Soil Microbial Legacy Overrides the Responses of a Dominant Grass and Nitrogen-Cycling Functional Microbes in Grassland Soil to Nitrogen Addition
Abstract
:1. Introduction
2. Results
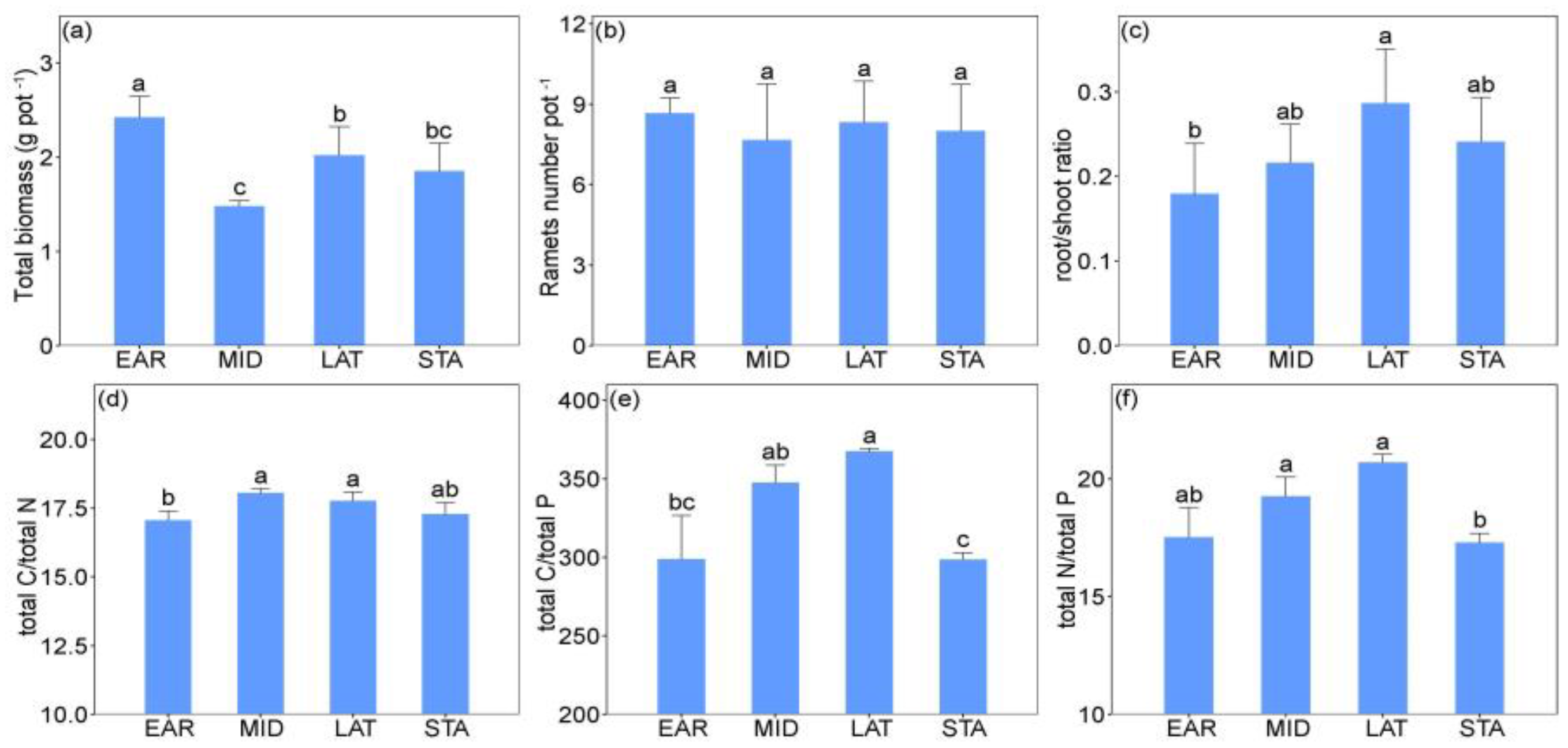
2.1. The Effects of Successional Stages and Sterilization on L. chinensis and NCFGs
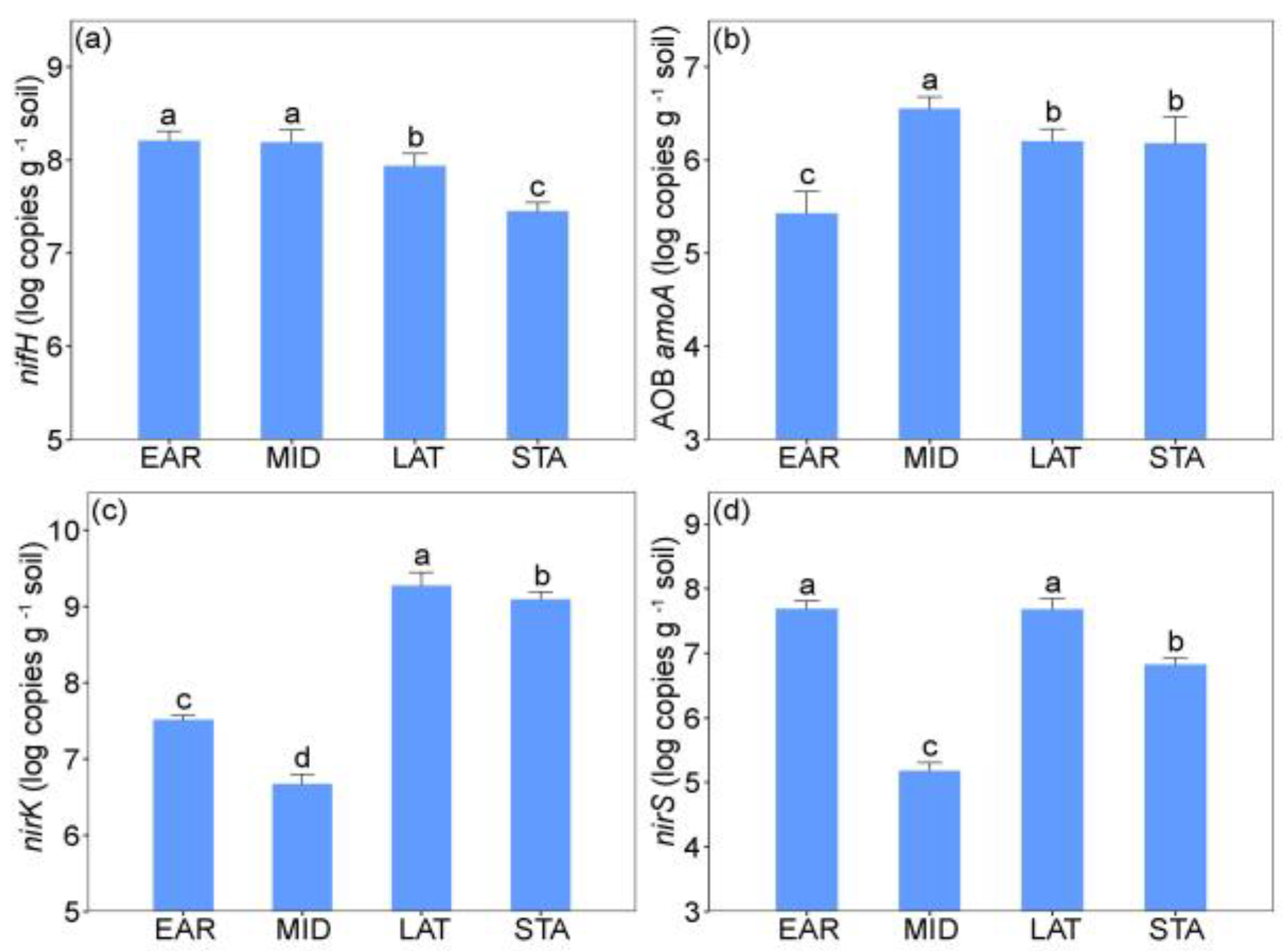
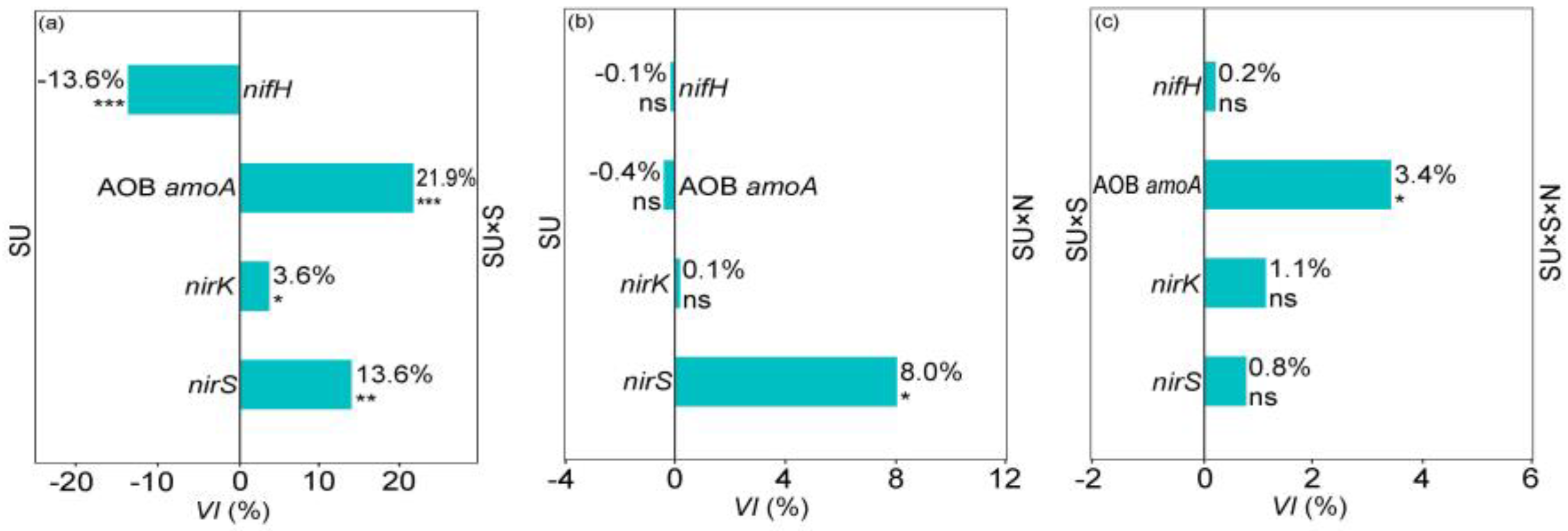
2.2. Soil Microbial Legacy Altered by N Addition
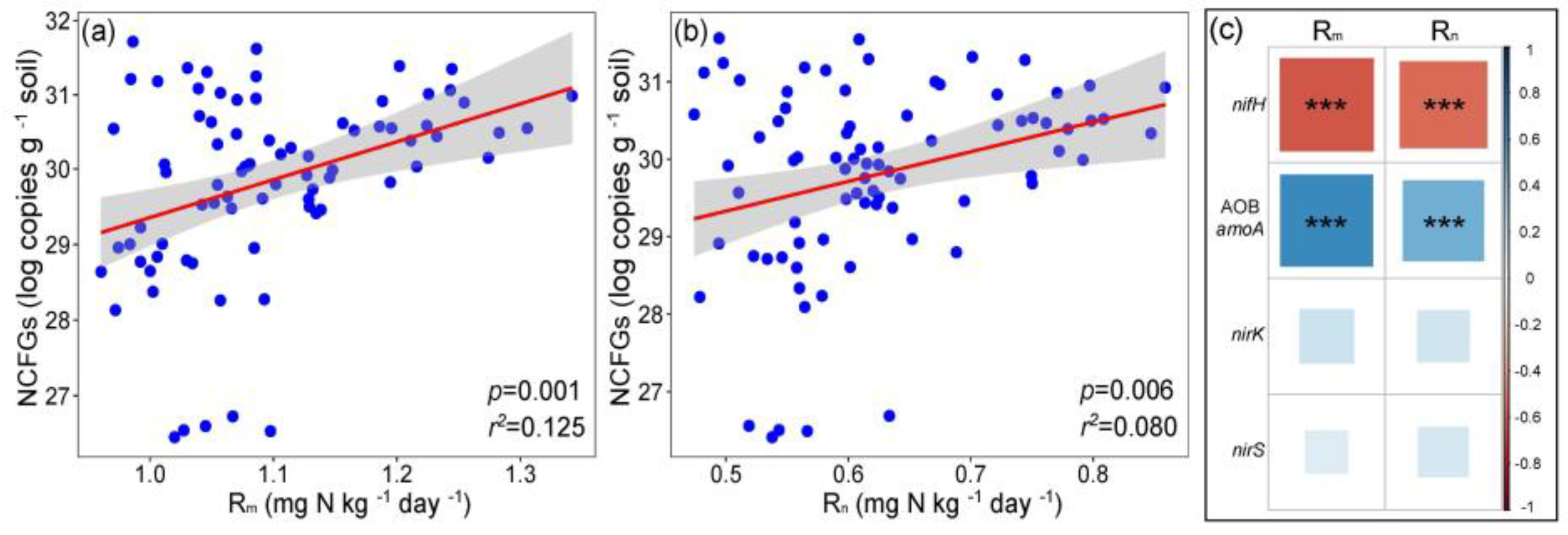
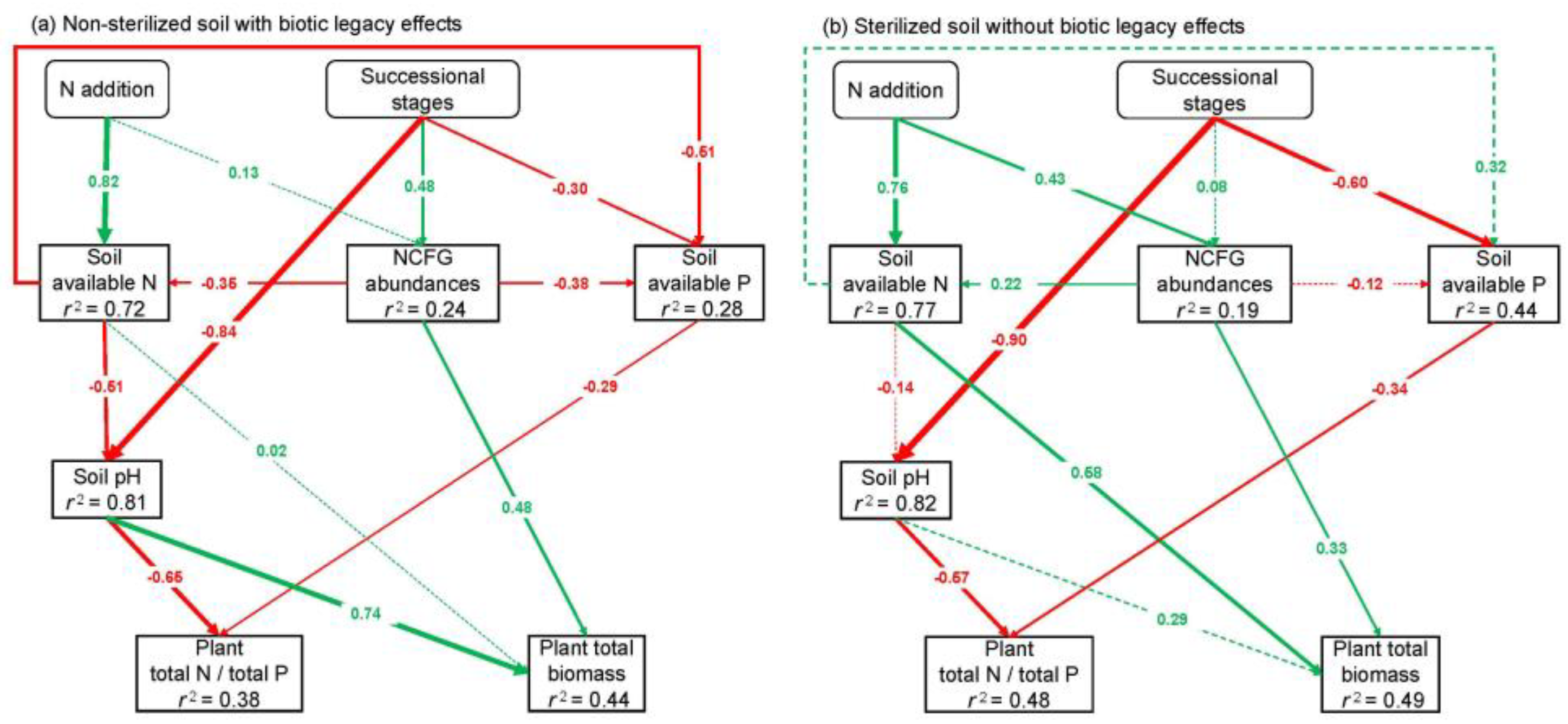
2.3. Key Factors and Pathways Influencing L. chinensis
3. Discussion
3.1. The Effects of Soil Microbial Legacy on L. chinensis and NCFGs
3.2. Soil Microbial Legacy Overrides N Addition Effects on L. chinensis and NCFGs
3.3. Key Factors Affecting L. chinensis and NCFGs
4. Materials and Methods
4.1. Sampling Area Description
4.2. Experimental Soil Preparation and Vegetation Survey
4.3. Mesocosm Experimental Design and Execution
4.4. Sampling and Measurements of the Plants and Soil
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van de Voorde, T.F.; Van der Putten, W.H.; Martijn, B.T. Intra-and interspecific plant-soil interactions, soil legacies and priority effects during old-field succession. J. Ecol. 2011, 99, 945–953. [Google Scholar] [CrossRef]
- Segnitz, R.M.; Russo, S.E.; Davies, S.J.; Peay, K.G. Ectomycorrhizal fungi drive positive phylogenetic plant-soil feedbacks in a regionally dominant tropical plant family. Ecology 2020, 101, e03083. [Google Scholar] [CrossRef] [PubMed]
- Ristok, C.; Poeschl, Y.; Dudenhöffer, J.H.; Ebeling, A.; Eisenhauer, N.; Vergara, F.; Wagg, C.; van Dam, N.M.; Weinhold, A. Plant species richness elicits changes in the metabolome of grassland species via soil biotic legacy. J. Ecol. 2019, 107, 2240–2254. [Google Scholar] [CrossRef] [Green Version]
- Kulmatiski, A.; Beard, K.H.; Stevens, J.R.; Cobbold, S.M. Plant-soil feedbacks: A meta-analytical review. Ecology 2008, 11, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Kardol, P.; Cornips, N.J.; Kempen, M.M.L.; Bakx-Schotman, J.M.T.; Van der Putten, W.H. Microbe-mediated plant-soil feedback causes historical contingency effects in plant community assembly. Ecol. Monogr. 2007, 77, 147–162. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, H.L.; Packer, A.; Bever, J.D.; Clay, K. Grassroots ecology: Plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology 2003, 84, 2281–2291. [Google Scholar] [CrossRef] [Green Version]
- Baxendale, C.; Orwin, K.H.; Pommier, P.T.; Bardgett, R.D. Are plant-soil feedback responses explained by plant traits? New Phytol. 2014, 204, 408–423. [Google Scholar] [CrossRef]
- Kaisermann, A.; de Vries, F.T.; Griffiths, R.I.; Bardgett, R.D. Legacy effects of drought on plant-soil feedbacks and plant-plant interactions. New Phytol. 2017, 215, 1413–1424. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; He, N.P.; Zhu, J.X.; Xu, L.; Yu, G.R.; Niu, S.L.; Sun, X.M.; Wen, X.F. Regional variation in the temperature sensitivity of soil organic matter decomposition in china’s forests and grasslands. Glob. Change Biol. 2017, 23, 3393–3402. [Google Scholar] [CrossRef]
- Jing, J.Y.; Bezemer, T.M.; Van der Putten, W.H. Complementarity and selection effects in early and mid-successional plant communities are differentially affected by plant-soil feedback. J. Ecol. 2015, 103, 641–647. [Google Scholar] [CrossRef]
- Kardol, P.; Bezemer, T.M.; Van der Putten, W.H. Temporal variation in plant-soil feedback controls succession. Ecol. Lett. 2006, 9, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Klironomos, J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Wren, I.F.; Herman, D.J.; Firestone, M.K. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol. Lett. 2005, 8, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Tharayil, N.; Alpert, P.; Bhowmik, P.; Gerard, P. Phenolic inputs by invasive species could impart seasonal variations in nitrogen pools in the introduced soils: A case study with Polygonum cuspidatum. Soil Biol. Biochem. 2013, 57, 858–867. [Google Scholar] [CrossRef]
- Nsikani, M.M.; Novoa, A.; Van Wilgen, B.W.; Keet, J.H.; Gaertner, M. Acacia saligna’s soil legacy effects persist up to 10 years after clearing: Implications for ecological restoration. Austral. Ecol. 2017, 42, 880–889. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Gan, Y.; Bainard, L.; Hamel, D.C.; St-Arnaud, M.; Hijri, M. Expression of N-cycling genes of root microbiomes provides insights for sustaining oilseed crop production. Environ. Microbiol. 2020, 22, 1–12. [Google Scholar] [CrossRef]
- Liu, W.; Ling, N.; Guo, J.; Yang, R.; Guo, S. Legacy effects of 8-year nitrogen inputs on bacterial assemblage in wheat rhizosphere. Biol. Fertil. Soils. 2020, 56, 583–596. [Google Scholar] [CrossRef]
- Lu, M.; Yang, Y.; Luo, Y.; Fang, C.; Zhou, X.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 2011, 189, 1040–1050. [Google Scholar] [CrossRef]
- Clark, C.M.; Tilman, D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef]
- Vankoughnett, M.R.; Henry, H.A.L. Soil freezing and N deposition: Transient vs multi-year effects on plant productivity and relative species abundance. New Phytol. 2014, 202, 1277–1285. [Google Scholar] [CrossRef]
- Van Paassen, J.G.; Britton, A.J.; Mitchell, R.J.; Street, L.E.; Johnson, D.; Coupar, A.; Woodin, S.J. Legacy effects of nitrogen and phosphorus additions on vegetation and carbon stocks of upland heaths. New Phytol. 2020, 228, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Z.L.; Zou, C.B.; Fan, Y.; Dittert, K.; Lin, S. Legacy effects of historical grazing affect the response of vegetation dynamics to water and nitrogen addition in semi-arid steppe. Appl. Veg. Sci. 2018, 21, 229–239. [Google Scholar] [CrossRef]
- De Long, J.R.; Fry, E.L.; Veen, G.F.; Kardol, P. Why are plant-soil feedbacks so unpredictable, and what to do about it? Funct. Ecol. 2019, 33, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Wu, J.; Clark, C.M.; Naeem, S.; Pan, Q.; Huang, J.; Zhang, L.X.; Han, X. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Change Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Fisher, J.B.; Phillips, R.P. Modeling the carbon cost of plant nitrogen acquisition: Mycorrhizal trade-offs and multipath resistance uptake improve predictions of retranslocation. J. Geophys. Res.-Biogeo. 2014, 119, 1684–1697. [Google Scholar] [CrossRef]
- Barel, J.M.; Kuyper, T.W.; De Boer, W.; Douma, J.C.; De Deyn, G.B. Legacy effects of diversity in space and time driven by winter cover crop biomass and nirogen concentration. J. Appl. Ecol. 2018, 55, 299–310. [Google Scholar] [CrossRef]
- Wang, R.Z.; Ripley, E.A. Effects of grazing on a Leymus chinensis grassland on the Songnen plain of north-eastern China. J. Arid Environ. 1997, 36, 307–318. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, L.H.; Han, X.G.; Ming, D. Do rhizome severing and shoot defoliation affect clonal growth of Leymus chinensis at ramet population level? Acta Oecol. 2004, 26, 255–260. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, S.; Xing, F.; Mu, X.; Bai, Y. Nitrogen addition interacted with salinity-alkalinity to modify plant diversity, microbial plfas and soil coupled elements: A 5-year experiment. Appl. Soil Ecol. 2019, 137, 78–86. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Mo, J.; Gilliam, F.S.; Zhou, G.; Luo, Y.; Zhang, W.; Huang, J. Divergent responses of soil buffering capacity to long-term N deposition in three typical tropical forests with different land-use history. Environ. Sci. Technol. 2015, 49, 4072–4080. [Google Scholar] [CrossRef]
- Jiang, S.C.; He, N.P.; Wu, L.; Zhou, D.W. Vegetation restoration of secondary bare saline-alkali patches in the Songnen plain, China. Appl. Veg. Sci. 2010, 13, 47–55. [Google Scholar] [CrossRef]
- Heinen, R.; Hannula, S.E.; De Long, J.R.; Huberty, M.; Jongen, R.; Kielak, A.; Steinauer, K.; Zhu, F.; Bezemer, T.M. Plant community composition steers grassland vegetation via soil legacy effects. Ecol. Lett. 2020, 23, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Katulanda, P.M.; Walley, F.L.; Janzen, H.H.; Helgason, B.L. Land use legacy regulates microbial community composition in transplanted chernozems. Appl. Soil Ecol. 2018, 129, 13–23. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Xu, Z.; Yan, Q.; Li, X.; Van Nostrand, J.D.; He, Z.; Yao, F.; Han, X.; Zhou, J.; et al. Responses of soil microbial functional genes to global changes are indirectly influenced by aboveground plant biomass variation. Soil Biol. Biochem. 2017, 104, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Shannon-Firestone, S.; Reynolds, H.L.; Phillips, R.P.; Flory, S.L.; Yannarell, A. The role of ammonium oxidizing communities in mediating effects of an invasive plant on soil nitrification. Soil Biol. Biochem. 2015, 90, 266–274. [Google Scholar] [CrossRef]
- Ribbons, R.R.; Levy-Booth, D.J.; Masse, J.; Grayston, S.J.; Mcdonald, M.A.; Vesterdal, L.; Prescott, C.E. Linking microbial communities, functional genes and nitrogen-cycling processes in forest floors under four tree species. Soil Biol. Biochem. 2016, 103, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Almaraz, M.; Wong, M.Y.; Yang, W.H. Looking back to look ahead: A vision for soil denitrification research. Ecology 2020, 101, e02917. [Google Scholar] [CrossRef] [Green Version]
- Hallin, S.; Jones, C.M.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef]
- Hamonts, K.; Clough, T.J.; Stewart, A.; Clinton, P.W.; Richardson, A.E.; Wakelin, S.A.; O’Callaghan, M.; Condron, L.M. Effect of nitrogen and waterlogging on denitrifier gene abundance, community structure and activity in the rhizosphere of wheat. FEMS Microbiol. Ecol. 2013, 83, 568–584. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H. Long-term plant growth legacies overwhelm short-term plant growth effects on soil microbial community structure. Soil Biol. Biochem. 2011, 43, 823–830. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Zhan, X.; Zhang, C.; Wu, D. Technical reports: Plant and environment interactions responses of a dominant temperate grassland plant (Leymus chinensis) to elevated carbon dioxide and nitrogen addition in China. J. Environ. Qual. 2010, 39, 251–259. [Google Scholar] [CrossRef]
- Wyngaard, N.; Franklin, D.H.; Habteselassie, M.Y.; Mundepi, A.; Cabrera, M.L. Legacy effect of fertilization and tillage systems on nitrogen mineralization and microbial communities. Soil Sci. Soc. Am. J. 2016, 80, 1262. [Google Scholar] [CrossRef]
- Brinkman, E.P.; Van der Putten, W.H.; Bakker, E.; Verhoeven, K.J.F. Plant-soil feedback: Experimental approaches, statistical analyses and ecological interpretations. J. Ecol. 2010, 98, 1063–1073. [Google Scholar] [CrossRef]
- Zhang, C.J.; Yang, Z.L.; Shen, J.P.; Sun, Y.F.; Wang, J.T.; Han, H.Y.; Wan, S.Q.; Zhang, L.M.; He, J.Z. Impacts of long-term nitrogen addition, watering and mowing on ammonia oxidizers, denitrifiers and plant communities in a temperate steppe. Appl. Soil Ecol. 2018, 130, 241–250. [Google Scholar] [CrossRef]
- Ye, C.; Chen, C.R.; Du, M.; Liu, W.Z.; Zhang, Q.F. Revegetation affects soil denitrifying communities in a riparian ecotone. Ecol. Eng. 2017, 103, 256–263. [Google Scholar] [CrossRef]
- Niklaus, P.A.; Roux, L.R.; Poly, F.; Buchmann, N.; Scherer-Lorenzen, M.; Weigelt, A.; Barnard, R.L. Plant species diversity affects soil-atmosphere fluxes of methane and nitrous oxide. Oecologia 2016, 181, 919–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coca-Salazar, A.; Richaume, A.; Florio, A.; Carnol, M. Response of ammonia-oxidizing bacteria and archaea abundance and activity to land use changes in agricultural systems of the central andes. Eur. J. Soil Biol. 2021, 102, 103263. [Google Scholar] [CrossRef]
- Regan, K.; Stempfhuber, B.; Schloter, M.; Rasche, F.; Prati, D.; Philippot, L.; Boeddinghaus, R.S.; Kandeler, E.; Marhan, S. Spatial and temporal dynamics of nitrogen fixing, nitrifying and denitrifying microbes in an unfertilized grassland soil. Soil Biol. Biochem. 2017, 109, 214–226. [Google Scholar] [CrossRef]
- Ordonez, J.C.; Bodegom, P.M.V.; Witte, J.P.M.; Wright, I.J.; Reich, P.B.; Aerts, A. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Vet, L.E.M.; Harvey, J.A.; Wäckers, F.L. Linking above and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends. Ecol. Evol. 2001, 16, 547–554. [Google Scholar] [CrossRef]
- Carlson, M.; Forcella, F.; Wortman, S.; Clay, D.; Clay, S.A. Organic fertilizer abrasive grits increase soil available nitrogen, plant height, and biomass. Agr. Geosci. Environ. 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Hortal, S.; Armas, C.; Pugnaire, F.I. Interactions among soil, plants, and microorganisms drive secondary succession in a dry environment. Soil Biol. Biochem. 2014, 78, 298–306. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Cammeraat, E.; Pérez-Cardiel, E.; Lasanta, T. Effects of secondary succession and afforestation practices on soil properties after cropland abandonment in humid Mediterranean mountain areas. Agr. Ecosyst. Environ. 2016, 228, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.A.; Rosenzweig, S.T.; Baer, S.G.; Blair, J.M. Changes in Potential Nitrous Oxide Efflux during Grassland Restoration. J. Environ. Qual. 2019, 48, 1913–1917. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Lin, H.; Zhang, Y.; Searle, E.B.; Yuan, Z. Phosphorus amendment mitigates nitrogen addition-induced phosphorus limitation in two plant species in a desert steppe, China. Plant Soil 2016, 399, 221–232. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in china. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef]
- De Long, J.R.; Semchenko, M.; Pritchard, W.J.; Cordero, I.; Fry, E.L.; Jackson, B.G.; Kurnosova, K.; Ostle, N.J.; Johnson, D.; Baggs, E.M.; et al. Drought soil legacy overrides maternal effects on plant growth. Funct. Ecol. 2019, 33, 1400–1410. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Pilbeam, D.J. The Utilization of Nitrogen by Plants: A Whole Plant Perspective. In Annual Plant Reviews. Nitrogen Metabolism in Plants in the Post-genomic Era; Foyer, C.H., Zhang, H., Eds.; Wiley-Blackwell: Oxford, UK, 2010; Volume 42, pp. 305–351. [Google Scholar]
- Hendriks, M.; Mommer, L.; de Caluwe, H.; Smit-Tiekstra, A.E.; van der Putten, W.H.; de Kroon, H. Independent variations of plant and soil mixtures reveal soil feedback effects on plant community overyielding. J. Ecol. 2013, 101, 287–297. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Quirk, H.; Yi, Z.; Oakley, S.; Ostle, N.J.; Bardgett, R.D. Vegetation composition promotes carbon and nitrogen storage in model grassland communities of contrasting soil fertility. J. Ecol. 2009, 97, 864–875. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, L.; Zhao, W.; Zhao, J.X. Rhododendron aureum Georgi formed a special soil microbial community and competed with above-ground plants on the tundra of the Changbai Mountain, China. Ecol. Evol. 2017, 7, 7503–7514. [Google Scholar] [CrossRef] [PubMed]
- Heinen, R.; Biere, A.; Bezemer, T.M. Plant traits shape soil legacy effects on individual plant-insect interactions. OIKOS 2020, 129, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Khanh, T.D.; Chung, M.I.; Xuan, T.D.; Tawata, S. The exploitation of crop allelopathy in sustainable agricultural production. J. Agron. Crop Sci. 2005, 191, 172–184. [Google Scholar] [CrossRef]
- Liu, X.W.; Li, X.L.; Li, X.T.; Ma, W.J.; Guo, Q.; Zhu, X.R.; Xing, F. Dominant plant identity determines the soil extracellular enzyme activities of entire communities in the semi-arid grassland. Appl. Soil Ecol. 2021, 161, 1–9. [Google Scholar] [CrossRef]
- Su, D.X.; Zhang, Z.H. Parameters for Degradation, Sandification and Salification of Rangelands; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic: Beijing, China, 2004; Available online: https://www.antpedia.com/standard/567725.html (accessed on 24 May 2019).
- Wang, D.L.; Guo, J.X.; Li, J.D. Theory and Technology of the Restoration in Songnen Alkali-Saline Grassland; Science Press: Beijing, China, 2019. [Google Scholar]
- Pellant, M.; Shaver, P.; Pyke, D.A.; Herrick, J.E. Interpreting Indicators of Rangeland Health, version 4; Department of the Interior, Bureau of Land Management, National Science and Technology Center: Denver, CO, USA, 2005. Available online: https://www.blm.gov/nstc/library/techref.htm (accessed on 24 May 2019).
- Wu, Z.Y.; Wang, X.B. The Vegetation of China; Science Press: Beijing, China, 1995. [Google Scholar]
- Aira, M.; G´omez-Brand´on, M.; Lazcano, C.; Bååth, E.; Domínguez, J. Plant genotype strongly modifies the structure and growth of maize rhizosphere microbial communities. Soil Biol. Biochem. 2010, 42, 2276–2281. [Google Scholar] [CrossRef]
- Hart, S.C.; Stark, J.M.; Davidson, E.A.; Firestone, M.K. Nitrogen mineralization, immobilization, and nitrification. Methods Soil Anal. Part 2 Microbiol. Biochem. Prop. 1994, 5, 985–1018. [Google Scholar] [CrossRef] [Green Version]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Schermelleh-Engel, K.; Moosbrugger, H.; Müller, H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. MPR-Online 2003, 8, 23–74. Available online: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.506.1827 (accessed on 26 March 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org (accessed on 10 July 2018).
- Vadakattu, G.; Zhang, B.; Penton, C.R.; Yu, J.; Tiedje, J.M. Diazotroph diversity and nitrogen fixation in summer active perennial grasses in a mediterranean region agricultural soil. Front. Mol. Biosci. 2019, 6, 115. [Google Scholar]
- Nogueira, C.B.; Menéndez, E.; Helena, M.; Bahena, R.; Velázquez, E.; Scotti, M.R. The N-fixing legume periandra mediterranea constrains the invasion of an exotic grass (Melinis minutiflora P. Beauv) by altering soil N cycling. Sci. Rep. 2019, 9, 11033. [Google Scholar] [CrossRef]
- Cuer, C.A.; Rodrigues, R.D.A.R.; Balieiro, F.C.; Jesus, J.; Silva, E.P.; Alves, B.J.R. Short-term effect of Eucalyptus plantations on soil microbial communities and soil-atmosphere methane and nitrous oxide exchange. Sci. Rep. 2018, 8, 15133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Hao, X.; Garcia-Lemos, A.M.; Nunes, I.; Nicolaisen, M.H.; Nybroe, O. Different Effects of Soil Fertilization on Bacterial Community Composition in the Penicillium canescens Hyphosphere and in Bulk Soil. Appl. Environ. Microbiol. 2020, 86, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Plant Traits | SU | S | N | SU × S | SU × N | S × N | SU × S × N | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| total biomass | 3.902 | 0.018 | 360.040 | <0.001 | 7.691 | 0.009 | 2.940 | 0.048 | 2.661 | 0.065 | 0.001 | 0.970 | 1.592 | 0.210 |
| ramets number | 0.801 | 0.502 | 23.043 | <0.001 | 0.064 | 0.802 | 3.241 | 0.035 | 0.461 | 0.711 | 0.574 | 0.454 | 1.161 | 0.340 |
| root/shoot | 2.178 | 0.110 | 9.278 | 0.005 | 0.014 | 0.907 | 1.959 | 0.140 | 2.755 | 0.059 | 0.224 | 0.639 | 4.882 | 0.007 |
| total C/total N | 14.387 | <0.001 | 3.128 | 0.086 | 0.452 | 0.506 | 3.232 | 0.035 | 2.951 | 0.047 | 0.535 | 0.470 | 1.404 | 0.260 |
| total C/total P | 26.907 | <0.001 | 3.691 | 0.064 | 0.802 | 0.377 | 4.776 | 0.007 | 1.457 | 0.245 | 0.550 | 0.464 | 4.727 | 0.008 |
| total N/total P | 15.960 | <0.001 | 15.303 | <0.001 | 2.940 | 0.096 | 5.482 | 0.004 | 0.307 | 0.820 | 0.148 | 0.703 | 12.648 | <0.001 |
| NCFG Abundances | SU | S | N | SU × S | SU × N | S × N | SU × S × N | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| nifH | 30.853 | <0.001 | 463.562 | <0.001 | 0.083 | 0.775 | 4.864 | 0.007 | 1.697 | 0.187 | 0.935 | 0.341 | 0.292 | 0.831 |
| AOB amoA | 30.525 | <0.001 | 440.357 | <0.001 | 3.752 | 0.062 | 12.933 | <0.001 | 1.325 | 0.283 | 4.397 | 0.044 | 0.846 | 0.479 |
| nirK | 329.179 | <0.001 | 20.557 | <0.001 | 1.091 | 0.304 | 20.197 | <0.001 | 0.183 | 0.907 | 1.245 | 0.273 | 1.802 | 0.167 |
| nirS | 48.667 | <0.001 | 58.224 | <0.001 | 11.266 | 0.002 | 30.589 | <0.001 | 11.870 | <0.001 | 7.535 | 0.010 | 12.241 | <0.001 |
| Successional Stages | Community Type | Main Plant Species | Aboveground Biomass (g/m2) | Density (ramet/m2) | Height (m) | Coverage (%) |
|---|---|---|---|---|---|---|
| EAR | Chloris virgata + Kochia sieversiana | C. virgata | 18.72 ± 11.67 | 192.50 ± 140.44 | 0.17 ± 0.05 | 34.50 ± 24.31 |
| K. sieversiana | 82.61 ± 12.09 | 528.00 ± 338.83 | 0.23 ± 0.07 | 63.75 ± 22.87 | ||
| Artemisia scoparia | 15.21 ± 7.55 | 72.50 ± 37.38 | 0.26 ± 0.07 | 15.00 ± 8.29 | ||
| MID | Leymus chinensis + forbs | L. chinensis | 148.69 ± 15.41 | 285.00 ± 46.35 | 0.35 ± 0.09 | 55.00 ± 14.72 |
| Calamagrostis macrolepis | 3.45 ± 2.78 | 4.25 ± 2.30 | 0.34 ± 0.10 | 8.00 ± 5.12 | ||
| Lespedeza daurica | 7.99 ± 6.08 | 10.50 ± 8.70 | 0.20 ± 0.08 | 3.75 ± 1.99 | ||
| K. sieversiana | 2.07 ± 1.01 | 1.50 ± 0.91 | 0.28 ± 0.10 | 1.38 ± 0.43 | ||
| A. scoparia | 1.31 ± 0.45 | 4.50 ± 2.65 | 0.26 ± 0.10 | 1.50 ± 1.00 | ||
| Allium mongolicum | 1.33 ± 0.53 | 2.50 ± 1.65 | 0.30 ± 0.06 | 1.38 ± 0.80 | ||
| LAT | L. chinensis + L. daurica | L. chinensis | 227.22 ± 36.47 | 384.00 ± 36.37 | 0.49 ± 0.09 | 48.75 ± 14.45 |
| C. macrolepis | 33.94 ± 41.58 | 62.00 ± 75.15 | 0.56 ± 0.09 | 8.75 ± 14.36 | ||
| L. daurica | 70.27 ± 20.43 | 76.50 ± 38.99 | 0.49 ± 0.13 | 31.25 ± 16.52 | ||
| STA | L. chinensis | L. chinensis | 234.64 ± 35.61 | 431.00 ± 164.90 | 0.47 ± 0.09 | 85.00 ± 8.16 |
| Carex duriuscula | 24.89 ± 12.96 | 612.00 ± 272.82 | 0.17 ± 0.05 | 30.00 ± 7.07 |
| Successional Stages | EAR | MID | LAT | STA |
|---|---|---|---|---|
| Soil pH | 9.71 ± 0.11 | 8.77 ± 0.43 | 8.13 ± 0.29 | 8.46 ± 0.13 |
| Soil moisture (%) | 10.04 ± 0.00 | 8.86 ± 0.01 | 8.58 ± 0.01 | 11.37 ± 0.01 |
| Soil total C (mg/kg) | 7182.50 ± 1065.00 | 117,25.00 ± 907.06 | 12,130.00 ± 2150.66 | 12,480.00 ± 2170.44 |
| Soil total N (mg/kg) | 175.95 ± 12.69 | 222.70 ± 30.59 | 278.75 ± 17.33 | 235.15 ± 15.29 |
| Soil total P (mg/kg) | 212.80 ± 22.99 | 251.75 ± 18.13 | 238.30 ± 20.77 | 270.30 ± 13.10 |
| Soil available N (mg/kg) | 14.29 ± 1.93 | 15.85 ± 2.59 | 13.60 ± 2.27 | 12.10 ± 0.56 |
| Soil available P (mg/kg) | 1.38 ± 0.23 | 2.32 ± 0.20 | 1.22 ± 0.18 | 1.50 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, X.; Xing, F.; Li, Z.; Liu, X.; Li, Y. Soil Microbial Legacy Overrides the Responses of a Dominant Grass and Nitrogen-Cycling Functional Microbes in Grassland Soil to Nitrogen Addition. Plants 2022, 11, 1305. https://doi.org/10.3390/plants11101305
Zhang M, Li X, Xing F, Li Z, Liu X, Li Y. Soil Microbial Legacy Overrides the Responses of a Dominant Grass and Nitrogen-Cycling Functional Microbes in Grassland Soil to Nitrogen Addition. Plants. 2022; 11(10):1305. https://doi.org/10.3390/plants11101305
Chicago/Turabian StyleZhang, Minghui, Xueli Li, Fu Xing, Zhuo Li, Xiaowei Liu, and Yanan Li. 2022. "Soil Microbial Legacy Overrides the Responses of a Dominant Grass and Nitrogen-Cycling Functional Microbes in Grassland Soil to Nitrogen Addition" Plants 11, no. 10: 1305. https://doi.org/10.3390/plants11101305





