Bioprospects of Endophytic Bacteria in Plant Growth Promotion and Ag-Nanoparticle Biosynthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Experimental Site
2.2. Inoculation of Endophytic Bacterial Strains on the Seeds and Seedlings of Tomatoes
2.3. Preparation of Inoculants and Slurry
2.4. Seed and Seedling Bacterization and Biometry
2.5. Internal Root Colonization by Bacteria (Scanning Electron Microscopy or SEM)
2.6. Estimation of Chlorophyll Content in Leaves
2.7. Synthesis of Nanoparticles
2.7.1. Nanoparticle Characterization
2.7.2. Antibacterial Activity
2.8. Statistical Analysis
3. Results
3.1. Effect of Endophytic Bacterial Inoculation on the Growth of Tomato Seedlings
3.2. Growth Parameters and Yields of Tomato after Endophytic Bacterial Inoculation
3.3. Estimation of Chlorophyll Content in Endophyte Inoculated Tomato Leaves
3.4. Root Colonization of Endophytic Bacterial Strains
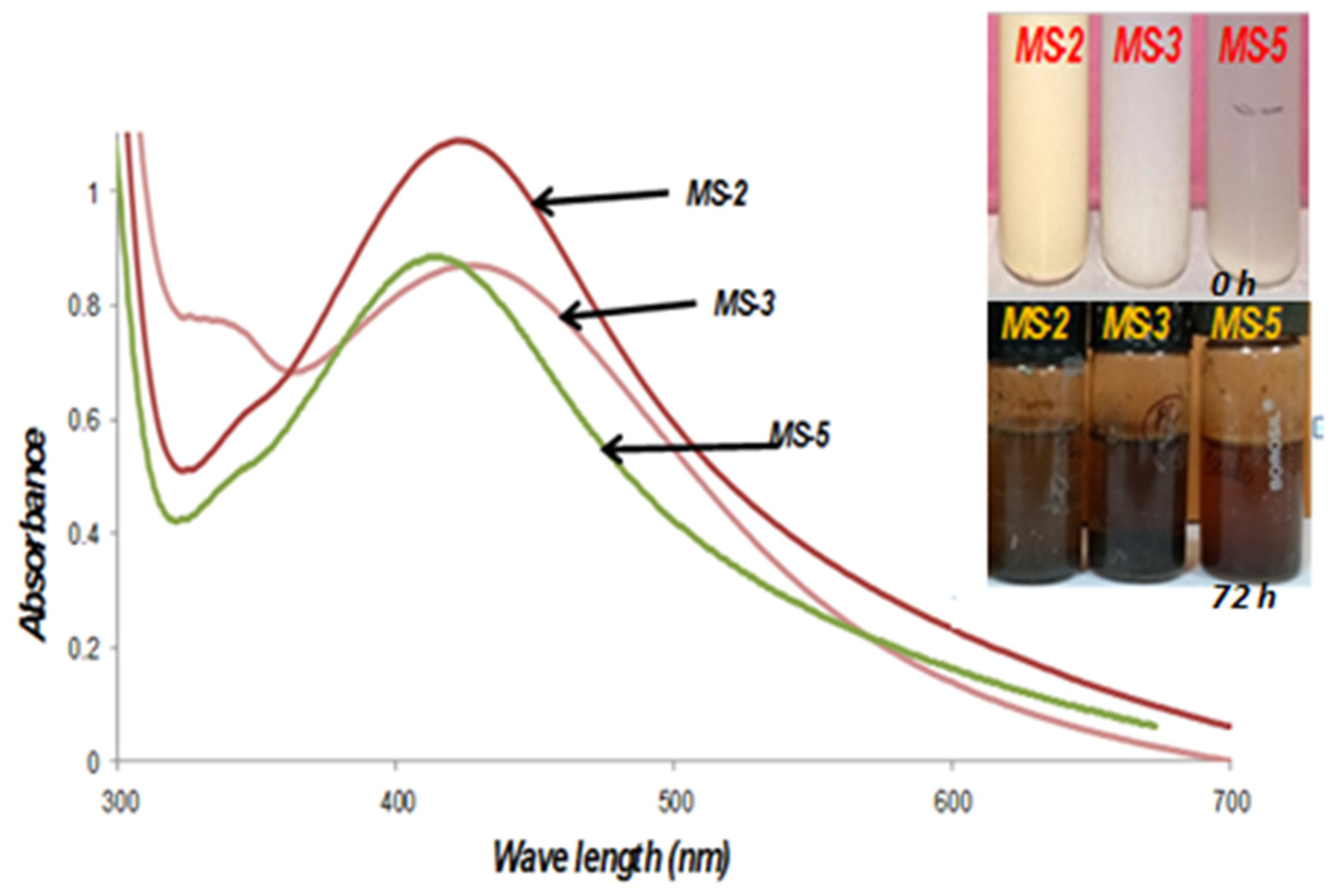
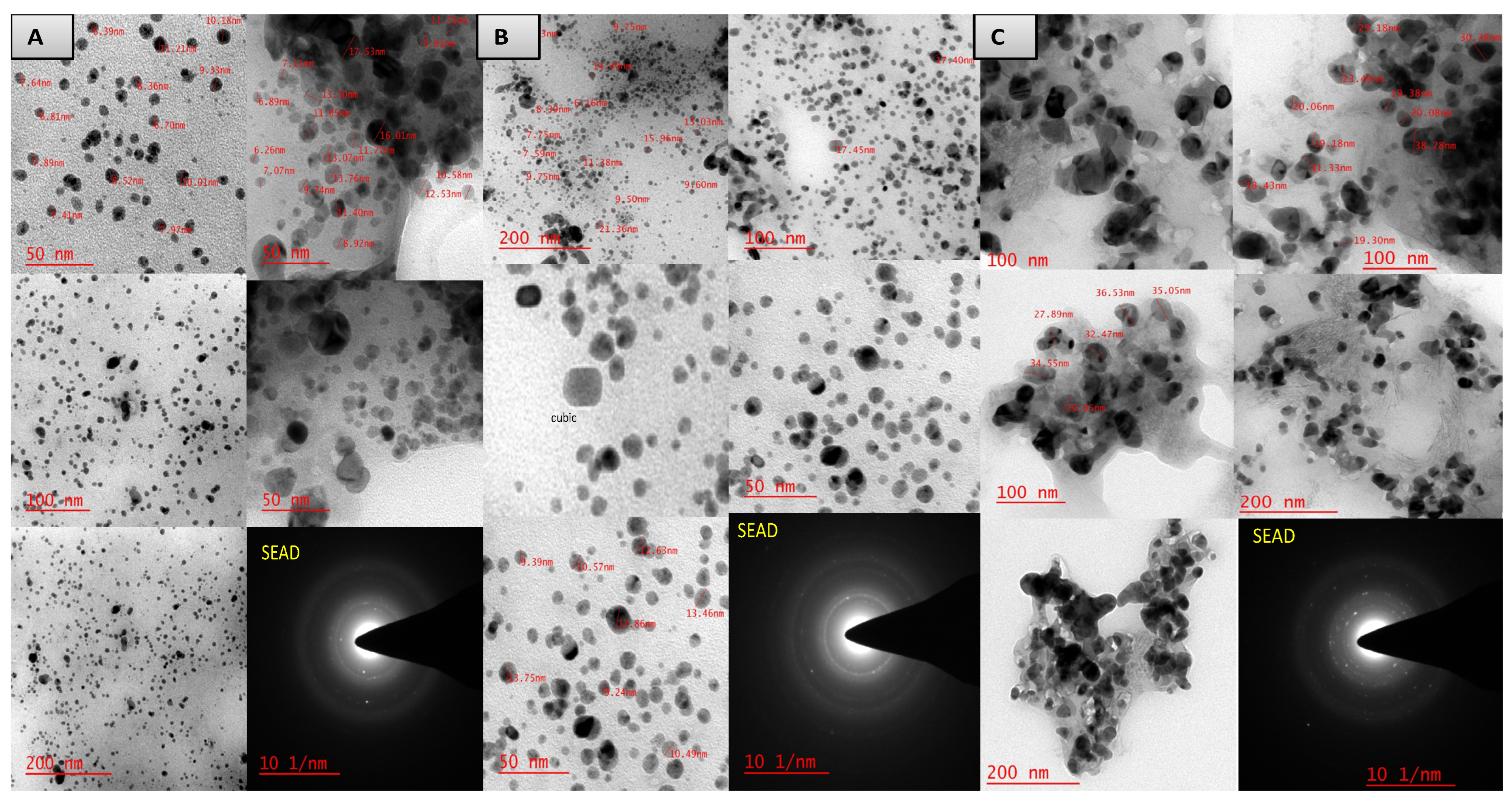
3.5. Biosynthesis and characterization of nanoparticles
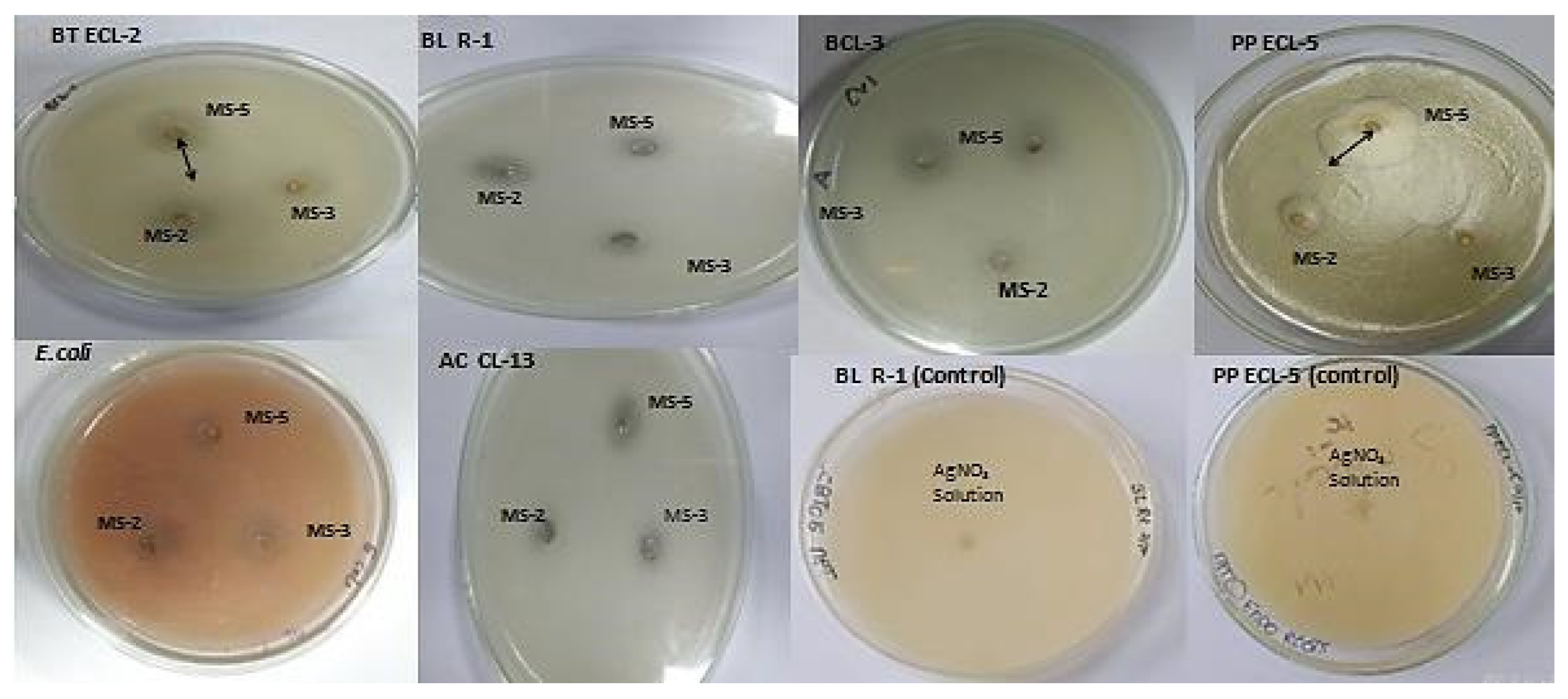
3.6. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Kumar, A. Microbial Biocontrol: Sustainable Agriculture and Phytopathogen Management; Springer Nature Chem: Cham, Switzerland, 2022; Volume 1, pp. 1–369. [Google Scholar] [CrossRef]
- Lopes, M.J.D.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A re-view. Cogent. Food Agric. 2016, 2, 1127500. [Google Scholar]
- Kobayashi, D.Y.; Palumbo, J.D. Bacterial endophytes and their effects on plants and uses in agriculture. In Microbial Endo-Phytes; Bacon, C.W., White, J.F., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 199–233. [Google Scholar]
- Petrini, O. Fungal Endophytes of Tree Leaves. In Microbial Ecology of Leaves; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef]
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Ait Barka, E. Endophytic colonization of Vitisvinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tommonaro, G.; Caporale, A.; DE Martino, L.; Popolo, A.; De Prisco, R.; Nicolaus, B.; Abbamondi, G.R.; Saturnino, C. Antioxidant and cytotoxic activities investigation of tomato seed extracts. Nat. Prod. Res. 2014, 28, 764–768. [Google Scholar] [CrossRef]
- Rania, A.A.; Hayfa, J.K.; Catalina, S.; Ahlem, N.; Kalliope, K.P.; Mejda, D.M. Tomato-associated endophyticbacteria with Fusarium Wilt suppression and tomato growth promotion abilities. J. Agric. Sci. Food. Res. 2018, 9, 1000246. [Google Scholar]
- Tian, B.; Zhang, C.; Ye, Y.; Wen, J.; Wu, Y.; Wang, H.; Li, H.; Cai, S.; Cai, W.; Cheng, Z.; et al. Beneficial traits of bacterial endophytes belonging to the core communities of the tomato root microbiome. Agric. Ecosyst. Environ. 2017, 247, 149–156. [Google Scholar] [CrossRef]
- Panigrahi, T. Synthesis and characterization of silver nanoparticles using leaf extract of AzadirachtaIndica. Ph.D. Thesis, National Institute of Technology Rourkela, Orissa, India, 2013. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.K.; Ma, W.; Labhasetwar, V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int. J. Cancer 2004, 112, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of Silver Nanoparticles and Its Applications. J. Nanotechnol. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.H.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Hemath Naveen, K.S.; Kumar, G.; Karthik, L.; Bhaskara Rao, K.V. Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp. Arch. Appl. Sci. Res. 2010, 2, 161–167. [Google Scholar]
- Natarajan, K.; Selvaraj, S.; Ramchandra, M. Microbial production of silver nanoparticles. Dig. J. Nanomat. Bios. 2010, 5, 135–140. [Google Scholar]
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Extracellular synthesis of silver nano-particles by the Bacillus strain CS 11 isolated from industrialized area. 3Biotech 2014, 4, 121–126. [Google Scholar]
- Minaeian, S.; Shahverdi, A.R.; Nohi, A.S.; Shahverdi, H.R. Extracellular biosynthesis of silver nanoparticles by some bacteria. J. Sci. 2008, 17, 1–4. [Google Scholar]
- Singh, M.; Singh, P.P.; Patel, A.K.; Pandey, K.; Singh, P. Enumeration of Culturable Endophytic Bacterial Population of Different Lycopersicum esculentum L. Varieties. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3344–3352. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Kumar, A.; Pandey, K. Biochemical and molecular identification of Solanum lycopersicum L. temperature tolerant bacterial endophytes. Biocatal. Agric. Biotechnol. 2019, 22, 101409. [Google Scholar] [CrossRef]
- Hungria, M.; Franchini, J.C.; Campo, R.J.; Graham, P.H. The importance of nitrogen fixation to soybean cropping in South America. In Nitrogen Fixation in Agriculture, Forestry, Ecology and Environment; Werner, D., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands; pp. 25–42.
- Marcos, F.C.C.; Iório, R.D.P.F.; Da Silveira, A.P.D.; Ribeiro, R.V.; Machado, E.C.; Lagôa, A.M.M.D.A. Endophytic bacteria affect sugarcane physiology without changing plant growth. Bragantia 2016, 75, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy, Principles and Techniques for Biologists, 2nd ed.; Jones and Bartlett: Sudbury, MA, USA, 1999. [Google Scholar]
- Kumari, R.; Ashraf, S.; Bagri, G.K.; Khatik, S.K.; Bagri, D.K.; Bagdi, D.L. Extraction and estimation of chlorophyll content of seed treated lentil crop using DMSO and acetone. J. Pharmacogn. Phytochem. 2018, 7, 249–250. [Google Scholar]
- Sunkar, S.; Nachiyar, V.; Namasivayam, S.K.R. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymu. Asian Pac. J. Trop. Biomedicine 2012, 2, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, S.; Kobori, T.; Ganesh, D.; Ogawa, K.; Aoyagi, H. Biosynthesis of Silver Nanoparticles Mediated by Extracellular Pigment from Talaromyces purpurogenus and Their Biomedical Applications. Nanomaterials 2019, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.; Paul, M.; Bazerque, P. Antibiotic assay by agar well diffusion method. Acta. Biol. Med. Exp. 1990, 15, 113–115. [Google Scholar]
- Zonooz, N.F.; Salouti, M. Extracellular biosynthesis of silver nanoparticles using cell filtrate of Streptomyces sp. ERI-3. Sci. Iran. 2011, 18, 1631–1635. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Pandey, K.D.; Singh, M.; Singh, S.K.; Hashem, A.; Al-Arjani, A.-B.F.; Abd_Allah, E.F.; Singh, P.K.; Kumar, A. Iso-lation and Characterization of Endophytes Bacterial Strains of Momordica charantia L. and Their Possible Approach in Stress Management. Microorganisms 2022, 10, 290. [Google Scholar] [CrossRef]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [Green Version]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Kuklinsky-Sobral, J.; Araujo, W.L.; Mendes, R.; Geraldi, I.O.; Pizzirani-Kleiner, A.A.; Azevedo, J.L. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 2004, 6, 1244–1251. [Google Scholar] [CrossRef]
- Horrigan, L.; Lawrence, R.S.; Walker, P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ. Health Perspect. 2002, 110, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Omomowo, O.I.; Babalola, O.O. Bacterial and Fungal Endophytes: Tiny Giants with Immense Beneficial Potential for Plant Growth and Sustainable Agricultural Productivity. Microorganisms 2019, 7, 481. [Google Scholar] [CrossRef] [Green Version]
- Viruel, E.; Erazzú, L.E.; MartínezCalsina, L.; Ferrero, M.A.; Lucca, M.E.; Siñeriz, F. Inoculation of maize with phosphate sol-ubilizing bacteria: Effect on plant growth and yield. J. Soil Sci. Plant Nutr. 2014, 14, 819–831. [Google Scholar]
- Kumar, A.; Singh, R.; Yadav, A.; Giri, D.D.; Singh, P.K.; Pandey, K.D. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3Biotech 2016, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.; Lin, J.; Yao, S.; Wang, Y.; Zhou, C.; Wang, H.; Xiao, M. Promotion of plant growth, biological control and induced systemic resistance in maize by Pseudomonas aurantiaca JD37. Ann. Microbiol. 2013, 63, 1177–1185. [Google Scholar] [CrossRef]
- Omer, Z.; Tombolini, R.; Broberg, A.; Gerhardson, B. Indole-3-acetic acid production by pink-pigmented facultative methylotrophic bacteria. Plant Growth Regul. 2004, 43, 93–96. [Google Scholar] [CrossRef]
- Tani, A.; Sahin, N.; Kimbara, K. Methylobacterium oxalidis sp. nov., isolated from leaves of Oxalis corniculata. Int. J. Syst. Evol. Microbiol. 2012, 62, 1647–1652. [Google Scholar] [CrossRef]
- Eevers, N.; Van Hamme, J.D.; Bottos, E.M.; Weyens, N.; Vangronsveld, J. Draft Genome Sequence of Methylobacterium radiotolerans, a DDE-Degrading and Plant Growth-Promoting Strain Isolated from Cucurbita pepo. Genome Announc. 2015, 3, e00488-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sy, A.; Giraud, E.; Jourand, P.; Garcia, N.; Willems, A.; de Lajudie, P.; Prin, Y.; Neyra, M.; Gillis, M.; Boivin-Masson, C.; et al. Methylotrophic Methylobacterium Bacteria Nodulate and Fix Nitrogen in Symbiosis with Legumes. J. Bacteriol. 2001, 183, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhaiyan, M.; Poonguzhali, S.; Kwon, S.-W.; Sa, T.-M. Methylobacterium phyllosphaerae sp. nov., a pink-pigmented, facultative methylotroph from the phyllosphere of rice. Int. J. Syst. Evol. Microbiol. 2009, 59, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardanov, P.; Sessitsch, A.; Häggman, H.; Kozyrovska, N.; Pirttilä, A.M. Methylobacterium-Induced Endophyte Community Changes Correspond with Protection of Plants against Pathogen Attack. PLoS ONE 2012, 7, e46802. [Google Scholar] [CrossRef] [PubMed]
- Yim, W.; Kim, K.; Lee, Y.; Sundaram, S.; Sa, T. Real time expression of ACC oxidase and PR-protein genes mediated by Methylobacterium spp. in tomato plants challenged with Xanthomonas campestris pv. vesicatoria. J. Plant Physiol. 2014, 171, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M. Plant perceptions of plant growth-promoting Pseudomonas. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 907–918. [Google Scholar] [CrossRef] [Green Version]
- Przemieniecki, S.; Kurowski, T.; Karwowska, A. Plant growth promoting potential of Pseudomonas sp. SP0113 isolated from potable water from a closed water well. Arch. Biol. Sci. 2015, 67, 663–673. [Google Scholar] [CrossRef]
- Mia, A.B.; Shamsuddin, Z.H.; Wahab, Z.; Marziah, M. High-yielding and quality banana production through plant growth-promoting rhizobacterial (PGPR) inoculation. Fruits 2005, 60, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M. Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom. Biotechnol. Lett. 2013, 18, 8132–8143. [Google Scholar]
- Chin-A-Woeng, T.F.C.; de Priester, W.; van der Bij, A.J.; Lugtenberg, B.J.J. Description of the Colonization of a Gnotobiotic Tomato Rhizosphere by Pseudomonas fluorescens Biocontrol Strain WCS365, Using Scanning Electron Microscopy. Mol. Plant-Microbe Interact.® 1997, 10, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Lu, G.; Gai, Y.; Gao, H.; Lu, B.; Kong, L.; Mu, Z. Colonization of Morus alba L. by the plant-growth-promoting and antagonistic bacterium Burkholderia cepacia strain Lu10-1. BMC Microbiol. 2010, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Bacilio-Jimenez, M.; Aguilar-Flores, S.; del Valle, M.V.; Perez, A.; Zepeda, A.; Zenteno, E. Endophytic bacteria in rice seeds inhibit early colonization of roots by Azospirillum brasiliense. Soil Biol. Biochem. 2001, 33, 167–172. [Google Scholar] [CrossRef]
- Kurokura, T.; Hiraide, S.; Shimamura, Y.; Yamane, K. PGPR Improves Yield of Strawberry Species under Less-Fertilized Conditions. Environ. Control Biol. 2017, 55, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Esitken, A.; Pirlak, L.; Turan, M.; Sahin, F. Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Sci. Hortic. 2006, 110, 324–327. [Google Scholar] [CrossRef]
- Algam, S.A.; Guan-lin, X.; Coosemans, J. Delivery methods for introducing endophytic Bacillus into tomato and their effect on growth promotion and suppression of tomato wilt. Plant Pathol. J. 2005, 4, 118–124. [Google Scholar]
- Ahirwar, N.K.; Gupta, G.; Singh, V.; Rawlley, R.K.; Ramana, S. Influence on growth and fruit yield of tomato (Lycopersicon esculentum Mill.) plants by inoculation with Pseudomonas fluorescence (SS5): Possible role of plant growth promotion. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 720–730. [Google Scholar]
- Kumar, A.; Ghosh, A. Biosynthesis and characterization of silver nanoparticles with bacterial isolate from gangetic-alluvial soil. Int. J. Biotechnol. Biochem. 2016, 12, 95–102. [Google Scholar]
- Meena, M.; Zehra, A.; Swapnil, P.; Harish; Marwal, A.; Yadav, G.; Sonigra, P. Endophytic Nanotechnology: An Approach to Study Scope and Potential Applications. Front. Chem. 2021, 9, 613343. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; et al. Synthesis of silver nanoparticles utilizing various biological systems: Mechanisms and applications—A review. Prog. Biomater. 2020, 9, 81–95. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C. In Vitro Antibacterial Activity and Mechanism of Silver Nanoparticles against Foodborne Pathogens. Bioinorg. Chem. Appl. 2014, 2014, 581890. [Google Scholar] [CrossRef] [Green Version]
- Bozanic, D.; Trandafilović, L.V.; Luyt, A.; Djoković, V. ‘Green’ synthesis and optical properties of silver—Chitosan complexes and nanocomposites. React. Funct. Polym. 2010, 70, 869–873. [Google Scholar] [CrossRef]
- Kora, A.J.; Arunachalam, J. Green Fabrication of Silver Nanoparticles by Gum Tragacanth (Astragalus gummifer): A Dual Functional Reductant and Stabilizer. J. Nanomater. 2012, 2012, 869765. [Google Scholar] [CrossRef] [Green Version]
- Sanghi, R.; Verma, P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009, 100, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Litvin, V.A.; Minaev, B. Spectroscopy study of silver nanoparticles fabrication using synthetic humic substances and their antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 108, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, S.; Nachiyar, C.V. Microbial synthesis and characterization of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus: A novel source in the benign synthesis. Glob. J. Med. Res. 2012, 12, 953–959. [Google Scholar]
- Durán, M.; Silveira, C.; Durán, N. Catalytic role of traditional enzymes for biosynthesis of biogenic metallic nanoparticles: A mini-review. IET Nanobiotechnol. 2015, 9, 314–323. [Google Scholar] [CrossRef]
- Picoli, S.U.; Durán, M.; Andrade, P.F.; Duran, N. Silver nanoparticles/silver chloride (Ag/AgCl) synthesized from Fusarium oxysporum acting against Klebsiella pneumouniae carbapenemase (KPC) and extended spectrum beta-lactamase (ESBL). Front. Nanosci. Nanotechnol. 2016, 2, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Vivek, M.; Kumar, P.S.; Steffi, S.; Sudha, S. Biogenic Silver Nanoparticles by Gelidiella acerosa Extract and their Antifungal Effects. Avicenna J. Med. Biotechnol. 2011, 3, 143–148. [Google Scholar]
- Singh, K.; Panghal, M.; Kadyan, S.; Yadav, J.P. Evaluation of Antimicrobial Activity of Synthesized Silver Nanoparticles using Phyllanthus amarus and Tinospora cordifolia Medicinal Plants. J. Nanomed. Nanotechnol. 2014, 5, 250. [Google Scholar] [CrossRef] [Green Version]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 3, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Endophytic Bacteria | Root Length (cm) ± SD | Shoot Length (cm) ± SD |
|---|---|---|
| Control (Hybrid) | 3.0 ± 0.4 d | 15.3 ± 2.5 e |
| Rhizobium pusense MS-1 (H) | 3.1 ± 0.5 d | 16.3 ± 3.0 e |
| Bacillus flexus MS-2 (H) | 4.5 ± 0.7 c | 19.3 ± 3.0 c |
| Bacillus cereus MS-3 (H) | 3.1 ± 0.8 d | 17.0 ± 4.0 d |
| Methylophilus flavus MS-4 (H) | 5.4 ± 0.5 a | 26.16 ± 3.5 a |
| Pseudomonas aeruginosa MS-5(H) | 4.9 ± 0.9 b | 21.83 ± 3.0 b |
| Control (Local) | 2.4 ± 0.2 d | 12.43 ± 1.5 e |
| R. pusense MS-1 (L) | 3.1 ± 0.3 c | 14.33 ± 2.2 d |
| B. flexus MS-2 (L) | 3.5 ± 0.3 c | 18.0 ± 3.0 b |
| B. cereus MS-3 (L) | 3.3 ± 0.7 c | 16.33 ± 2.3 c |
| M. flavus MS-4 (L) | 4.2 ± 0.7 a | 21.83 ± 3.0 a |
| P. aeruginosa MS-5 (L) | 3.6 ± 0.5 b | 20.86 ± 2.0 a |
| Variety | Treatments | Time Taken for Germination (in Days) | Percent Germination | Plant Height (cm) | Number of Fruits/Plant | Mean Weight (gm) of Fruit /Plant | Weight of Total Fruit (kg/Plant) after 1st Flowering |
|---|---|---|---|---|---|---|---|
| Hybrid | Control | 5 | 62% | 50 ± 4 a | 19 ± 3 a | 28 ± 4 a | 0.532 a |
| MS-1 | 5 | 66% | 51 ± 3 a | 22 ± 4 a | 29 ± 5 a | 0.638 a | |
| MS-2 | 5 | 75% | 61 ± 4 b | 25 ± 3 b | 40 ± 3 b | 1.000 b | |
| MS-3 | 4 | 74% | 56 ± 4 a | 28 ± 2 b | 35 ± 3 c | 0.980 b | |
| MS-4 | 3 | 78% | 75 ± 8 c | 48 ± 4 c | 50 ± 3 d | 2.400 c | |
| MS-5 | 3 | 69% | 64 ± 5 b | 27 ± 4 b | 44 ± 3 b | 1.188 b | |
| Local | Control | 3 | 78% | 63 ± 7 a | 25 ± 5 a | 23 ± 1 a | 0.575 a |
| MS-1 | 2 | 90% | 65 ± 4 a | 28 ± 3 a | 29 ± 3 b | 0.812 b | |
| MS-2 | 3 | 85% | 70 ± 6 a | 38 ± 2 b | 34 ± 3 b | 1.292 c | |
| MS-3 | 3 | 96% | 68 ± 7 a | 34 ± 2 a | 31 ± 4 b | 1.054 c | |
| MS-4 | 2 | 99% | 75 ± 5 a | 40 ± 3 b | 44 ± 4 c | 1.760 d | |
| MS-5 | 2 | 95% | 73 ± 4 a | 36 ± 5 a | 40 ± 4 c | 1.440 c |
| (100 µL/well) | Zone of Inhibition in mm | |||||
|---|---|---|---|---|---|---|
| Bacillus thuringiensis | Pseudomonas putida | Azotobacter chroococcum | Escherichia coli | Bacillus licheniformis | Rhizobium sp. | |
| B. flexus MS-2 AgNPs | 17 ± 2 a | 8 ± 0.5 a | 5 ± 0.4 a | 7 ± 0.6 a | 7 ± 0.5 a | 7 ± 0.7 a |
| B. cereus MS-3 AgNPs | 5 ± 0.4 b | 9 ± 0.5 a | 7 ± 0.6 a | 5 ± 0.3 a | 10 ± 0.8 a | 9 ± 0.9 a |
| P. aeruginosa MS-5 AgNPs | 21 ± 2 a | 28 ± 2 b | 9 ± 0.8 a | 6 ± 0.5 a | 7 ± 0.6 a | 11 ± 1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Qureshi, K.A.; Jaremko, M.; Rajput, M.; Singh, S.K.; Kaushalendra; Pandey, K.D.; Ferreira, L.F.R.; Kumar, A. Bioprospects of Endophytic Bacteria in Plant Growth Promotion and Ag-Nanoparticle Biosynthesis. Plants 2022, 11, 1787. https://doi.org/10.3390/plants11141787
Singh M, Qureshi KA, Jaremko M, Rajput M, Singh SK, Kaushalendra, Pandey KD, Ferreira LFR, Kumar A. Bioprospects of Endophytic Bacteria in Plant Growth Promotion and Ag-Nanoparticle Biosynthesis. Plants. 2022; 11(14):1787. https://doi.org/10.3390/plants11141787
Chicago/Turabian StyleSingh, Monika, Kamal A. Qureshi, Mariusz Jaremko, Minakshi Rajput, Sandeep Kumar Singh, Kaushalendra, Kapil D. Pandey, Luiz Fernando Romanholo Ferreira, and Ajay Kumar. 2022. "Bioprospects of Endophytic Bacteria in Plant Growth Promotion and Ag-Nanoparticle Biosynthesis" Plants 11, no. 14: 1787. https://doi.org/10.3390/plants11141787
APA StyleSingh, M., Qureshi, K. A., Jaremko, M., Rajput, M., Singh, S. K., Kaushalendra, Pandey, K. D., Ferreira, L. F. R., & Kumar, A. (2022). Bioprospects of Endophytic Bacteria in Plant Growth Promotion and Ag-Nanoparticle Biosynthesis. Plants, 11(14), 1787. https://doi.org/10.3390/plants11141787








