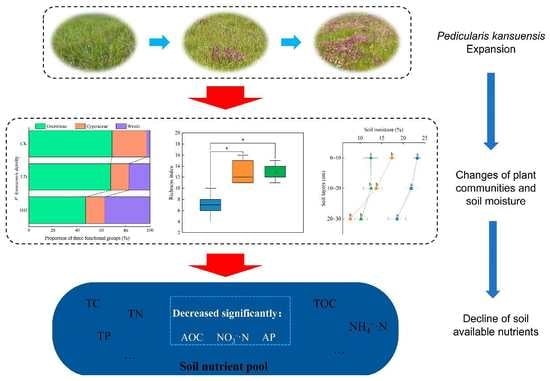
Effects of Pedicularis kansuensis Expansion on Plant Community Characteristics and Soil Nutrients in an Alpine Grassland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
3.1. Responses of Plant Communities to P. kansuensis Expansion
3.1.1. Change of Community Characteristics
3.1.2. Change of Community Biomasses
3.1.3. Change of the ABR of Different Functional Groups
3.2. Responses of Soil Physicochemical Properties to P. kansuensis Expansion
3.2.1. Change of Soil Moisture and pH
3.2.2. Change of Soil Nutrients
3.3. Relationship between Community Characteristics and Soil Nutrients
4. Discussion
4.1. Community Characteristics and Biomass
4.2. Soil Physicochemical Properties
4.3. Mechanism Analysis of the P. kansuensis Expansion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, F.; Xue, X.; You, Q.G.; Sun, J.; Zhou, J.; Wang, T.; Tsunekawa, A. Change in the trade-off between aboveground and belowground biomass of alpine grassland: Implications for the land degradation process. Land Degrad. Dev. 2019, 31, 105–117. [Google Scholar] [CrossRef]
- Dong, S.K.; Shang, Z.H.; Gao, J.X.; Boone, R. Enhancing the ecological services of the Qinghai-Tibetan Plateau’s grasslands through sustainable restoration and management in era of global change. Agric. Ecosyst. Environ. 2022, 326, 107756. [Google Scholar] [CrossRef]
- Chen, D.D.; Zhao, L.; He, F.Q.; Chen, X.; Huo, L.L.; Zhao, X.Q.; Xu, S.X.; Li, Q. Analysis of nutritional components of common edible forages from different area of Sanjiangyuan alpine grassland. Grassl. Turf. 2021, 41, 134–142. [Google Scholar]
- Sun, H.L.; Zheng, D.; Yao, T.D.; Zhang, Y.L. Protection and construction of the national ecological security shelter zone on Tibetan Plateau. Acta Geophys. Sin. 2012, 67, 3–12. [Google Scholar]
- Chen, B.X.; Zhang, X.Z.; Tao, J.; Wu, J.S.; Wang, J.S.; Shi, P.L.; Zhang, Y.J.; Yu, C.Q. The impact of climate change and anthropogenic activities on alpine grassland over the Qinghai-Tibet Plateau. Agric. For. Meteorol. 2014, 189–190, 11–18. [Google Scholar] [CrossRef]
- Fu, B.J.; Ouyang, Z.Y.; Shi, P.; Fan, J.; Wang, X.D.; Zheng, H.; Zhao, W.W.; Wu, F. Current condition and protection strategies of Qinghai-Tibet Plateau ecological security barrier. Bull. Chin. Acad. Sci. 2021, 36, 1298–1306. [Google Scholar]
- Zhang, Z.C.; Liu, M.; Sun, J.; Wei, T. Degradation leads to dramatic decrease in topsoil but not subsoil root biomass in an alpine meadow on the Tibetan Plateau, China. J. Arid Land 2020, 12, 806–818. [Google Scholar] [CrossRef]
- Li, X.L.; Gao, J.; Brierley, G.; Qiao, Y.M.; Zhang, J.; Yang, Y.W. Rangeland degradation on the Qinghai-Tibet Plateau: Implications for rehabilitation. Land Degrad. Dev. 2013, 24, 72–80. [Google Scholar] [CrossRef]
- Chen, L.L.; Shi, J.J.; Wang, Y.L.; Ma, Y.S.; Dong, Q.M.; Hou, X.K. Study on different degraded degrees grassland community structure characteristics of the alpine area. Acta Agrestia Sin. 2016, 24, 210–213. [Google Scholar]
- She, Y.D.; Yang, X.Y.; Ma, L.; Zhang, Z.H.; Wang, D.J.; Huang, X.T.; Ma, Z.; Yao, B.Q.; Zhou, H.K. Study on the characteristics and interrelationship of plant community and soil in degraded alpine meadow. Acta Agrestia Sin. 2021, 29, 62–71. [Google Scholar]
- Li, J.H.; Yang, G.J.; Wang, S.P. Vegetation and soil characteristics of degraded alpine meadows on the Qinghai-Tibet Plateau, China: A review. Chin. J. Appl. Ecol. 2020, 31, 2109–2118. [Google Scholar]
- Zhou, H.K.; Zhao, X.Q.; Wen, J.; Chen, Z.; Yao, B.Q.; Yang, Y.W.; Xu, W.X.; Duan, J.C. The characteristics of soil and vegetation of degenerated alpine steppe in the Yellow River Source Region. Acta Pratac. Sin. 2012, 21, 1–11. [Google Scholar]
- Wang, X.X.; Dong, S.K.; Yang, B.; Li, Y.Y.; Su, X.K. The effects of grassland degradation on plant diversity, primary productivity, and soil fertility in the alpine region of Asia's headwaters. Environ. Monit. Assess. 2014, 186, 6903–6917. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Law, D.J.; Caplan, J.S.; Barron-Gafford, G.A.; Dontsova, K.M.; Espeleta, J.F.; Villegas, J.C.; Okin, G.S.; Breshears, D.D.; Huxman, T.E. Biological invasions and climate change amplify each other’s effects on dryland degradation. Glob. Chang. Biol. 2022, 28, 285–295. [Google Scholar] [CrossRef]
- Sun, H.Q.; Lin, G.J.; Li, X.L.; Li, C.H. Analysis of vegetation community structure and productivity of different degraded grasslands of alpine meadows in the Sanjiangyuan area. Heilongjiang Anim. Sci. Vet. Med. 2013, 19, 1–3. [Google Scholar]
- Bao, G.S.; Suetsugu, K.; Wang, H.S.; Yao, X.; Liu, L.; Ou, J.; Li, C.J. Effects of the hemiparasitic plant Pedicularis kansuensis on plant community structure in a degraded grassland. Ecol. Res. 2015, 30, 507–515. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, H.K.; Zhao, X.Q.; Wen, J.; Ye, X.; Yang, Y. The effect of alpine meadow degradation on Pedicularis kansuensis’s growth and resource allocation in blooming date. Ecol. Environ. Sci. 2010, 19, 2800–2807. [Google Scholar]
- Wei, W.D. Effect of allelopathic action of Pedicularis kansuensis Maxim on seed germination and seedling growth in graminaceous grass species. Seed 2010, 29, 48–51. [Google Scholar]
- Qiu, Z.Q.; Ma, Y.S.; Shi, J.J.; Pan, D.F.; Li, R.J. Influence of Pedicularis kansuensis on Elymus nutans artificial grassland in “Black Soil Type”: Degenerated alpine grassland. Grassl. Turf. 2006, 118, 26–29. [Google Scholar]
- Hou, Y. Allelopathy Effects of Poisonous Plant in the “Black Soil Land” of Tibetan-Plateau and its Inhibitory Mechanism to Pedicularis kansuensis. Master’s Thesis, Lanzhou University, Lanzhou, China, 2011. [Google Scholar]
- Liu, Y.Y. Ecological Factors of Pedicularis kansuensis Maxim. Expansion in Bayanbulak Grassland. Doctoral Dissertation, Xinjiang University, Urumqi, China, 2018. [Google Scholar]
- Tian, Y.Q.; Sui, X.L.; Zhang, T.; Li, Y.M.; Li, A.R. Effects of soil nitrogen heterogeneity and parasitism by Pedicularis species on growth and root spatial distribution of Polypogon monspeliensis. Guihaia 2020, 40, 1838–1848. [Google Scholar]
- Zhao, Z.Z.; Dong, S.K.; Jiang, X.M.; Liu, S.L.; Ji, H.Z.; Li, Y.; Han, Y.H.; Sha, W. Effects of warming and nitrogen deposition on CH4, CO2 and N2O emissions in alpine grassland ecosystems of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2017, 592, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, S.K.; Zhao, Z.Z.; Li, S.; Han, Y.H.; Sha, W.; Shen, H.; Liu, S.L.; Dong, Q.M.; Zhou, H.K.; et al. Effect of simulated nitrogen deposition on the community composition and stability of alpine grasslands in the Qinghai Lake Area. Pratac. Sci. 2019, 36, 2733–2741. [Google Scholar]
- Ma, L.; Zhang, Q.; Zhang, Z.H.; Guo, J.; Yang, X.Y.; Zhou, B.R.; Deng, Y.F.; Wang, F.; She, Y.D.; Zhou, H.K. Effects of gradient warming on species diersity and bomass in apine madows. Acta Agrestia Sin. 2020, 28, 1395–1402. [Google Scholar]
- She, Y.D.; Zhang, Z.H.; Ma, L.; Xu, W.H.; Huang, X.T.; Zhou, H.K. Vegetation attributes and soil properties of alpine grassland in different degradation stages on the Qinghai-Tibet Plateau, China: A meta-analysis. Arab. J. Geosci. 2022, 15, 193. [Google Scholar] [CrossRef]
- Wang, F.S.; He, Y.T.; Shi, P.L.; Niu, B.; Zhang, X.Z.; Xu, X.L. Stellera chamaejasme as an indicator for alpine meadow degradation on the Tibetan Plateau. Chin. J. Appl. Environ. Biol. 2016, 22, 567–572. [Google Scholar]
- Shi, G.X.; Wang, W.Y.; Jiang, S.J.; Cheng, G.; Yao, B.Q.; Feng, H.Y.; Zhou, H.K. Effects of the spreading of Ligularia virgaurea on soil physicochemical property and microbial functional diversity. Chin. J. Plant Ecol. 2018, 42, 126–132. [Google Scholar]
- Chen, N.; Zhang, Y.J.; Zhu, J.T.; Li, J.X.; Liu, Y.J.; Zu, J.X.; Cong, N.; Huang, K.; Wang, L. Nonlinear responses of productivity and diversity of alpine meadow communities to degradation. Chin. J. Plant Ecol. 2018, 42, 50–65. [Google Scholar]
- Xu, H.P.; Zhang, J.; Pang, X.P.; Wang, Q.; Zhang, W.N.; Wang, J.; Guo, Z.G. Responses of plant productivity and soil nutrient concentrations to different alpine grassland degradation levels. Environ. Monit. Assess. 2019, 191, 678. [Google Scholar] [CrossRef]
- Zhan, T.Y.; Hou, G.; Liu, M.; Sun, J.; Fu, S. Different characteristics of vegetation and soil properties along degraded gradients of alpine grasslands in the Qinghai-Tibet Plateau. Pratac. Sci. 2019, 36, 1010–1021. [Google Scholar]
- Irving, L.J.; Cameron, D.D. You are what you eat: Interactions between root parasitic plants and their hosts. Adv. Bot. Res. 2009, 50, 87–138. [Google Scholar]
- Li, A.R.; Smith, F.A.; Smith, S.E.; Guan, K.Y. Two sympatric root hemiparasitic Pedicularis species differ in host dependency and selectivity under phosphorus limitation. Funct. Plant Biol. 2012, 39, 784–794. [Google Scholar] [CrossRef] [PubMed]
- You, Q.G.; Xue, X.; Peng, F.; Dong, S.Y. Alpine meadow degradation effect on soil thermal and hydraulic properties and its environmental impacts. J. Desert Res. 2015, 35, 1183–1192. [Google Scholar]
- Cui, X.; Pan, Y.; Wang, Y.N.; Zheng, X.N.; Gao, Y. Effects of Stellera chamaejasme on small-scale community composition and soil physical and chemical properties in degraded grassland. Chin. J. Ecol. 2020, 39, 2581–2592. [Google Scholar]
- Qin, R.M.; Wen, J.; Zhang, S.X.; Yang, X.Y.; Xu, M.H. Impacts of simulated warming on C, N, and P stoichiometric characteristics of alpine meadow soil in the Qinghai-Tibetan Plateau. Arid Zone Res. 2020, 37, 908–916. [Google Scholar]
- Li, J.J.; Fan, M.C.; Shangguan, Z.P. Ecological stoichiometry characteristics of soil carbon, nitrogen, and phosphorus of the Robinia pseudoacacia forest on the north-south strip of the Loess Plateau. Acta Ecol. Sin. 2019, 39, 7996–8002. [Google Scholar]
- McKane, R.B.; Johnson, L.C.; Shaver, G.R.; Nadelhoffer, K.J.; Rastetter, E.B.; Fry, B.; Giblin, A.E.; Kielland, K.; Kwiatkowski, B.L.; Laundre, J.A.; et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 2002, 415, 68–71. [Google Scholar] [CrossRef]
- Tao, S.; Hua, X.Y.; Wang, Y.N.; Guo, N.; Yan, X.F.; Lin, J.X. Research advance in effects of different nitrogen forms on growth and physiology of plants. Guizhou Agric. Sci. 2017, 45, 64–68. [Google Scholar]
- Fisher, J.P.; Phoenix, G.K.; Childs, D.Z.; Press, M.C.; Smith, S.W.; Pilkington, M.G.; Cameron, D.D. Parasitic plant litter input: A novel indirect mechanism influencing plant community structure. New Phytol. 2013, 198, 222–231. [Google Scholar] [CrossRef]
- Yuan, K.N. Soil Chemistry for Plant Nutrition; Science Press: Beijing, China, 1983; pp. 35–38. [Google Scholar]
- Zhao, J.; Shen, X.; Li, X.; Hu, J.J.; Wang, H.Q.; Chen, L.B. Correlation between soil pH and the contents of available nutrients in selected soils from three pear orchards in Wendeng. North Horticult. 2009, 11, 5–8. [Google Scholar]







| Patch | Number of P. kansuensis | Coverage of P. kansuensis (%) | Community Coverage (%) |
|---|---|---|---|
| CK | 0 | 0 | 93.8 ± 0.9 |
| LD | 3.8 ± 0.8 | 6.2 ± 1.8 | 92.6 ± 1.2 |
| HD | 14.4 ± 1.7 | 20.2 ± 1.3 | 91 ± 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, R.; Wei, J.; Ma, L.; Zhang, Z.; She, Y.; Su, H.; Chang, T.; Xie, B.; Li, H.; Wang, W.; et al. Effects of Pedicularis kansuensis Expansion on Plant Community Characteristics and Soil Nutrients in an Alpine Grassland. Plants 2022, 11, 1673. https://doi.org/10.3390/plants11131673
Qin R, Wei J, Ma L, Zhang Z, She Y, Su H, Chang T, Xie B, Li H, Wang W, et al. Effects of Pedicularis kansuensis Expansion on Plant Community Characteristics and Soil Nutrients in an Alpine Grassland. Plants. 2022; 11(13):1673. https://doi.org/10.3390/plants11131673
Chicago/Turabian StyleQin, Ruimin, Jingjing Wei, Li Ma, Zhonghua Zhang, Yandi She, Hongye Su, Tao Chang, Beilong Xie, Honglin Li, Wenying Wang, and et al. 2022. "Effects of Pedicularis kansuensis Expansion on Plant Community Characteristics and Soil Nutrients in an Alpine Grassland" Plants 11, no. 13: 1673. https://doi.org/10.3390/plants11131673