Eupatorin and Salviandulin-A, with Antimicrobial and Anti-Inflammatory Effects from Salvia lavanduloides Kunth Leaves
Abstract
:1. Introduction
2. Results
2.1. Structural Elucidation of Compound (1), Salviandulin A (2), and Eupatorin (3)
2.2. Antimicrobial Activity of Extract, Fractions, and Compounds of S. lavanduloides
2.3. Anti-Inflammatory Activity of Extracts, Fractions, and Compounds of S. lavanduloides
3. Discussion
4. Materials and Methods
4.1. Equipment and Reagents
4.2. Plant Material
4.3. Extracts
4.4. Isolation and Identification of Compounds (1–3)
4.5. Antimicrobial Activity
4.5.1. Bacterial
4.5.2. Minimal Inhibitory Concentration (MIC)
4.6. Anti-Inflammatory Activity
4.6.1. Animals
4.6.2. 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced Mouse Ear Edema
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ceravantes, G.E.; García, G.R.; Salazar, S.P.M. Importance of Staphylococcus aureus methicillin resistant in-hospital and acquired in the community. Mex. J. Clin. Pathol. Lab. Med. 2014, 61, 196–204. [Google Scholar]
- CONAMED-PAHO Bulletin; 2018; Volume 3, p. 17.
- World Health Organization (WHO). WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: http://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 June 2017).
- Harris, L.G.; Foster, S.J.; Richards, R.G. An introduction to Staphylococcus aureus, and techniques for identifying and quantifying S. aureus adhesins in relation to adhesion to biomaterials: Review. Eur. Cells Mater. 2002, 4, 100–120. [Google Scholar] [CrossRef] [PubMed]
- Sordé, R.; Pahissa, A.; Rello, J. Management of refractory Pseudomonas aeruginosa infection in cystic fibrosis. Infect. Drug. Resis. 2011, 4, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, A.H.; García, O.S.; Mata, R.R.; Pichardo, M.C.M.; Aguirre, V.M.B. The enzyme glycogen synthase kinase 3 regulates the activity of transcription factors NF-kB and creb during the inflammatory response caused by Staphylococcus aureus. J. Biochem. Educ. 2018, 36, 111–117. [Google Scholar]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Suarez, C.; Godiol, F. Antibioticos betalactamicos. Enferm. Infecc. Y Microbiol. Clin. 2009, 27, 116–129. [Google Scholar] [CrossRef]
- Loza, E. NSAIDs in clinical practice: What you need to know. Inf. Ter. Nat. Sys. Health 2011, 35, 88–95. [Google Scholar]
- Guzmán, S.H.; Maldonado, R.S.; Díaz, H.M.; González, C.M. Medicinal Plants the Reality of an Ancestral Tradition; National Institute of Agricultural and Livestock Forestry Research Regional Research Center Bajío Celaya Experimental Field Center: Guanajuato, Mexico, 2017.
- López, L.T. Anti-inflammatory medicinal plants used in the treatment of rheumatism. Phytotherapy 2003, 22, 118–122. [Google Scholar]
- Jash, S.K.; Gorai, D.; Roy, R. Salvia genus and triterpenoids. Int. J. Pharm. Sci. Res. 2016, 7, 4710. [Google Scholar]
- Walker, J.B.; Elisens, W.J. A revision of Salvia section Heterosphace (Lamiaceae) in western North America. AIDS Contrib. Bot. 2001, 19, 571–589. [Google Scholar]
- Ramamoorthy, T.P.; Elliott, M. Lamiaceae of Mexico: Diversity, distribution, endemism and evolution. In Biological Diversity of Mexico: Origins and Distribution; Ramamoorthy, T.P., Bye, R., Lot, A., Fa, J., Eds.; Institute of Biology, National Autonomous University of Mexico: Coyoacán, Mexico, 1993; pp. 501–526. [Google Scholar]
- Villaseñor, J.L. The vascular plant genera of the flora of Mexico. Bol. Soc. Bot. Méx. 2004, 75, 105–135. [Google Scholar]
- Cornejo, T.G.; Ibarra, M.G. Diversity and distribution of the genus Salvia (Lamiaceae) in Michoacán, Mexico. Rev. Mex. De Biodivers. 2011, 82, 1279–1296. [Google Scholar]
- González, G.J.G.; Bedolla, G.B.Y.; Cornejo, T.G.; Fernández, A.J.L.; Fragoso, M.I.; García, P.M.D.R.; Harley, R.M.; Klitgaard, B.; Martínez, G.M.J.; Wood, J.R.I.; et al. Richness and distribution of Salvia subg. Calosphace (Lamiaceae). Int. J. Plant Sci. 2020, 181, 831–856. [Google Scholar] [CrossRef]
- Baricevic, D.; Sosa, S.; Della, L.R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Todorov, S.; Philianos, S.; Petkov, V.; Harvala, C.; Zamfirova, R.; Olimpiou, H. Experimental pharmacological study of three species from genus Salvia. Acta Physiol. Pharmacol. Bul. 1984, 10, 13–20. [Google Scholar]
- Ulubelen, A. Cardioactive and antibacterial terpenoids from some Salvia species. Phytochemistry 2003, 64, 395–399. [Google Scholar] [CrossRef]
- Gaitán, F.I.C. Activity of Twelve Native Guatemalan Plants against Sporothrix Schenckii. Biology Chemestry Thesis. University of San Carlos de Guatemala: Guatemala, 2005. Available online: http://biblioteca.usac.edu.gt/tesis/06/06_2289.pdf (accessed on 18 April 2022).
- Monroy, O.C.; Castillo, E.P. Medicinal Plants Used in the State of Morelos, 2nd ed.; Autonomous University of the State of Morelos: Cuernavaca, Mexico, 2007; p. 405. [Google Scholar]
- Del Cid, A.N.E. Activity of Seventeen Extracts of Twelve Native Guatemalan Plants against Fonsecaea Pedrosoi; USAC: Guatemala, 2005. [Google Scholar]
- Vázquez, G.D.; Castro, R.A.E. Medicinal uses of the Labiatae family in Chiapas, Mexico. Ethnobiology 2002, 2, 19–31. [Google Scholar]
- Arellano, R.B. Medicinal Ethnobotany of the Me’phaa Culture in La Ciénega, Municipality of Malinaltepec, Guerrero, Mexico; UAGro: Chilpancingo, Mexico, 2017. [Google Scholar]
- Palacios, V.M.C. Inhibition of the Growth of Gardnerella Vaginalis by Six Medicinal Plants of the Southwestern Guatemalan Flora; University of San Carlos de Guatemala: Guatemala, 2004; Available online: https://biblioteca-farmacia.usac.edu.gt/Tesis/QB782.pdf (accessed on 20 April 2022).
- Ortega, A.; Cárdenas, J.; Toscano, A.; Maldonado, E.; Aumelas, A.; Van, C.M.R.; Jankowski, C. Salviandulines A and B. Two secoclerodane diterpenoids from Salvia lavanduloides. Phytochemistry 1991, 30, 3357–3360. [Google Scholar] [CrossRef]
- Maldonado, E.; Cardenas, J.; Salazar, B.; Ortega, A.; Jankowski, C.K.; Van Calsteren, M.R. Salvianduline C, a 5, 6-secoclerodane diterpenoid from Salvia lavanduloides. Phytochemistry 1992, 31, 217–220. [Google Scholar] [CrossRef]
- González, C.M.M.; Ramos, V.C.S.; Serrano, V.R.; Pérez, G.C.; Sánchez, M.E.; Pérez, G.S. Anti-inflammatory activity of standardized dichloromethane extract of Salvia connivens on macrophages stimulated by LPS. Pharm. Biol. 2017, 55, 1467–1472. [Google Scholar] [CrossRef] [Green Version]
- Bisio, A.; Damonte, G.; Fraternale, D.; Giacomelli, E.; Salis, A.; Romussi, G.; Cafaggi, S.; Ricci, D.; De Tommasi, N. Phytotoxic clerodane diterpenes from Salvia miniata Fernald (Lamiaceae). Phytochemistry 2011, 72, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Abe, F.; Kinjo, J.; Okabe, H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis B RIQ. and consideration of structure–activity relationship. Biol. Pharm. Bull. 2002, 25, 875–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argueta, A.; Cano, A.L.M.; Rodarte, G.M.E. Atlas of the Plants of Traditional Mexican Medicine; Instituto Nacional Indigenista: Mexico City, Mexico, 1994.
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S. The pharmacology of indomethacin. Headache J. Head Face Pain 2016, 56, 436–446. [Google Scholar] [CrossRef]
- Laavola, M.; Nieminen, R.; Yam, M.F.; Sadikun, A.; Asmawi, M.Z.; Basir, R.; Welling, J.; Vapaatalo, H.; Korhonen, R.; Moilanen, E. Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation. Planta Med. 2012, 78, 779–786. [Google Scholar] [CrossRef]
- De La Cruz, S.N.G.; Gómez, R.A.; Alvarez, F.P.; Ventura, Z.E.; Pérez, G.M.D.; Avilés, F.M.; Gutiérrez, R.A.S.; González, C.M. Antibacterial activity of Morinda citrifolia Linneo sedes against Methicillin Resistant Staphylococcus spp. Microb. Pathog. 2019, 128, 347–353. [Google Scholar] [CrossRef]
- Monterrosas, B.N.; Ocampo, M.L.A.; Jiménez, F.E.; Jiménez, A.A.R.; Zamilpa, A.; Gonzalez, C.M.; Tortoriello, J.; Herrera, R.M. Anti-inflammatory activity of different Agave plants and the compound Cantalasaponin-1. Molecules 2013, 18, 8136–8146. [Google Scholar] [CrossRef]
- Salinas, S.D.O.; Herrera, R.M.; Pérez, S.; Jiménez, F.E.; Zamilpa, A. Anti-inflammatory activity of hautriwaic acid isolated from Dodonaea viscosa leaves. Molecules 2012, 17, 4292–4299. [Google Scholar] [CrossRef]
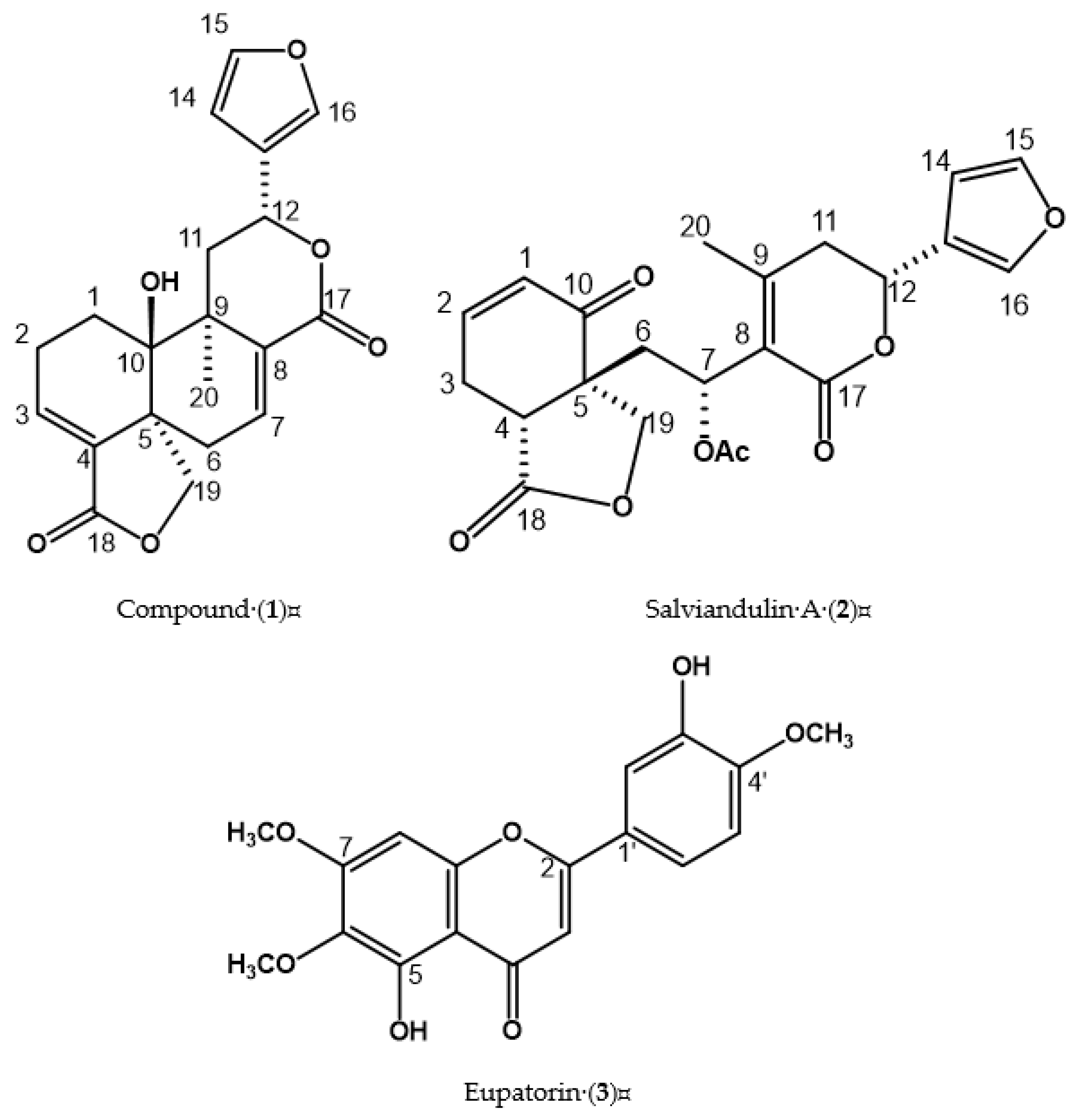

| Position | δ1H (J in Hz) 1 | δ13C 1 | δ1H (J in Hz) 2 | δ13C 2 |
|---|---|---|---|---|
| 1a b | 1.79, dd(8.0, 14.3) 1.72, ddd(4.0, 11.3, 15.4) | 25.3 | 6.13, ddd(1.1, 2.5, 10.2) | 129.7 |
| 2a b | 2.40, dd(4.0, 8.0) 2.41, dd(4.0, 8.0) | 22.9 | 6.98, dddd(0.7, 2.5, 5.5, 10.2) | 149.3 |
| 3a b | 6.79, dd(3.3, 3.6) | 135.6 | 3.01, dd(br, 5.1, 20.1) 2.76, ddd(2.5, 6.9, 20.5) | 23.2 |
| 4 | 131.84 | 3.07, d(br, 6.9) | 43.3 | |
| 5 | 44.6 | 51.6 | ||
| 6a b | 2.79, dd(4.4, 20.5) 2.33, d(1.8, 20.5) | 39.10 | 2.69, dd(8.0, 14.6) 2.33, d(4.4, 14.6) | 39.1 |
| 7 | 6.42, dd(3.3, 4.0) | 133.1 | 5.63, d(4.4, 8.0) | 67.3 |
| 8 | 134.8 | 124.9 | ||
| 9 | 40.82 | 154.8 | ||
| 10 | 71.7 | 197.4 | ||
| 11a b | 2.66, dd (2.2, 15.4) 1.70, dd(12.4, 15.4) | 38.5 | 2.74, m 2.51, dd(3.6, 17.9) | 37.5 |
| 12 | 5.03, dd(1.83, 12.4) | 71.91 | 5.3, d(3.6, 11) | 71.0 |
| 13 | 123.7 | 123.5 | ||
| 14 | 6.63 d(br, 1.4) | 109.03 | 6.42, d(br, 1.1) | 108.5 |
| 15 | 7.69, dd(1.4, 1.8) | 144.0 | 7.42, dd(1.8, 1.8) | 143.9 |
| 16 | 7.79, d(br, 0.7) | 140.58 | 7.47, dd(0.7, 0.7) | 140.1 |
| 17 | 167.8 | 163.0 | ||
| 18 | 168.96 | 176.5 | ||
| 19a b | 4.29, d(8.0) 4.33, d(8.0) | 73.35 | 4.74, d(8.8) 3.84, d(8.8) | 73.6 |
| 20 | 1.10, s | 29.20 | 2.13, s | 20.4 |
| MeCO2- | 2.03, s | 170.2, 21.1 |
| Position | δ1H (in ppm, J in Hz) 3 | δ 13C 3 |
|---|---|---|
| 2 | 165.3 | |
| 3 | 6.67(s) | 104.2 |
| 4 | 183.5 | |
| 5 | 154.0 | |
| 6 | 133.4 | |
| 7 | 160.0 | |
| 8 | 6.86(s) | 92.0 |
| 9 | 153.5 | |
| 10 | 106.3 | |
| 1′ | 124.4 | |
| 2′ | 7.48 (d, 1.4) | 112.6 |
| 3′ | 148.0 | |
| 4′ | 152.1 | |
| 5′ | 7.55 (dd, 1.4, 8.4) | 113.7 |
| 6′ | 7.10 (d, 8.4) | 119.6 |
| 6-OCH3 | 3.79 (s) | 56.3 |
| 7-OCH3 | 3.92(s) | 56.8 |
| 4′-OCH3 | 3.97(s) | 60.5 |
| Extracts μg/mL | |||||
|---|---|---|---|---|---|
| Bacterium | Sl-Hex | Sl-AcOEt | Sl-D | Control (+) | Control (−) |
| Sa | >200 | 200 | 100 | --- | † |
| SaR | >200 | 200 | 100 | --- | † |
| Se-1 | 100 | <25 | 100 | --- | † |
| Se-2 | >200 | 200 | 200 | --- | † |
| Se-3 | >200 | 200 | 200 | --- | † |
| Sh | >200 | 200 | 200 | --- | † |
| Ef | <25 | <25 | <25 | --- | † |
| Pa | <25 | <25 | <25 | --- | † |
| Sd | <25 | <25 | <25 | --- | † |
| Ca | <25 | <25 | <25 | † | † |
| Fractions μg/mL | μg/mL Compounds | ||||||
|---|---|---|---|---|---|---|---|
| Bacterium | SlD-2 | SlD-3 | 1 | 2 | 3 | Control (+) | Control (−) |
| Sa | 100 | 200 | 16 | 16 | 16 | --- | † |
| SaR | 50 | 200 | 16 | 16 | 16 | --- | † |
| Se-1 | 50 | <25 | 16 | 16 | 16 | --- | † |
| Se-2 | 100 | 200 | 16 | 16 | 16 | --- | † |
| Se-3 | 100 | 200 | 16 | 16 | 16 | --- | † |
| Sh | 100 | 100 | 16 | 16 | 16 | --- | † |
| Ef | <25 | <25 | <2 | <2 | <2 | --- | † |
| Pa | <25 | <25 | <2 | <2 | <2 | --- | † |
| Sd | <25 | >200 | <2 | 16 | <2 | --- | † |
| Ca | >200 | >200 | <2 | 16 | 16 | † | † |
| Treatments (1.0 mg/ear) | Edema (mg) | Inflammation Inhibition (%) |
|---|---|---|
| VEH | 11.76 ± 1.40 | 0 |
| INDO | 2.72 ± 0.75 | 76.87 |
| Extracts | ||
| Sl-Hex | 4.46 ± 2.04 | 62.02 |
| Sl-D | 6.50 ± 1.23 | 44.73 |
| Sl-AcOEt | 6.45 ± 1.10 | 45.15 |
| Fractions | ||
| SlD-2 | 9.73 ± 2.14 | 17.23 |
| SlD-3 | 4.56 ± 1.40 | 61.22 |
| Compounds | ||
| Salviandulin A (2) | 3.50 ± 0.67 | 70.24 |
| Eupatorin (3) | 3.25 ± 1.01 * | 72.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cortazar, M.; Salinas-Sánchez, D.O.; Herrera-Ruiz, M.; Román-Ramos, D.C.; Zamilpa, A.; Jiménez-Ferrer, E.; Ble-González, E.A.; Álvarez-Fitz, P.; Castrejón-Salgado, R.; Pérez-García, M.D. Eupatorin and Salviandulin-A, with Antimicrobial and Anti-Inflammatory Effects from Salvia lavanduloides Kunth Leaves. Plants 2022, 11, 1739. https://doi.org/10.3390/plants11131739
González-Cortazar M, Salinas-Sánchez DO, Herrera-Ruiz M, Román-Ramos DC, Zamilpa A, Jiménez-Ferrer E, Ble-González EA, Álvarez-Fitz P, Castrejón-Salgado R, Pérez-García MD. Eupatorin and Salviandulin-A, with Antimicrobial and Anti-Inflammatory Effects from Salvia lavanduloides Kunth Leaves. Plants. 2022; 11(13):1739. https://doi.org/10.3390/plants11131739
Chicago/Turabian StyleGonzález-Cortazar, Manasés, David Osvaldo Salinas-Sánchez, Maribel Herrera-Ruiz, Dionisio Celerino Román-Ramos, Alejandro Zamilpa, Enrique Jiménez-Ferrer, Ever A. Ble-González, Patricia Álvarez-Fitz, Ricardo Castrejón-Salgado, and Ma. Dolores Pérez-García. 2022. "Eupatorin and Salviandulin-A, with Antimicrobial and Anti-Inflammatory Effects from Salvia lavanduloides Kunth Leaves" Plants 11, no. 13: 1739. https://doi.org/10.3390/plants11131739
APA StyleGonzález-Cortazar, M., Salinas-Sánchez, D. O., Herrera-Ruiz, M., Román-Ramos, D. C., Zamilpa, A., Jiménez-Ferrer, E., Ble-González, E. A., Álvarez-Fitz, P., Castrejón-Salgado, R., & Pérez-García, M. D. (2022). Eupatorin and Salviandulin-A, with Antimicrobial and Anti-Inflammatory Effects from Salvia lavanduloides Kunth Leaves. Plants, 11(13), 1739. https://doi.org/10.3390/plants11131739










