Cold Stress, Freezing Adaptation, Varietal Susceptibility of Olea europaea L.: A Review
Abstract
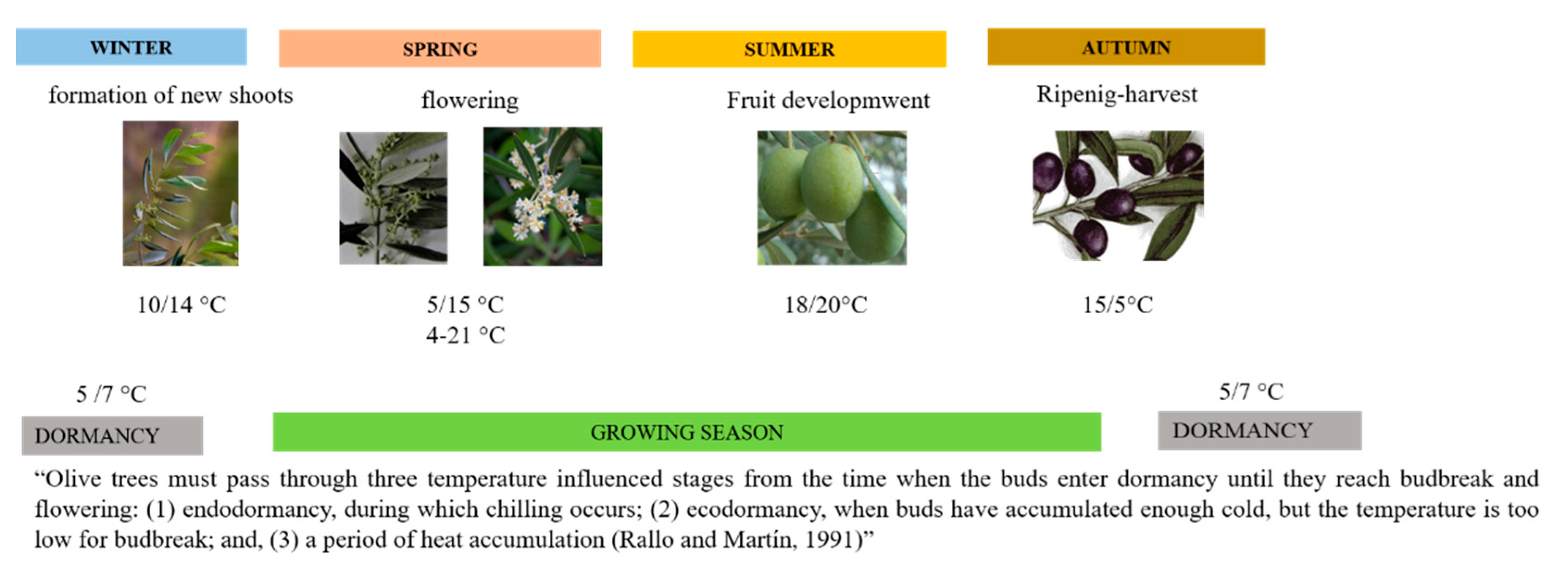
:1. Introduction
2. Low Temperature Stress in Olive Tree
2.1. Chilling Stress
2.2. Freezing Stress
2.3. Morphological and Anatomical Consequences of Frost Damage
2.4. Physiological and Biochemical Consequences of Cold Stress
3. Responses, Adaptions and Recovery at Low Temperature
3.1. Biochemical Responses
3.2. Antioxidant Responses
3.3. Molecular Responses
4. Evaluation of Freezing Tolerance in Olive Tree
5. Agronomic Management for Frost Protection
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breton, C.M.; Warnock, P.; Berville, A.J. Origin and History of the Olive. In Olive Germplasm-The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Diez, C.M.; Trujillo, I.; Martinez-Urdiroz, N.; Barranco, D.; Rallo, L.; Marfil, P.; Gaut, B.S. 2015 Olive domestication and diversification in the Mediterranean Basin. New Phytol. 2015, 206, 436–447. [Google Scholar] [CrossRef]
- Besnard, G.; Terral, J.F.; Cornille, A. On the origins and domestication of the olive: A review and perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef] [Green Version]
- Bartolini, G.; Prevost, G.; Messeri, C.; Carignani, C. Olive Germplasm: Cultivars and World-Wide Collections; FAO/Plant Production and Protection: Rome, Italy, 2005; Available online: www.oleadb.it (accessed on 24 February 2022).
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.) A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- FAOSTAT Food and Agriculture Organization of the United Nations Statistical Dataset Food and Agriculture Data 2020. Available online: https://www.fao.org›faostat (accessed on 24 February 2022).
- International Olive Council Changes in Olive Oil and Table Olive Production. Newsletter 2019, 144, 18–28. Available online: https://www.internationaloliveoil.org (accessed on 24 February 2022).
- He, S.A.; Liu, L.; Fu, Y. Olive Introduction and Breeding in China. I. The Theory and Practice of Olive Introduction in China. Riv. Ortoflorofruttic. Ital. 1981, 65, 413–432. Available online: http://www.jstor.org/stable/42878471 (accessed on 12 December 2021).
- Bartolucci, P.; Raj Dhakal, B.; Kumar Shrestha, D. Prospects for Olive Growing in Nepal; Project, TCP/NEP/6713; Food and Agriculture Organization-FAO: Kathmandu, Nepal, 1999; pp. 1–57. Available online: https://www.fao.org/3/af106e/af106e.pdf (accessed on 24 February 2022).
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Conteras, C.; Maestri, D. Olive Cultivation in the Southern Hemisphere: Flowering, Water Requirements and Oil Quality Responses to New Crop Environments. Front. Plant Sci. 2017, 8, 1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.W.; Ma, L.Y.; Gómez-del-Campo, M.; Zhang, D.; Deng, Y.; Jia, Z. Youth tree behavior of olive (Olea europaea L.) cultivars in Wudu, China: Cold and drought resistance, growth, fruit production, and oil quality. Sci. Hortic. 2018, 236, 106–122. [Google Scholar] [CrossRef]
- Fiorino, P. L’evoluzione globale dell’olivicoltura. In Atti Giornata di Studio L’olivo e il suo olio, Pontificia Academia Scientiarum, Città del Vaticano16 Dicembre 2008; Accademia dei Georgofili: Firenze, Italy, 2009; pp. 23–51. Available online: https://www.georgofili.net (accessed on 24 February 2022).
- Morettini, A. Avversità dell’olivo. In Olivicoltura, 2nd ed.; REDA: Roma, Italy, 1972; Volume 23, pp. 453–5407. ISBN 738781. [Google Scholar]
- Fabbri, A. The olive in Northern Italy. A Mediterranean tale. Riv. Stor. Dell’agricoltura 2017, LVII, 1–32. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Gonçalves, J.F.; Moreno, M.; Villar, R. Projected climate changes are expected to decrease the suitability and production of olive varieties in southern Spain. Sci. Total Environ. 2020, 709, 136161. [Google Scholar] [CrossRef] [PubMed]
- Graniti, A.; Faedda, R.; Cacciola, S.O.; San Lio, M. Olive diseases in a changing ecosystem. In Olive Diseases and Disorders; Schena, L., Agosteo, G.E., Cacciola, S.O., Eds.; Transworld Research Network: Kerala, India, 2011; pp. 1–31. ISBN 978-81-7895-539-1. [Google Scholar]
- Gencer, D.C.; Okatan, V.; Korkmaz, N. 2019 Olive growing and importance of plant nutrition in olive cultivars. TURJAF 2019, 1, 34–38. Available online: https://dergipark.org.tr/en/pub/turjfas/issue/51298/649088 (accessed on 12 November 2021).
- Ozturk, M.; Altay, V.; Gönenç, T.M.; Unal, B.T.; Efe, R.; Akçiçek, E.; Bukhari, A. An Overview of Olive Cultivation in Turkey: Botanical Features, Eco-Physiology and Phytochemical Aspects. Agronomy 2021, 11, 295. Available online: https://doi.org/10.3390/agronomy11020295 (accessed on 10 December 2021). [CrossRef]
- Therios, I.N. Climatic and soil conditions. In Olives; Crop Production Science in Horticulture; CABI: Oxfordshire, UK, 2009; pp. 51–81. [Google Scholar] [CrossRef]
- Conde-Innamorato, P.; Arias-Sibillotte, M.; Villamil, J.J.; Bruzzone, J.; Bernaschina, Y.; Ferrari, V.; Zoppolo, R.; Villamil, J.; Leoni, C. It is Feasible to Produce Olive Oil in Temperate Humid Climate Regions. Front. Plant Sci. 2019, 27, 1544. [Google Scholar] [CrossRef]
- Hartmann, H.T. Effect of winter chilling on fruitfulness and vegetative growth in the olive. Proc. Amer. Soc. Hortic. Sci. 1953, 62, 184–190. [Google Scholar]
- Rallo, L.; Martin, G.C. The role of chilling in releasing olive floral buds from dormancy. Hortic. Sci. 1991, 26, 1058–1062. [Google Scholar] [CrossRef] [Green Version]
- Lavee, S. Biennial bearing in olive (Olea europaea L). Olea 2006, 25, 5–13. [Google Scholar]
- Marone, E.; Fiorino, P. Oleiculture in progress. Adv. Hortic. Sci. 2012, 26, 163–175. [Google Scholar] [CrossRef]
- Rojo, J.; Orlandi, F.; Ben Dhiab, A.; Lara, B.; Picornell, A.; Oteros, J.; Msallem, M.; Fornaciari, M.; Pérez-Badia, R. Estimation of Chilling and Heat Accumulation Periods Based on the Timing of Olive Pollination. Forests 2020, 11, 835. [Google Scholar] [CrossRef]
- Osborne, C.P.; Chuine, I.; Viner, D.; Woodward, F.I. Olive phenology as a sensitive indicator of future climatic warming in the Mediterranean. Plant Cell Environ. 2000, 23, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, D.; Ribas, F.; Moriana, A.; Rapoport, H.F.; Juan, A.D. Influence of temperatureon the growth and development of olive (Olea europaea L.) trees. J. Hortic. Sci. Biotechnol. 2008, 83, 171–176. [Google Scholar] [CrossRef]
- Orlandi, F.; Ruga, L.; Romano, B.; Fornaciari, M. Olive flowering as an indicator of local climatic changes. Theor. Appl. Climatol. 2005, 81, 169–176. [Google Scholar] [CrossRef]
- Connor, D.; Fereres, E. The physiology of adaptation and yield expression in olive. Hortic. Rev. 2005, 31, 155–229. [Google Scholar]
- Garrido, A.; Fernández-González, M.; Álvarez-López, S.; González-Fernández, E.; Rodríguez-Rajo, F.J. First phenological and aerobiological assessment of olive orchards at the Northern limit of the Mediterranean bioclimatic area. Aerobiologia 2020, 36, 641–656. [Google Scholar] [CrossRef]
- Fiorino, P.; Mancuso, S. Differential thermal analysis, supercooling and cell viability in organs of Olea europaea at subzero temperatures. Adv. Hortic. Sci. 2000, 14, 23–27. Available online: https://www.researchgate.net/publication/267416904-539 (accessed on 12 January 2022).
- Agosteo, G.E. Olive disease, a historical account. In Olive Diseases and Disorders; Schena, L., Agosteo, G.E., Cacciola, S.O., Eds.; Transworld Research Newtwork: Kerala, India, 2011; pp. 403–433. ISBN 978-81-7895. [Google Scholar]
- Sanzani, S.M.; Schena, L.; Nigro, F.; Sergeeva, V.; Ippolito, A.; Salerno, M.G. Abiotic diseases of olive. J. Plant Pathol. 2012, 294, 469–491. [Google Scholar]
- Lodolini, E.M.; Alfei, B.; Santinelli, A.; Cioccolanti, T.; Polverigiani, S.; Neri, D. Frost tolerance of 24 olive cultivars and subsequent vegetativere-sprouting as indication of recovery ability. Sci. Hortic. 2016, 211, 152–157. [Google Scholar] [CrossRef]
- Paradiso, N. Attenzione ai danni da gelo su olivo in Puglia. L’Informatore Agrar. 2018, 16, 74–75. [Google Scholar]
- IPCC Climate Change 2014. In Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team; Pachauri, R.K.; Meyer, L.A. (Eds.) IPCC: Geneva, Switzerland, 2016; p. 151. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 12 January 2022).
- Wen, Y.; Qin, D.-W.; Leng, B.; Zhu, Y.-F.; Cao, K.-F. The physiological cold tolerance of warm-climate plants is correlated with their latitudinal range limit. Biol. Lett. 2018, 14, 20180277. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2021, 11, 56. [Google Scholar] [CrossRef]
- Mancuso, S. Electrical resistance changes during exposure to low temperature measure chilling and freezing tolerance in olive tree (Olea europaea L.) plants. Plant Cell Environ. 2000, 23, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Mougiou, N.; Baalbaki, B.; Doupis, G.; Kavroulakis, N.; Poulios, S.; Vlachonasios, K.E.; Koubouris, G.C. The Effect of Low Temperature on Physiological, Biochemical and Flowering Functions of Olive Tree in Relation to Genotype. Sustainability 2020, 12, 10065. [Google Scholar] [CrossRef]
- Larcher, W. Low temperature effects on Mediterranean sclerophylls: An unconventional viewpoint. In Components of Productivity of Mediterranean-Climate Regions–Basic and Applied Aspects; Margaris, N., Mooney, H., Eds.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1981; pp. 259–266. [Google Scholar]
- iScaramuzzi, F.; Pisani, P.L.; Fiorino, P. I danni da freddo all’olivicoltura Toscana nel 1985. In Ricostruzione Degli Olivi Danneggiati dal Freddo; Accademia dei Georgofili: Firenze, Italy, 1989; Volume 2, pp. 43–47. [Google Scholar]
- Palliotti, A.; Bongi, G. Freezing injury in the olive leaf and effects of mefluidide treatment. J. Hortic. Sci. 1996, 71, 57–63. [Google Scholar] [CrossRef]
- Barranco, D.; Ruiz, N.; Gomez-del Campo, M. Frost tolerance of eight olive cultivars. HortScience 2005, 40, 558–560. [Google Scholar] [CrossRef] [Green Version]
- Rahemi, M.; Yazdani, F.; Sedaghat, S. Evaluation of freezing tolerance in olive cultivars by stomatal density and freezing stress. Int. J. Hortic. Sci. Technol. 2016, 3, 145–153. [Google Scholar] [CrossRef]
- Bini, G.; Corvi, A.; Fabbri, A. Osservazioni sui danni causati dalla gelata del 1985 agli olivi dei vivai del pesciatino. L’informatore Agrario 1987, 9, 211–217. [Google Scholar]
- Levitt, J. Chilling, Freezing, and High Temperature Stresses. In Responses of Plants to Environmental Stresses, 2nd ed.; Academic Press: New York, NY, USA, 1980; Volume 1, pp. 23–283. ISBN 9780124455023. [Google Scholar]
- Lyons, J.M. Chilling injury in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Lara, I.; Drincovich, M.F.; Beckles, D.M.; Shifeng, C. Physiological, Molecular and Genetic Perspectives of Chilling Tolerance in Horticultural Crop. Plant Sci. 2020, 11, 1916. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.; Qin, Y. Plant Low-Temperature Stress: Signaling and Response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef]
- Hackett, W.P.; Hartmann, H.T. The influence of temperature on floral initiation in the olive. Physiol. Plant. 1967, 20, 430–436. [Google Scholar] [CrossRef]
- Malik, N.S.A.; Bradford, J.M. Inhibition of flowering in “Arbequina” olives from chilling at lower temperatures. J. Food Agric. Environ. 2009, 7, 429–431. [Google Scholar]
- Ippolito, A.; Sanzani, S. Non-parasitic diseases of olive. In Olive Diseases and Disorders; Schena, L., Agosteo, G.E., Cacciola, S.O., Eds.; Transworld Research Network: Kerala, India, 2011; pp. 43–70. ISBN 978-81-7895-539-1. [Google Scholar]
- Bongi, G.; Long, S.P. Light-dependent damage to photosynthesis in olive leaves during chilling and high temperature stress. Plant Cell Environ. 1987, 10, 241–249. [Google Scholar]
- Gucci, R.; Caruso, G. Environmental stresses and sustainable olive growing. Acta Hortic. 2011, 924, 19–30. [Google Scholar] [CrossRef]
- Centeno, A.; Memmi, H.; Moreno, M.M.; Moreno, C.; Pérez-López, D. Water relations in olive trees under cold conditions. Sci. Hortic. 2018, 235, 1–8. [Google Scholar] [CrossRef]
- Pavel, E.W.; Fereres, E. Low soil temperatures induce water deficits in olive (Olea europaea) trees. Physiol. Plant. 1998, 104, 525–532. [Google Scholar] [CrossRef]
- Pérez-López, D.; Gijón, M.C.; Mariño, J.; Moriana, A. Water relation response to soil chilling of six olive (Olea europaea L.) cultivars with different frost resistance. Span. J. Agric. Res. 2010, 8, 780–789. [Google Scholar] [CrossRef] [Green Version]
- López-Bernal, A.; García-Tejera, O.; Testi, L.; Orgaz, F. Low winter temperatures induce a disturbance of water relations in field olivet rees. Trees 2015, 29, 1247–1257. [Google Scholar] [CrossRef]
- Rejšková, A.; Patková, L.; Stodůlková, E.; Lipavska, H. The effect of abiotic stresses on carbohydrate status of olive shoots (Olea europaea L.) under in vitro conditions. J. Plant Physiol. 2007, 164, 174–184. [Google Scholar] [CrossRef]
- Pearce, R.S. Plant Freezing and Damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Wisniewski, M.; Nassuth, A.; Arora, R. Cold hardiness in trees: A mini-review. Front. Plant Sci. 2018, 9, 1394. [Google Scholar] [CrossRef]
- Ashworth, E.N.; Davis, G.A.; Anderson, J.A. Factors Affecting Ice Nucleation in Plant Tissues. Plant Physiol. 1985, 79, 1033–1037. Available online: http://www.jstor.org/stable/4269656 (accessed on 12 December 2021). [CrossRef] [Green Version]
- Wisniewski, M.; Fuller, M.; Palta, J.; Carter, J.; Arora, R. Ice Nucleation, Propagation, and Deep Supercooling in Woody Plants. J. Crop Improv. 2014, 10, 5–16. [Google Scholar] [CrossRef]
- Surico, G.; Lavermicocca, P. Presenza di nuclei di cristallizzazione del ghiaccio du piante di olivo. Phytopathol. Mediterr. 1988, 27, 84–89. Available online: wwwjstororg/stable/42684902 (accessed on 12 December 2021).
- Arias, N.S.; Bucci, S.J.; Fabián, G.; Goldstein, G. Freezing avoidance by supercooling in Olea europaea cultivars: The role of apoplastic water, solute content and cell wall rigidity. Plant Cell Environ. 2015, 38, 2061–2070. [Google Scholar] [CrossRef]
- Arias, N.S.; Fabián, G.; Scholz, F.G.; Goldstein, G.; Bucci, S.J. The cost of avoiding freezing in stems: Trade-off between xylem resistance to cavitation and supercooling capacity in woody plants. Tree Physiol. 2017, 37, 1251–1262. [Google Scholar] [CrossRef]
- Arias, N.S.; Gustavo, F.; Guillermo Goldstein, G.; Bucci, S. Low temperature acclimation and legacy effects of summer water deficits in olive freezing resistance. Tree Physiol. 2021, 41, 1836–1847. [Google Scholar] [CrossRef]
- Larcher, W. Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst. 2000, 134, 279–295. [Google Scholar] [CrossRef]
- Rapoport, H.F. The reproductive biology of the olive tree and its relationship to extreme environmental conditions. Acta Hortic. 2014, 1057, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Morettini, A. Sulla ricostruzione degli olivi daneggiati dalle basse temperature del 1956. In Ricostruzione Degli Olivi Danneggiati dal Freddo; Accademia dei Georgofili: Firenze, Italy, 1989; Volume 2, pp. 1–42. [Google Scholar]
- Antognozzi, E.; Famiani, F.; Proietti, P.; Pannelli, G.; Alfei, B. Frost resistance of some olive cultivars during the winter. Acta Hortic. 1994, 356, 152–155. [Google Scholar] [CrossRef]
- Sergeeva, V. Frost and chilling injuries in olive. Aust. N. Z. Olive Grow. Processor 2010, 74, 23–34. [Google Scholar]
- Zelasco, S.; Foianini, I.; Enrietta, S.; Biancat, A.; Ganino, T.; Bazzini, S.; Salimonti, A.; Murada, G.; Conforti, F.L.; Cantini, C. Genetic and agronomic selection of olive germplasm after Burian effect. In Proceedings of the 6th International Conference on Olive Tree and Olive Products, Olivebioteq, Sevilla, Spain, 15–19 October 2018. [Google Scholar]
- Scaramuzzi, F.; Andreucci, E. Indagini e osservazioni sui danni provocati dalle nime termiche del febbraio 1956 agli olivi nei vivai di Pescia. Plant Biosyst. 1957, 64, 19–124. [Google Scholar]
- Denney, J.O.; Ketchie, D.O.; Osgood, J.W.; Martin, G.C.; Connell, J.H.; Sibbett, G.S.; Nour, G.A. Freeze damage and cold hardiness in olive: Findings from the 1990 freeze. Lessons from a record-breaking freeze: Some olives show damage; many, cold hardiness. Calif. Agric. 1993, 47, 3–12. [Google Scholar]
- Ruiz, N.; Barranco, D.; Rapoport, H.F. Anatomical response of olive (Olea europaea L.) to freezing temperatures. J. Hortic. Sci. Biotechnol. 2006, 81, 783–790. [Google Scholar] [CrossRef]
- Keramatlou, I.; Navabpour, S.; Zainilnejad, K.; Tavakol, E.; Mazinani, M.H. Morphological Evaluation and Selection of Tolerant Trees to Freezing Stress at The Olive Orchards in Golestan Province. J. Hortic. Sci. 2019, 33, 287–299. [Google Scholar] [CrossRef]
- Bongi, G. Freezing avoidance in olive tree (Olea europaea L.): From proxies to targets of action. Adv. Hortic. Sci. 2002, 16, 117–124. [Google Scholar]
- Sergeeva, V.; Spooner-Hart, R. Diseases and disorders associated with environmental stress in sustainable olive orchards in Australia. Acta Hortic. 2011, 924, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Morelló, J.-R.; Motilva, M.-J.; Ramo, T.; Romero, M.-P. Effect of freeze injuries in olive fruit on virgin olive oil composition. Food Chem. 2003, 81, 547–553. [Google Scholar] [CrossRef]
- Di Vaio, C.; Nocerino, S.; Paduano, A.; Sacchi, R. Influence of some environmental factors on drupe maturation and olive oil composition. J. Sci. Food Agric. 2013, 93, 1134–1139. [Google Scholar] [CrossRef]
- Tombesi, A. Modalità di ristrutturazione degli olivi danneggiati dal gelo. Riv. Fruttic. 1986, 8, 27–35. [Google Scholar]
- Fabbri, A.; Ferrini, F. Osservazioni anatomiche sui danni da freddo invernali in rami di piante adulte di olivo. In Proceedings of the Agrometeorologia; Agricoltura e Ambiente, Firenze, Italy, 21–22 November 1990; Serie VII–XXXVII; Accademia dei Georgofili: Firenze, Italy, 1990; p. 443. [Google Scholar]
- Jacoboni, N.; Bartoletti, F. Tipologia del danno ed interventi riparatori. In Aspetti Tecnici ed Economici dell’Olivicoltura Viterbese; ENEA: Roma, Italy, 1985; pp. 161–191. [Google Scholar]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef]
- Adam, S.; Murthy, S.D.S. Effect of cold stress on photosynthesis of plants and possible protection mechanisms. In Approaches to Plant Stress and their Management; Gaur, R.K., Sharma, P., Eds.; Springer: New Delhi, India, 2014; pp. 207–218. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Chapter 2: Cold stress and photosynthesis. In Photosynthesis, Productivity and Environmental Stress; Ahmad, P., Ahanger, M.A., Alyemeni, M.N., Alam, P., Eds.; John Wiley & Sons, Ltd.: Chennai, India, 2020; pp. 27–37. [Google Scholar]
- Afshar, M.M.; Rezaei, S.; Malekroudi, R.M. The Impact of cold stress on two olive cultivars. J. Plant Process Funct. 2012, 1, 1–11. Available online: https://www.sid.ir/en/journal/ViewPaper.aspx?id=305233 (accessed on 11 November 2021). (Article in Persian with an Abstract in English).
- Leonardo, B.; Picardi, E.; Pacenza, M.; Chiappetta, A.; Muto, A.; Gagliardi, O.; Muzzalupo, I.; Pesole, G.; Bitonti, M.B. Changes in gene expression and metabolic profile of drupes of Olea europaea L. cv Carolea in relation to maturation stage and cultivation area. BMC Plant Biol. 2019, 19, 428. [Google Scholar] [CrossRef]
- Simkeshzadeh, N.; Mobli, M.; Etemadi, N.; Baninasab, B. Assessment of the Frost Resistance in Some Olive Cultivars Using Visual Injuries and Chlorophyll Fluorescence. J. Hortic. Sci. 2011, 2, 163–169, (Article in Persian with an Abstract in English). [Google Scholar]
- Saadati, S.; Baninasab, B.; Mobli, M.; Gholami, M. Measurements of freezing tolerance and their relationship with some biochemical and physiological parameters in seven olive cultivars. Acta Physiol. Plant. 2019, 41, 51. [Google Scholar] [CrossRef]
- Guerra, D.; Lamontanara, A.; Bagnaresi, P.; Orrù, L.; Rizza, F.; Zelasco, S.; Beghè, D.; Ganino, T.; Pagani, D.; Cattivelli, L.; et al. Transcriptome changes associated with cold acclimation in leaves of olive tree (Olea europaea L.). Tree Genet. Genomes 2015, 11, 2–24. [Google Scholar] [CrossRef]
- Gubanova, T.; Paliy, A.E. Physiological and Biochemical Aspects of Frost Resistance in Olea europaea L. Russ. J. Plant Physiol. 2020, 67, 671–679. [Google Scholar] [CrossRef]
- Bartolozzi, F.; Fontanazza, G. Assessment of frost tolerance in olive (Olea europaea L.). Sci. Hortic. 1999, 8, 309–319. [Google Scholar] [CrossRef]
- Gulen, H.; Cansev, A.; Eris, A. Cold hardiness of olive (Olea Europaea L.) cultivars in cold-acclimated and non-acclimated stages: Seasonal alteration of soluble sugars and phospholipids. J. Agric. Sci. 2009, 147, 459–467. [Google Scholar] [CrossRef]
- Rezaei, S.; Afshar Mohammadian, M.; Ramezani Malekroudi, M. The impact of cold stress on two olive cultivars. J. Plant Proc. Func. 2013, 1, 1–11. [Google Scholar]
- Pfeiffer, T.Ž.; Štolfa, I.; Žanić, M.; Pavičić, N.; Cesar, V.; Lepeduš, H. Oxidative stress in leaves of two olive cultivars under freezing conditions. Acta Biol. Hung. 2013, 64, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, K.; He, T.; Li, F.; Zhang, D.; Liu, J. The Low Temperature Induced Physiological Responses of Avena nuda L., a Cold-Tolerant Plant Species. Sci. World J. 2013, 7, 2013. [Google Scholar] [CrossRef] [Green Version]
- Awasth, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Hashempour, A.; Ghasemnezhad, M.; Fotouhi Ghazvini, R.; Sohani, M.M. Olive (Olea europaea L.) freezing tolerance related to antioxidant enzymes activity during cold acclimation and non acclimation. Acta Physiol. Plant 2014, 36, 3231–3241. [Google Scholar] [CrossRef]
- Paliy, A.E.; Paliy, I.N. Some biochemical features of Olea europaea L. frost resistance. Acta Hortic. 2021, 1324, 395–400. [Google Scholar] [CrossRef]
- Nayyar, H.; Chander, S. Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J. Agron. Crop Sci. 2004, 190, 355–365. [Google Scholar] [CrossRef]
- Solecka, D. Role of phenylpropanoid compounds in plant responses to different stress factors. Acta Physiol. Plant 1997, 19, 257–268. [Google Scholar] [CrossRef]
- Ortega-García, F.; Peragón, J. The response of phenylalanine ammonia-lyase, flavonoid oxidase and phenols to cold stress in the olive tree (Olea europaea L. Cv. Picual). J. Sci. Food Agric. 2009, 89, 1565–1573. [Google Scholar] [CrossRef]
- Francini, A.; Giro, A.; Ferrante, A. Biochemical and Molecular Regulation of Phenylpropanoids Pathway Under Abiotic Stresses. In Plant Signaling Molecules: Role and Regulation under Stressful Environments; Khan, M., Iqbal, R., Reddy, P.S., Ferrante, A., Khan, N., Eds.; Woodhead Publishing: Chennai, India, 2019; Volume 11, pp. 183–192. ISBN 978-0-12-816452-5. [Google Scholar]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.; Rozhon, W.; Poppenberger, B. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA 2016, 113, E5982–E5991. [Google Scholar] [CrossRef] [Green Version]
- Steponkus, P.L. Role of the Plasma Membrane in Freezing Injury and Cold Acclimation. Annu. Rev. Plant. Physiol. 1984, 38, 543–584. [Google Scholar] [CrossRef]
- Arora, R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018, 270, 301–312. [Google Scholar] [CrossRef]
- Takahashi, D.; Willick, I.R.; Kasuga, J.; Livingston, D.P., III. Responses of the Plant Cell Wall to Sub-Zero Temperatures: A Brief Update. Plant Cell Physiol. 2021, 62, 1858–1866. [Google Scholar] [CrossRef]
- Palta, J.P. Stress interactions at the cellular and membrane levels. HortScience 1990, 25, 1377–1381. [Google Scholar] [CrossRef]
- D’Angeli, S.; Malhó, R.; Altamura, M.M. Low-temperature sensing in olive tree: Calcium signalling and cold acclimation. Plant Sci. 2003, 165, 1303–1313. [Google Scholar] [CrossRef]
- D’Angeli, S.; Altamura, M.M. Osmotin induces cold protection in olive trees by affecting programmed cell death and cytoskeleton organization. Planta 2007, 225, 1147–1163. [Google Scholar] [CrossRef]
- Matteucci, M.; D’Angeli, S.; Errico, S.; Lamanna, R.; Perrotta, G.; Altamura, M.M. Cold affects the transcription of fatty acid desaturases and oil quality in the fruit of Olea europaea L. genotypes with different cold hardiness. J. Exp. Bot. 2011, 62, 3403–3420. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, J.; Roychoudhury, A. Chapter 1: Cold-Induced Injuries and Signaling Responses in Plants. In Cold Tolerance in Plants; Wani, S., Herath, V., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–35. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Al-Issawi, M.; Fuller, M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 2017, 12, 143–157. [Google Scholar] [CrossRef]
- Gusta, L.V.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2013, 147, 4–14. [Google Scholar] [CrossRef]
- López-Bernal, Á.; García-Tejera, O.; Testi, L.; Orgaz, F.; Villalobos, F.J. Studying and modelling winter dormancy in olive trees. Agric. For. Meteorol. 2020, 280, 107776. [Google Scholar] [CrossRef]
- Gucci, R.; Mancuso, S.; Sebastiani, L. Resistenza agli stress ambientali. In Olea—Trattato di Olivicoltura; Fiorino, P., Ed.; Edagricole Srl: Bologna, Italy, 2003; pp. 91–111. [Google Scholar]
- Sebastiani, L.; Gucci, R.; Kerem, Z.; Fernández, J.E. Physiological Responses to Abiotic Stresses. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; Volume 978, pp. 99–122. [Google Scholar]
- Bongi, G.; Paliotti, A. Olive. In Handbook of Environmental Physiology of Fruit Crops: Temperate Crops; Schaffer, B., Anderson, P.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994; Volume I, pp. 165–187. [Google Scholar]
- Fiorino, P.; Mancuso, S. Olivo e basse temperature: Danni da freddo, adattamento e resistenza. L’Informatore Agrar. 2000, 22, 55–59. [Google Scholar]
- Roselli, G.; La Porta, N.; Morelli, D. Valutazione del germoplasma di olivo per la tolleranza a stress da freddo. In Atti Convegno Germoplasma Frutticolo; Springer: Alghero, Italy, 1992; pp. 107–112. [Google Scholar]
- Bartolozzi, F.; Rocchi, P.; Camerini, F.; Fontanazza, G. Paramtri biochimici in foglie di olvo (Olea europaea L.) e loro potenziale relazione con la resistenza al cogelamento. Olivo Olio 1999, 6, 18–26. [Google Scholar]
- Bartolozzi, F.; Cerquaglia, F.; Coppari, L.; Fontanazza, G. Frost tolerance induced by cold acclimation in olive (Olea europaea L.). Acta Hortic. 2002, 586, 473–477. [Google Scholar] [CrossRef]
- Soleimani, A.; Lessani, H.; Tabaeiaghdaei, M. A suevey of cold Hardiness in some olive (Olea europaea L.) cultivar. Acta Hortic. 2008, 791, 519–522. [Google Scholar] [CrossRef]
- Eris, A.; Gulen, H.; Barut, E.; Cansev, A. Annual patterns of total soluble sugars and proteins related to coldhardiness in olive (Olea europaea L.‘Gemlik’). J. Hortic. Sci. Biotechnol. 2007, 82, 597–604. [Google Scholar] [CrossRef]
- Hashempour, A.; Ghasemnezhad, M.; Fotouhi Ghazvini, R.; Sohani, M.M. Eff ect of Freezing Stress on Lipid Peroxidation and Antioxidant Enzyme Activities of Olive cvs. ‘Fishomi’ and ‘Roughani’. Agric. Conspec. Sci. 2014, 79, 245–252. [Google Scholar]
- Saadati, S.; Baninasab, B.; Mobli, M.; Gholami, M. Cold tolerance in olive leaves of three cultivars related to some physiological parameters during cold acclimation and de-acclimation stages. J. Agric. Sci. Technol. 2020, 22, 1313–1326. [Google Scholar]
- Cansev, A.; Gulen, H.; Eris, A. Cold-Hardiness of Olive (Olea Europaea L.) Cultivars In Cold-Acclimated and non-acclimated stages: Seasonal alteration of antioxidative enzymes and dehydrin-like proteins. J. Agric. Sci. 2009, 147, 51–61. [Google Scholar] [CrossRef]
- Cansev, A.; Gulen, H.; Eris, A. The activities of catalase and ascorbate peroxidase in olive (Olea europaea L. cv. Gemlik) under low temperature stress. Hort. Environ. Biotechnol. 2011, 52, 113–120. [Google Scholar] [CrossRef]
- Leyva-Perez, M.d.L.O.; Valverde-Corredor, A.; Valderrama, R.; Jimenez-Ruiz, J.; Munoz-Merida, A.; Trelles, O.; Barroso, J.B.; Mercado-Blanco, J.; Luque, F. Early and delayed long-term transcriptional changes and short-term transient responses during cold acclimation in olive leaves. DNA Res. 2015, 22, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, R.; Adamo, S.; Fontana, M.; Manzo, M.; Salvini, M.; Durante, M.; Petruccelli, R.; Bartolini, G. Differential gene expression induced by cold stress in Olea europaea L. Acta Hortic. 2008, 791, 55–59. [Google Scholar] [CrossRef]
- Bernardi, R.; Bartolini, G.; Petruccelli, R.; Salvini, M.; Durante, M. Modulated gene expression during the cold acclimation process in tolerant and sensitive clones of cultivar Leccino (Olea europaea L.). POJ 2015, 8, 405–411. [Google Scholar]
- Yurtsever, M.; Vural Korkut, S. Cloning of lipase from Olea europaea cv. Gemlik leaves and expression analysis in response to cold stress. Turk. J. Bot. 2019, 43, 290–297. Available online: Http://journaltubitakgovtrbotany/ (accessed on 6 December 2021). [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.N.A.; Azzeme, A.M.; Yousefi, K. Fine-Tuning Cold Stress Response Through Regulated Cellular Abundance and Mechanistic Actions of Transcription Factors. Front. Plant Sci. 2022, 13, 850216. [Google Scholar] [CrossRef]
- Kim, J.M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef] [Green Version]
- Hoang, X.L.T.; Nhi, D.N.H.; Thu, N.B.A.; Thao, N.P.; Tran, L.P. Transcription Factors and Their Roles in Signal Transduction in Plants under Abiotic Stresses. Curr. Genom. 2017, 18, 483–497. [Google Scholar] [CrossRef]
- Drescher, A.; Ruf, S.; Calsa, T., Jr.; Carrer, H.; Bock., R. The two largest chloroplast genome-encoded open reading frames of higher plants are essential genes. Plant J. 2000, 22, 97–104. [Google Scholar] [CrossRef]
- Kikuchi, S.; Asakura, Y.; Imai, M.; Nakahira, Y.; Kotani, Y.; Hashiguchi, Y.; Nakai, Y.; Takafuji, K.; Bédard, J.; Hirabayashi, Y.; et al. A Ycf2-FtsHi Heteromeric AAA-ATPase Complex Is Required for Chloroplast Protein Import. Plant Cell 2018, 30, 2677–2703. [Google Scholar] [CrossRef] [Green Version]
- Unver, T.; Wu, Z.; Sterck, L.; Turktas, M.; Lohaus, R.; Li, Z.; Yang, M.; He, L.; Deng, T.; Escalante, F.J.; et al. Genome of wild olive and the evolution of oil biosynthesis. Proc. Natl. Acad. Sci. USA 2017, 114, E9413–E9422. [Google Scholar] [CrossRef] [Green Version]
- Hernández, M.L.; Padilla, M.N.; Sicardo, M.D.; Mancha, M.; MartínezRivas, J.M. Effect of different environmental stresses on the expression of oleate desaturase genes and fatty acid composition in olive fruit. Phytochemistry 2011, 72, 178–187. [Google Scholar] [CrossRef]
- D’Angeli, S.; Matteucci, M.; Fattorini, L.; Gismondi, A.; Ludovici, M.; Canini, A.; Altamura, M.M. OeFAD8, OeLIP and OeOSM expression and activity in cold-acclimation of Olea europaea, a perennial dicot without winter-dormancy. Planta 2016, 243, 1279–1296. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.; Jaiswal, A.; Taj, G.; Jaiswal, J.P.; Qureshi, M.I.; Singh, N.K. DREB1/CBF transcription factors: Their structure function and role in abiotic stress tolerance in plants. J. Genet. 2012, 91, 385–395. [Google Scholar] [CrossRef]
- Eom, S.H.; Ahn, M.A.; Kim, E.; Lee, H.J.; Wi, S.H.; Kim, S.K.; Lim, H.B.; Hyun, T.K. Plant Response to Cold Stress: Cold Stress Changes Antioxidant Metabolism in Heading Type Kimchi Cabbage (Brassica rapa L. ssp. Pekinensis). Antioxidants 2022, 11, 700. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P. MAP Kinase Cascades Regulate the Cold Response by Modulating ICE1 Protein Stability. Dev. Cell 2017, 43, 618–629. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhang, Z.; Xie, S.; Tong, S.; Li, Y.; Zhu, J.K. Mutational Evidence for the Critical Role of CBF Transcription Factors in Cold Acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Chen, S.; Cai, K.; Lu, Z.; Yang, Y.; Tigabu, M.; Zhao, X. Transcriptome sequencing and gene expression profiling of Pinus sibirica under different cold stresses. Breed. Sci. 2021, 71, 550–563. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Tong, Q.; Xu, G.; Xu, M.; Li, H.; Fan, P.; Li, S.; Liang, Z. Transcriptomic analysis of grapevine Dof transcription factor gene family in response to cold stress and functional analyses of the VaDof17d gene. Planta 2021, 253, 55. [Google Scholar] [CrossRef]
- Sun, S.; Hu, C.; Qi, X.; Chen, J.; Zhong, Y.; Muhammad, A.; Lin, M.; Fang, J. The AaCBF4-AaBAM3.1 module enhances freezing tolerance of kiwifruit (Actinidia arguta). Hortic. Res. 2021, 8, 97. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sharma, P.C. DeepSAGE Based Differential Gene Expression Analysis under Cold and Freeze Stress in Seabuckthorn (Hippophae rhamnoides L.). PLoS ONE 2015, 10, e0121982. [Google Scholar] [CrossRef] [Green Version]
- Fernando Cruz, F.; Julca, I.; Gómez-Garrido, J.; Loska, D.; Marcet-Houben, M.; Cano, E.; Galán, B.; Frias, L.; Ribeca, P.; Derdak, S.; et al. Genome sequence of the olive tree, Olea Europaea. GigaScience 2016, 5, 29. [Google Scholar] [CrossRef]
- Azzarello, E.; Mugnai, S.; Pandolfi, C.; Masi, E.; Marone, E.; Mancuso, S. Comparing Image (Fractal Analysis) and Electrochemical Impedance Spectroscopy and Electrolyte Leakage) Techniques for the Assessment of the Freezing Tolerance in Olive. Trees 2009, 23, 159–167. [Google Scholar] [CrossRef]
- Yu, D.J.; Lee, H.J. Evaluation of freezing injury in temperate fruit trees. Hortic. Environ. Biotechnol. 2020, 61, 787–794. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K +-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Wisniewski, M.; Bassett, C.; Gusta, L. An overview of cold hardiness in woody plants: Seeing the forest through the trees. Hortic. Sci. 2003, 38, 952–959. [Google Scholar] [CrossRef]
- Roselli, G.; Benelli, G.; Morelli, D. Relationship between stomatal density and winter hardiness in olive (Olea europaea L.). J. Hortic. Sci. Biotech. 1989, 64, 199–203. [Google Scholar] [CrossRef]
- Roselli, G.; Venora, G. Relationship between stomatal size and winter hardiness in the olive. Acta Hortic. 1990, 286, 89–92. [Google Scholar] [CrossRef]
- Soleimani, A.; Lessani, H.; Talaie, A. Relationship between stomatal density and ionic leakage as indicators of cold hardiness in olive (Olea europaea L.). Acta Hortic. 2003, 618, 521–525. [Google Scholar] [CrossRef]
- Bartolozzi, F.; Mencuccini, M.; Fontanazza, G. Enhancement of frost tolerance in olive shoots in vitro by cold acclimation and sucrose increase in the culture medium. Plant Cell Tissue Organ Cult. 2001, 67, 299–302. [Google Scholar] [CrossRef]
- Wisniewski, M.; Demid, L.; Neuner, G. Adaptive mechanisms of freeze avoidance in plants: A brief update. Environ. Exp. Bot. 2014, 99, 133–140. [Google Scholar] [CrossRef]
- Duman, J.G.; Wisniewski, M.J. The use of antifreeze proteins for frost protection in sensitive crop plants. Environ. Exp. Bot. 2014, 106, 60–69. [Google Scholar] [CrossRef]
- Kumar, S. Epigenomics of Plant Responses to Environmental Stress. Epigenomes 2018, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Pannelli, G. Olivo: La potatura di risanameno dopo i danni causati dal gelo. Vita Campogna 2013, 1, 30–34. [Google Scholar]
- Fernández-Escobar, R.; Navarro, S.; Melgar, J.C. Effect of nitrogen status on frost tolerance of olive trees. Acta Hortic. 2011, 924, 41–45. [Google Scholar] [CrossRef]
- Fernández-Escobar, R. Olive Nutritional Status and Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1151. [Google Scholar] [CrossRef]
- Saadati, S.; Baninasab, B.; Mobli, M.; Gholami, M. Foliar application of potassium to improve the freezing tolerance of olive leaves by increasing some osmolite compounds and antioxidant activity. Sci. Hortic. 2021, 276, 109765. [Google Scholar] [CrossRef]
- Roelofs, D.; Aarts, M.G.M.; Schat, H.; van Straalen, N.M. Functional ecological genomics to demonstrate general and specific responses to abiotic stress. Funct. Ecol. 2008, 22, 8–18. [Google Scholar] [CrossRef]
- Charrier, G.; Martin-StPaul, N.; Damesin, C.; Delpierre, N.; Hänninen, H.; Torres-Ruiz, J.M.; Davi, H. Interaction of drought and frost in tree ecophysiology: Rethinking the timing of risks. Ann. For. Sci. 2021, 78, 40. [Google Scholar] [CrossRef]
- Turchetti Iturrieta, J.P.; Ruiz, M.; Vita Serman, F. Frost tolerance in young plants of olea europaea l. ‘Arbequina’ and ‘Barnea’: Role of tissue water status. Acta Hortic. 2014, 1057, 155–162. [Google Scholar] [CrossRef]
- Hashempour, A.; Ghasemnezhad, M.; Fotouhi Ghazvini, R.; Sohani, M.M. The Physiological and Biochemical Responses to Freezing Stress of Olive Plants Treated with Salicylic Acid. Russ. J. Plant Physiol. 2014, 61, 443–450. [Google Scholar] [CrossRef]
- Saadati, S.; Baninasab, B.; Mobli, M.; Gholami, M. Enhancement of freezing tolerance of olive leaves by foliar application of methyl jasmonate and 24–epibrassinolide through changes in some metabolites and antioxidant activity. Sci. Hortic. 2021, 284, 110127. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Baldini, E. Notizie Sull’olivicoltura Bolognese; Accademia Nazionale di Agricoltura: Bologna, Italy, 2003. [Google Scholar]
- Breviglieri, N. Osservazioni sui Danni Causati all’Olivo Dalle Basse Temperature Dell’inverno 1939-40 nel Mugello; L’Olivicoltore: Roma, Italy, 1940. [Google Scholar]
- Caruso, G. Monografia Dell’olivo; Unione Tipografico-Editrice: Torino, Italy, 1882; Volume 3, pp. 501–533. [Google Scholar]
- Cuppari, P. Alcune osservazioni intorno agli effetti del gelo sugli olivi. Bull. Agrar. 1848, 5, XXII. [Google Scholar]
- Denney, J.O.; McEachern, G.R. An analysis of several climatic temperature variables dealing with olive reproduction. J. Amer. Soc. Hort. Sci. 1983, 108, 578–581. [Google Scholar]
- Francolini, F. Olivicoltura; Un. Tip.: Torino, Italy, 1923. [Google Scholar]
- Fontanazza, G.; Preziosi, P. L’olivo e le basse temperature. Osservazioni su 37 cultivar da olio e 20 cultivar da mensa. L’Italia Agric. 1969, 7–8, 737–745. [Google Scholar]
- Gaetani, L. I danni del gelo degli olivi nel 1929 in Umbria. La Metereologia Prat. 1938. [Google Scholar]
- Giovine, G.M. Relazione del Danno Cagionato Agli Olivi Della Campagna di Molfetta Dalla Gelata del 30–31 Dicembre 1788; Opere: Bari, Italy, 1788; Volume II. [Google Scholar]
- Denney, J.O. Special Section Insert: Lessons from a record-breaking freeze: Some olives show damage; many, coldhardiness. Calif. Agric. 1993, 47, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mariti, G. L’Odeporico, Ossia Itinerario per le Colline Pisane; A Spese di Giovacchino Pagani: Firenze, Italy, 1797; p. 54. [Google Scholar]
- Morettini, A.; Marinucci, M.; Jacoboni, N. Olivi Colpiti Dal Gelo; REDA: Rome, Italy, 1965; p. 69. [Google Scholar]
- Naser, I.; Hermogino, R.; Angeles, C.; Abu Kashem, A. Effect of frost and salts dissolved after heavy rain on the productivity of olive trees under desert growing conditions. J. Agric. Allied Sci. 2018, 7, 85–103. [Google Scholar]
- Panbuffetti, P. Ieri Come Oggi. La galaverna del. Agricoltore 1956, 1568, 15. [Google Scholar]
- Pellini, U. Alberi Nella Storia di Reggio; AGE: Reggio Emilia, Italy, 1996. [Google Scholar]
- Pecori, R. La Coltura Dell’olivo; Italia Ricci M: Florence, Italy, 1889; p. 428. [Google Scholar]
- Presta, G. Memoria su i saggi diversi di olio e su della ragia di ulivo della penisola salentina. In Memoria Intorno a Sessantadue Saggi Diversi di Olio, 1st ed.; GS Romano: Lecce, Italy, 1786; Volume I, pp. 1–42. [Google Scholar]
- Presta, G. Degli Ulivi, Delle Ulive e Della Maniera di Cavar L’olio; Stamperia Reale: Parma, Italy, 1794; Volume II, p. 619. [Google Scholar]
- Ridolfi, C. Del danno provato dagli olivi pel gelo del dicembre 1846. G. Agrar. Toscano 1847, XXI, 71–731847. [Google Scholar]
- Tavanti, G. Trattato Teorico-Pratico Completo Sull’Ulivo; Piatti: Firenze, Italy, 1819; p. 259. [Google Scholar]
- Trinci, C. Trattato degli Ulivi. In L’Agricoltore Sperimentato, Ovvero Regole Generali Sopra L’agricoltura, 7th ed.; G. Dorigoni: Venezia, Italy, 1763; Volume 1, p. 526. [Google Scholar]
- Xoplaki, E.; Panagiotis Maheras, P.; Juerg Luterbacher, J. Variability of climate in meridional balkans During the periods 1675–1715 and 1780–1830 And its impact on human life. Clim. Change 2001, 48, 581–615. [Google Scholar] [CrossRef]
- Zamparotti, P. L’inverno Più Freddo Degli Ultimi 500 Anni. Meteo Clima e Storia 2004. Available online: https://news.meteogiornale.it/meteo-clima-e-storia/linverno-piu-freddo-degli-ultimi-500-anni/ (accessed on 24 February 2022).
- Zorzi, A. La Repubblica del Leone; Bompiani: Milan, Italy, 2001; p. 766. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruccelli, R.; Bartolini, G.; Ganino, T.; Zelasco, S.; Lombardo, L.; Perri, E.; Durante, M.; Bernardi, R. Cold Stress, Freezing Adaptation, Varietal Susceptibility of Olea europaea L.: A Review. Plants 2022, 11, 1367. https://doi.org/10.3390/plants11101367
Petruccelli R, Bartolini G, Ganino T, Zelasco S, Lombardo L, Perri E, Durante M, Bernardi R. Cold Stress, Freezing Adaptation, Varietal Susceptibility of Olea europaea L.: A Review. Plants. 2022; 11(10):1367. https://doi.org/10.3390/plants11101367
Chicago/Turabian StylePetruccelli, Raffaella, Giorgio Bartolini, Tommaso Ganino, Samanta Zelasco, Luca Lombardo, Enzo Perri, Mauro Durante, and Rodolfo Bernardi. 2022. "Cold Stress, Freezing Adaptation, Varietal Susceptibility of Olea europaea L.: A Review" Plants 11, no. 10: 1367. https://doi.org/10.3390/plants11101367
APA StylePetruccelli, R., Bartolini, G., Ganino, T., Zelasco, S., Lombardo, L., Perri, E., Durante, M., & Bernardi, R. (2022). Cold Stress, Freezing Adaptation, Varietal Susceptibility of Olea europaea L.: A Review. Plants, 11(10), 1367. https://doi.org/10.3390/plants11101367







