An Interplay of Light and Smoke Compounds in Photoblastic Seeds
Abstract
:1. Introduction
2. The Impact of Smoke Formulations and Isolated Smoke Compounds on the Germination of Photoblastic Seeds
| Plant Species | Smoke Formulation | Active Substance of Smoke | Light and Illumination Parameters | Mode of Action | Reference |
|---|---|---|---|---|---|
| Lactuca sativa (Asteraceae, different seed lots) P | SW of Australian grass Themeda triandra Diluted from 1:2 to 1:10,000 | Not specified (mixture of smoke compounds) | Darkness, R 1.8 μmol (quantum) m−2 s−1, FR 4.5 μmol (quantum) m−2 s−1, 10-min illumination | 1:100, 1:500 and 1:1000 dilutions increased seed germination in darkness, 1:500–1:1000 dilutions substituted for R in the presence of FR in germination stimulation | [10] |
| Lactuca sativa P | SW of Themeda triandra, Acacia mearnsii, Eucalyptus grandis, Hypoxis colchicifolia, Pinus patula, SW diluted from 1:1 to 1:100 on tissue paper. | Not specified (mixture of smoke compounds) | Darkness | Inhibition at 1:1 concentration, stimulation when dilutions of 1:10 or 1:100 were used (depending on the plant source) | [11] |
| Angianthus tomentosus, Gnephosis tenuissima, Myriocephalus guerinae, Podolepis canescens, Rhodanthe citrina (Australian annual Asteraceae) | Butenolide and SW | Butenolide (probably KAR1) and mixture of smoke compounds | 570 nm (0.8 μmol (quantum) m−2 s−1); 640 nm (1.24 μmol (quantum) m−2 s−1); 720 nm (0.2 μmol (quantum) m−2 s−1); white light (15.4, μmol (quantum) m−2 s−1, 1400–700 nm); and continuous darkness. | [25] | |
| Juniperus procera (Cupressaceae, Gymnosperms) | SW of Alnus incana, a tree belonging to Angiosperms | Not specified (mixture of smoke compounds) | White light/continuous | No effect | [34] |
| Arabidopsis thaliana (Brassicaceae) ecotypes | Karrikin | KAR1 | 100 μmol (quantum) m−2 s−1, continuous.For Ler and gal-3 ecotypes, FR 6 μmol (quantum) m−2 s−1 | Stimulation in light (?) | [35] |
| Avena fatua (Poaceae) | SW of Australian grass Themeda triandra (Poaceae) Butenolide | Not specified (mixture of smoke compounds) Butenolide (probably KAR1) | 8 and 50 μmol (quantum) m−2 s−1, 16/8 h | Stimulation in darkness | [32] |
| Avena fatua, Lolium rigidum, Eragrostis curvula, Phalaris minor, Hordeum glaucum, Ehrharta calycina and Bromus diandrus (Poaceae, Australia) | Karrikinolide | KAR1 (?) | 30 μmol (quantum) m−2 s−1, 12/12 h | Stimulation/inhibition depending on the species | [33] |
| Hibbertia species (Dilleniaceae, Australia) | Karrikin, aerosol smoke | KAR1 (?), mixture of smoke compounds | Fluorescent tubes, 400–700 nm, 50 μmol (quantum) m−2 s−1, 12/12 h | Stimulation | [36] |
| Chrysanthemoides monilifera, (Asteraceae) Australian weed (two populations) | Karrikins | KAR1 and KAR2 | Breakdown of physiological dormancy by KAR1 and germination stimulation in field trials | [20] | |
| Ansellia africana (Orchidaceae) | SW, KAR1, TMB | Mixture of smoke compounds, KAR1, TMB | 30 μmol (quantum) m−2 s−1), 16/8 h photoperiod | Stimulation of seed germination and protocorm development by SW, inhibition by TMB | [37] |
| Heteropogon contortus (Poaceae) P | Food-grade liquid smoke, SW of xylose, H. contortus, KAR1, cyanide, potassium cyanide, MAN (benzaldehyde) | Mixture of smoke compounds, KAR1, benzaldehyde, cyanide, potassium cyanide, benzaldehyde | Daylight supplied by a 60-W incandescent plant light bulb | Stimulation of seed germination by all treatments, with the exception of KAR1 | [18] |
| Matricaria chamomilla, Solidago sp., (Asteraceae) P | SW of mixture of different European meadow plant species | Not specified (mixture of smoke compounds) | Natural (not specified) | Stimulation in darkness (both species), inhibition in the light (Solidago) | [30] |
| Bauhinia variegata (Fabaceae) N | SW and KAR1 | Mixture of smoke compounds and KAR1 | Cool-white fluorescent lamps (approx. 70 µmol (quantum) m−2 s−1) | Stimulated germination in light conditions | [38] |
| Chaenorhinum rubrifolium (Plantaginaceae) | Smoke, KAR1, MAN, NO and nitrates | Smoke, KAR1, MAN | Daylight (?, approx. 100 µmol (quantum) m−2 s−1, 12/12 h | Stimulation | [21] |
| Heteropogon contortus (Poaceae) P | SW | Not specified (mixture of smoke compounds) | Natural (?), 12/12 h | Stimulation | [39] |
| Lactuca sativa P | KAR1, SW and TMB | KAR1, SW and TMB | R (660 nm) and FR(730 nm) for 1 h, | TMB significantlyinhibited germination (33%) in R light Stimulation by KAR1 while FR was used | [28] |
| Solidago chinensis, Pterocaulon balansae, Stenachaenium megapotamicum (forbs) Acanthostyles buniifolius (shrub) (Asteraceae, South America) Erianthus angustifolius, Aristida laevis (grasses, South America) | Smoke fumigation | Not specified (mixture of smoke compounds) | White (cool daylight, 300 and 80 lx), 12/12 h | Germination stimulation by low light and smoke | [40] |
| Cleome gynandra (Brassicales), native to Africa N | SW and KAR1 | Mixture of smoke compounds and KAR1 | Fluorescent cool white lamps, 108 W, and R (1.6 μmol (quantum) m−2 s−1), FR μmol (1.4 quantum) m−2 s−1), green (0.3 μmol (quantum) m−2 s−1), blue (0.2 μmol (quantum) m−2 s−1) | Stimulation by SW in darkness | [8] |
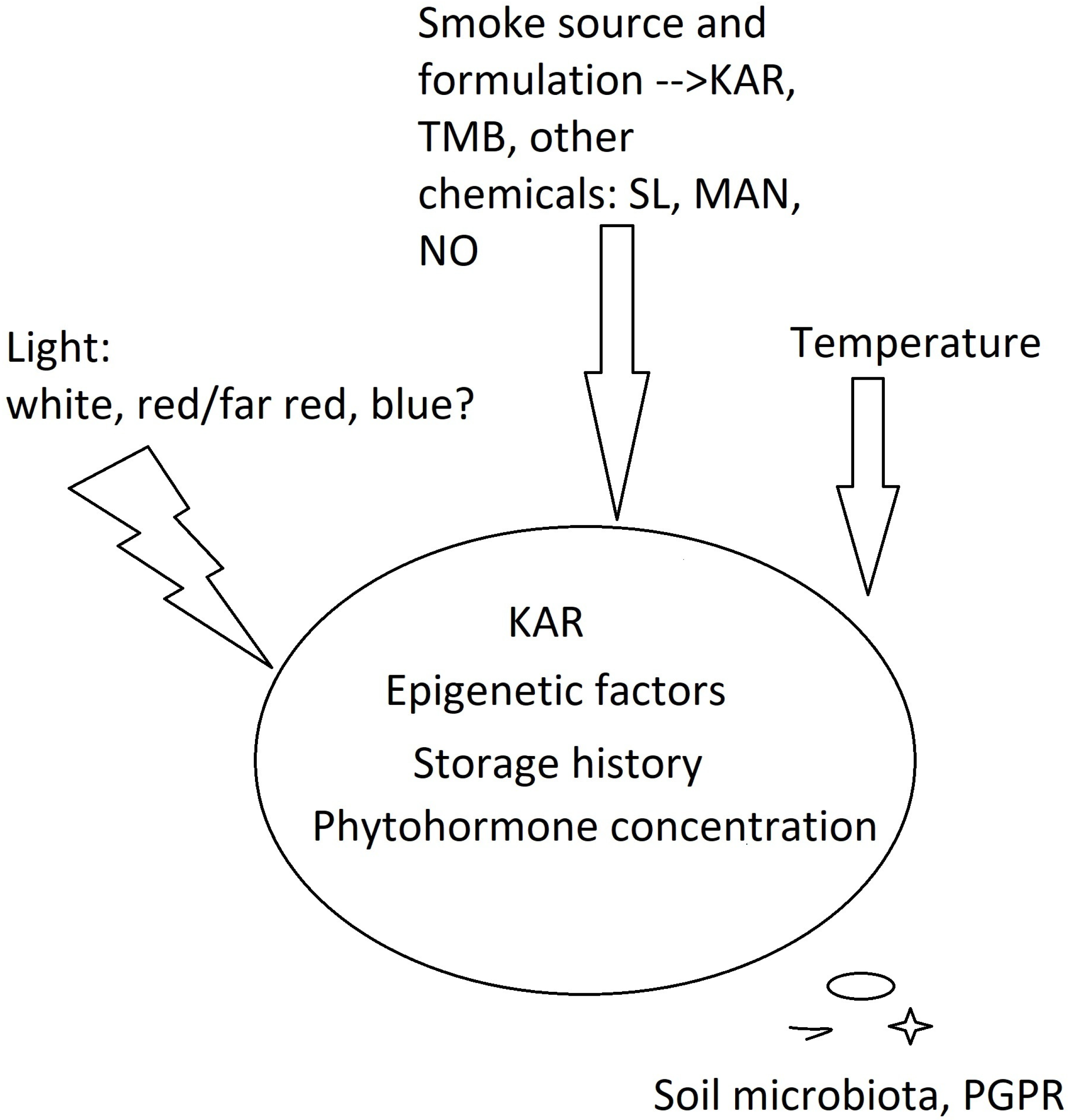
3. Physiological Window of KAR1 Perception by Seeds
4. Ecological and Practical Implications
5. Disentangling Molecular Mechanism of KAR Action in Photoblastic Seeds
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Milberg, P.; Andersson, L.; Thompson, K. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Sci. Res. 2000, 10, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Ando, M.; Seiwa, K. Interaction of seed size with light quality and temperature regimes as germination cues in 10 temperate pioneer tree species. Funct. Ecol. 2016, 30, 866–874. [Google Scholar] [CrossRef]
- Luna, B.; Moreno, J.M. Range-size, local abundance and germination niche-breadth in Mediterranean plants of two life-forms. Plant Ecol. 2010, 210, 85–95. [Google Scholar] [CrossRef]
- Donohue, K.; Heschel, M.S.; Chiang, G.C.; Butler, C.M.; Barua, D. Phytochrome mediates germination responses to multiple seasonal cues. Plant Cell Environ. 2007, 30, 202–212. [Google Scholar] [CrossRef]
- Nelson, D.C.; Risenborough, J.-A.; Flematti, G.R.; Stevens, J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins Discovered in Smoke Trigger Arabidopsis Seed Germination by a Mechanism Requiring Gibberellic Acid Synthesis and Light. Plant Physiol. 2009, 149, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Tognacca, R.S.; Botto, J.F. Post-transcriptional regulation of seed dormancy and germination: Current understanding and future directions. Plant Commun. 2021, 2, 100169. [Google Scholar] [CrossRef]
- Gubler, F.; Hughes, T.; Waterhouse, P.; Jacobsen, J. Regulation of dormancy in barley by blue light and after-ripening: Effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008, 147, 886–896. [Google Scholar] [CrossRef] [Green Version]
- Nemahunguni, N.K.; Gupta, S.; Kulkarni, M.; Finnie, J.F.; van Staden, J. The effect of biostimulants and light wavelengths on the physiology of Cleome gynandra seeds. Plant Growth Regul. 2020, 90, 467–474. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Lin, R. The role of light in regulating seed dormancy and germination. J. Integr. Plant Biol. 2020, 62, 1310–1326. [Google Scholar] [CrossRef]
- Drewes, F.E.; Smith, M.T.; Van Staden, J. The effect of a plant derived smoke extract on the germination of light-sensitive lettuce seed. Plant Growth Regul. 1995, 16, 205–209. [Google Scholar] [CrossRef]
- Jäger, A.K.; Light, M.E.; van Staden, J. Effects of source of plant material and temperature on the production of smoke extracts that promote germination of light-sensitive lettuce seeds. Environ. Exp. Bot. 1996, 36, 421–429. [Google Scholar] [CrossRef]
- Keeley, J.E.; Babr-Keeley, M. Role of charred wood, heat-shock, and light in germination of postfire phrygana species from the eastern Mediterranean basin. Israel J. Plant Sci. 1999, 47, 11–16. [Google Scholar] [CrossRef]
- Van Staden, J.; Jäger, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef] [PubMed]
- Flematti, G.R.; Merritt, D.J.; Piggott, M.J.; Trengove, R.D.; Smith, S.M.; Dixon, K.W.; Ghisalberti, E.L. Burning vegetation produces cyanohydrins that liberate cyanide and stimulate seed germination. Nat. Commun. 2011, 2, 360. [Google Scholar] [CrossRef]
- Cao, D.; Schöttner, M.; Halitschke, R.; Baldwin, I.T. Syringaldehyde is a novel smoke-derived germination cue for the native fire-chasing tobacco, Nicotiana attenuata. Seed Sci. Res. 2021, 31, 292–299. [Google Scholar] [CrossRef]
- Light, M.E.; Burger, B.V.; Staerk, D.; Kohout, L.; Van Staden, J. Butenolides from plant-derived smoke: Natural plant-growth regulators with antagonistic actions on seed germination. J. Nat. Prod. 2010, 73, 267–269. [Google Scholar] [CrossRef] [Green Version]
- Baldos, O.C.; DeFrank, J.; Sakamoto, G.S. Germination response of dormant tanglehead (Heteropogon contortus) seeds to smoke-infused water and the smoke-associated stimulatory compounds, karrikinolide and cyanide. HortScience 2015, 50, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Kamran, M.; Khan, A.L.; Ali, L.; Hussain, J.; Waqas, M.; Al-Harrasi, A.; Lee, I.J. Hydroquinone; a novel bioactive compound from plant-derived smoke can cue seed germination of lettuce. Front. Chem. 2017, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.J.; Long, R.L.; Flematti, G.R.; Cherry, H.; Turner, S.R. Karrikins promote germination of physiologically dormant seeds of Chrysanthemoides monilifera ssp. monilifera (boneseed). Weed Res. 2013, 54, 48–57. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ç.; Ergan, G.; Çatav, Ş.S.; Zar, G.; Küçükakyüz, K.; Özüdoğru, B. Multiple fire-related cues stimulate germination in Chaenorhinum rubrifolium (Plantaginaceae), a rare annual in the Mediterranean Basin. Seed Sci. Res. 2017, 27, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Çatav, Ş.S.; Küçükakyüz, K.; Tavşanoğlu, Ç.; Pausas, J.G. Effect of fire-derived chemicals on germination and seedling growth in Mediterranean plant species. Basic Appl. Ecol. 2018, 30, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Papenfus, H.B.; Naidoo, D.; Pošta, M.; Finnie, J.F.; Van Staden, J. The effects of smoke derivatives on in vitro seed germination and development of the leopard orchid Ansellia africana. Plant Biol. 2016, 18, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Pošta, M.; Papenfus, H.B.; Light, M.E.; Beier, P.; Van Staden, J. Structure–activity relationships of N- and S-analogs of the seed germination inhibitor (3,4,5-trimethylfuran-2(5H)-one) for mode of action elucidation. Plant Growth Regul. 2017, 82, 47–53. [Google Scholar] [CrossRef]
- Merritt, D.J.; Kristiansen, M.; Flematti, G.R.; Turner, S.R.; Ghisalberti, E.L.; Trengove, R.D.; Dixon, K.W. Effects of a butenolide present in smoke on light-mediated germination of Australian Asteraceae. Seed Sci. Res. 2006, 16, 29–35. [Google Scholar] [CrossRef]
- Dayamba, S.D.; Sawadogo, L.; Tigabu, M.; Savadogo, P.; Zida, D.; Tiveau, D.; Oden, P.C. Effects of aqueous smoke solutions and heat on seed germination of herbaceous species of the Sudanian savanna-woodland in Burkina Faso. Flora 2010, 205, 319–325. [Google Scholar] [CrossRef]
- Gupta, S.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Role of Smoke Stimulatory and Inhibitory Biomolecules in Phytochrome-Regulated Seed Germination of Lactuca sativa. Plant Physiol. 2019, 181, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Hrdlička, J.; Ngoroyemoto, N.; Nemahunguni, N.K.; Gucký, T.; Novák, O.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Preparation and Standardisation of Smoke-Water for Seed Germination and Plant Growth Stimulation. J. Plant Growth Regul. 2020, 39, 338–345. [Google Scholar] [CrossRef]
- Elsadek, M.A.; Yousef, E.A.A. Smoke-Water Enhances Germination and Seedling Growth of Four Horticultural Crops. Plants 2019, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Bączek-Kwinta, R. Swailing affects seed germination of plants of European bio-and agricenosis in a different way. Open Life Sci. 2017, 12, 62–75. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Cacco, G.; Sorgona, A.; Marabottini, R.; Paolacci, A.R.; Ciaffi, M.; Badiani, M. The inhibitory effects of coumarin on the germination of durum wheat (Triticum turgidum ssp. durum, cv. Simeto) seeds. J. Chem. Ecol. 2006, 32, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Kępczyński, J.; Cembrowska, D.; Van Staden, J. Releasing primary dormancy in Avena fatua L. caryopses by smoke-derived butenolide. Plant Growth Regul. 2010, 62, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Long, R.L.; Stevens, J.C.; Griffiths, E.M.; Adamek, M.; Powles, S.B.; Merritt, D.J. Detecting karrikinolide responses in seeds of the Poaceae. Austral J. Bot. 2011, 59, 610–620. [Google Scholar] [CrossRef]
- Tigabu, M.; Fjellström, J.; Odén, P.C.; Teketay, D. Germination of Juniperus procera seeds in response to stratification and smoke treatments, and detection of insect-damaged seeds with VIS + NIR spectroscopy. New For. 2007, 33, 155–169. [Google Scholar] [CrossRef]
- Ooi, M.K.; Auld, T.D.; Whelan, R.J. Dormancy and the fire-centric focus: Germination of three Leucopogon species (Ericaceae) from South-eastern Australia. Ann. Bot. 2006, 98, 421–430. [Google Scholar] [CrossRef]
- Hidayati, S.N.; Walck, J.L.; Merritt, D.J.; Turner, S.R.; Turner, D.W.; Dixon, K.W. Sympatric species of Hibbertia (Dilleniaceae) vary in dormancy break and germination requirements: Implications for classifying morphophysiological dormancy in Mediterranean biomes. Ann. Bot. 2012, 109, 1111–1123. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Kulkarni, G.; Stirk, W.A.; Rengasamy, K.R.R.; Salomon, M.V.; Piccoli, P.; Bottini, R.; van Staden, J. Interactions between a plant growth-promoting rhizobacterium and smoke-derived compounds and their effect on okra growth. J. Plant Nutr. Soil Sci. 2015, 178, 741–747. [Google Scholar] [CrossRef]
- Pendota, S.C.; Kulkarni, M.G.; Rengasamy, K.R.R.; Van Staden, J. Seed germination and seedling growth of Bauhinia variegata in response to smoke-water and synthesised smoke-isolated karrikinolide (KAR1). Seed Sci. Technol. 2017, 45, 306–318. [Google Scholar] [CrossRef]
- Leperlier, C.; Riviere, J.-N.E.; Allibert, A.; Dessau, D.; Lacroix, S.; Fock-Bastide, I. Overcoming dormancy and light requirements in seeds of Heteropogon contortus, a target species for savanna restoration. Ecol. Eng. 2018, 122, 10–15. [Google Scholar] [CrossRef]
- López-Mársico, L.; Farías-Moreira, L.; Altesor, A.; Rodríguez, C. Light intensity triggers different germination responses to fire-related cues in temperate grassland species. Folia Geobot. 2019, 54, 53–63. [Google Scholar] [CrossRef]
- Dave, G.S.; Galvadiya, B.; Bariya, H.; Vyas, S.R. A dataset of LC-MS QTOF analysis of potato and mustard crop residue smoke water. Data Brief 2018, 21, 343–350. [Google Scholar] [CrossRef]
- Jurado, E.; Márquez-Linares, M.; Flores, J. Effect of cold storage, heat, smoke and charcoal on breaking seed dormancy of Arctostaphylos pungens HBK (Ericaceae). FYTON 2011, 80, 4–11. [Google Scholar]
- Flores, J.; Jurado, E.; Arredondo, A. Effect of light on germination of seeds of Cactaceae from the Chihuahuan Desert, México. Seed Sci. Res. 2006, 16, 149–155. [Google Scholar] [CrossRef]
- De Cuyper, C.; Struk, S.; Braem, L.; Gevaert, K.; De Jaeger, G.; Goormachtig, S. Strigolactones, karrikins and beyond. Plant Cell Environ. 2017, 40, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.C.; Flematti, G.R.; Riseborough, J.A.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 7095–7100. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Chen, F.; Shuai, H.; Luo, X.; Ding, J.; Tang, S.; Xu, S.; Liu, J.; Liu, W.; Du, J.; et al. Karrikins delay soybean seed germination by mediating abscisic acid and gibberellin biogenesis under shaded conditions. Sci. Rep. 2016, 6, 22073. [Google Scholar] [CrossRef] [Green Version]
- Bose, U.; Juhász, A.; Broadbent, J.A.; Komatsu, S.; Colgrave, M.L. Multi-Omics Strategies for Decoding Smoke-Assisted Germination Pathways and Seed Vigour. Int. J. Mol. Sci. 2020, 21, 7512. [Google Scholar] [CrossRef]
- Guercio, A.M.; Torabi, S.; Cornu, D.; Dalmais, M.; Bendahmane, A.; Le Signor, C.; Pillot, J.P.; Le Bris, P.; Boyer, F.D.; Rameau, C.; et al. Structural and functional analyses explain Pea KAI2 receptor diversity and reveal stereoselective catalysis during signal perception. Commun. Biol. 2022, 5, 126. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.C.; Scaffidi, A.; Dun, E.A.; Waters, M.T.; Flematti, G.R.; Dixon, K.W.; Beveridge, C.A.; Ghisalberti, E.L.; Smith, S.M. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2013, 108, 8897–8902. [Google Scholar] [CrossRef] [Green Version]
- Ochuodho, J.O.; Modi, A.T. Temperature and light requirements for the germination of Cleome gynandra seeds. S. Afric. J. Plant Soil 2005, 22, 49–54. [Google Scholar] [CrossRef]
- Lariguet, P.; Ranocha, P.; De Meyer, M.; Barbier, O.; Penel, C.; Dunand, C. Identification of a hydrogen peroxide signalling pathway in the control of light-dependent germination in Arabidopsis. Planta 2013, 238, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Smith, M.W.; Brown, R.G.; Kamiya, Y.; Sun, T. Phytochrome regulation and differential expression of gibberellin 3beta-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell 1998, 10, 2115–2126. [Google Scholar] [PubMed] [Green Version]
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.P.; Koshiba, T.; et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006, 48, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Aoki, M.; Nakaminami, K.; Mitsuhashi, W.; Tatematsu, K.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Inoue, Y.; Nambara, E.; et al. Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiol. 2008, 146, 1386–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentsink, L.; Koornneef, M. Seed Dormancy and Germination, The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2008; p. 6. [Google Scholar]
- Cho, J.N.; Ryu, J.Y.; Jeong, Y.M.; Park, J.; Song, J.J.; Amasino, R.M.; Noh, B.; Noh, Y.S. Control of seed germination by light-induced histone arginine demethylation activity. Dev. Cell 2012, 22, 736–748. [Google Scholar] [CrossRef] [Green Version]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef]
- Hoang, H.H.; Sechet, J.; Bailly, C.; Leymarie, J.; Corbineau, F. Inhibition of germination of dormant barley (Hordeum vulgare L.) grains by blue light as related to oxygen and hormonal regulation. Plant Cell Environ. 2014, 37, 1393–1403. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bączek-Kwinta, R. An Interplay of Light and Smoke Compounds in Photoblastic Seeds. Plants 2022, 11, 1773. https://doi.org/10.3390/plants11131773
Bączek-Kwinta R. An Interplay of Light and Smoke Compounds in Photoblastic Seeds. Plants. 2022; 11(13):1773. https://doi.org/10.3390/plants11131773
Chicago/Turabian StyleBączek-Kwinta, Renata. 2022. "An Interplay of Light and Smoke Compounds in Photoblastic Seeds" Plants 11, no. 13: 1773. https://doi.org/10.3390/plants11131773
APA StyleBączek-Kwinta, R. (2022). An Interplay of Light and Smoke Compounds in Photoblastic Seeds. Plants, 11(13), 1773. https://doi.org/10.3390/plants11131773






