Synergistic Practicing of Rhizobacteria and Silicon Improve Salt Tolerance: Implications from Boosted Oxidative Metabolism, Nutrient Uptake, Growth and Grain Yield in Mung Bean
Abstract
:1. Introduction
2. Results
2.1. Combined Si and PGPR Treatment Positively Affects Plant Growth and Yield under Saline Conditions
2.2. Leaf Mineral Contents Are Enhanced by Si and PGPR Treatment
2.3. Seed Mineral Content Is Enhanced by Si and PGPR Treatment
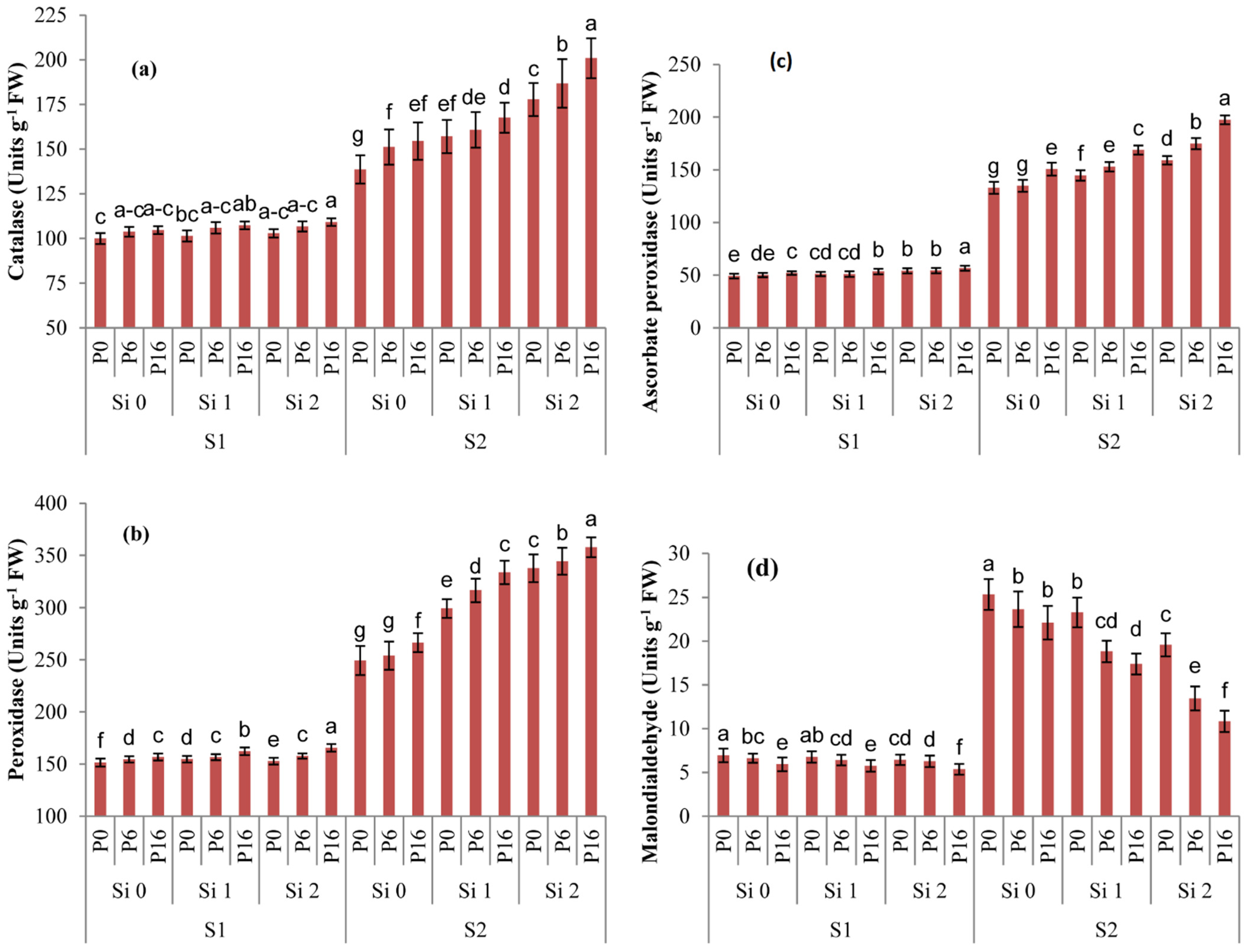
2.4. Changes in Redox Metabolism by Si and PGPR Treatment
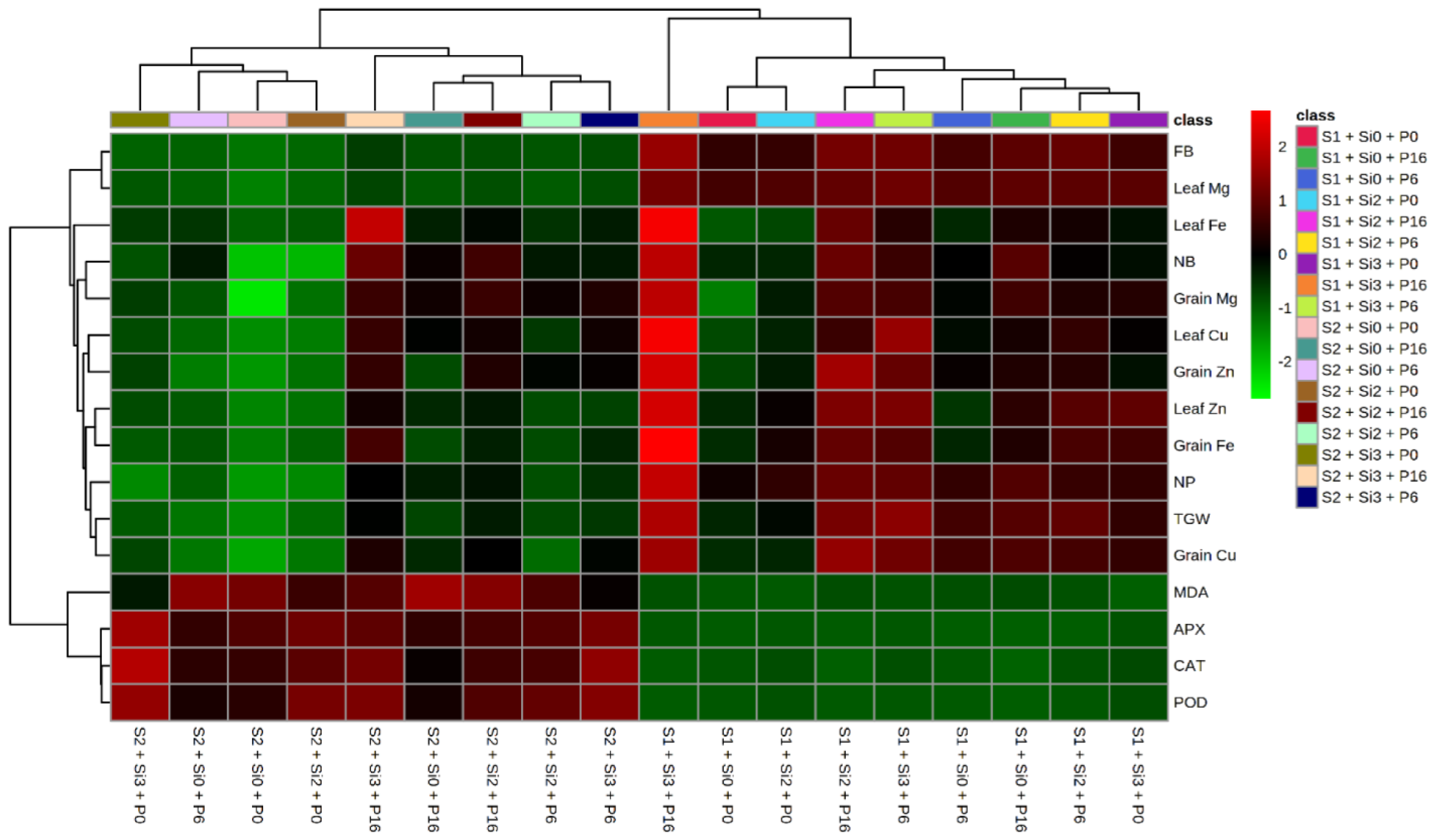
2.5. Heat Map and Correlation Analysis of Mung Bean Traits by Si and PGPR Treatment
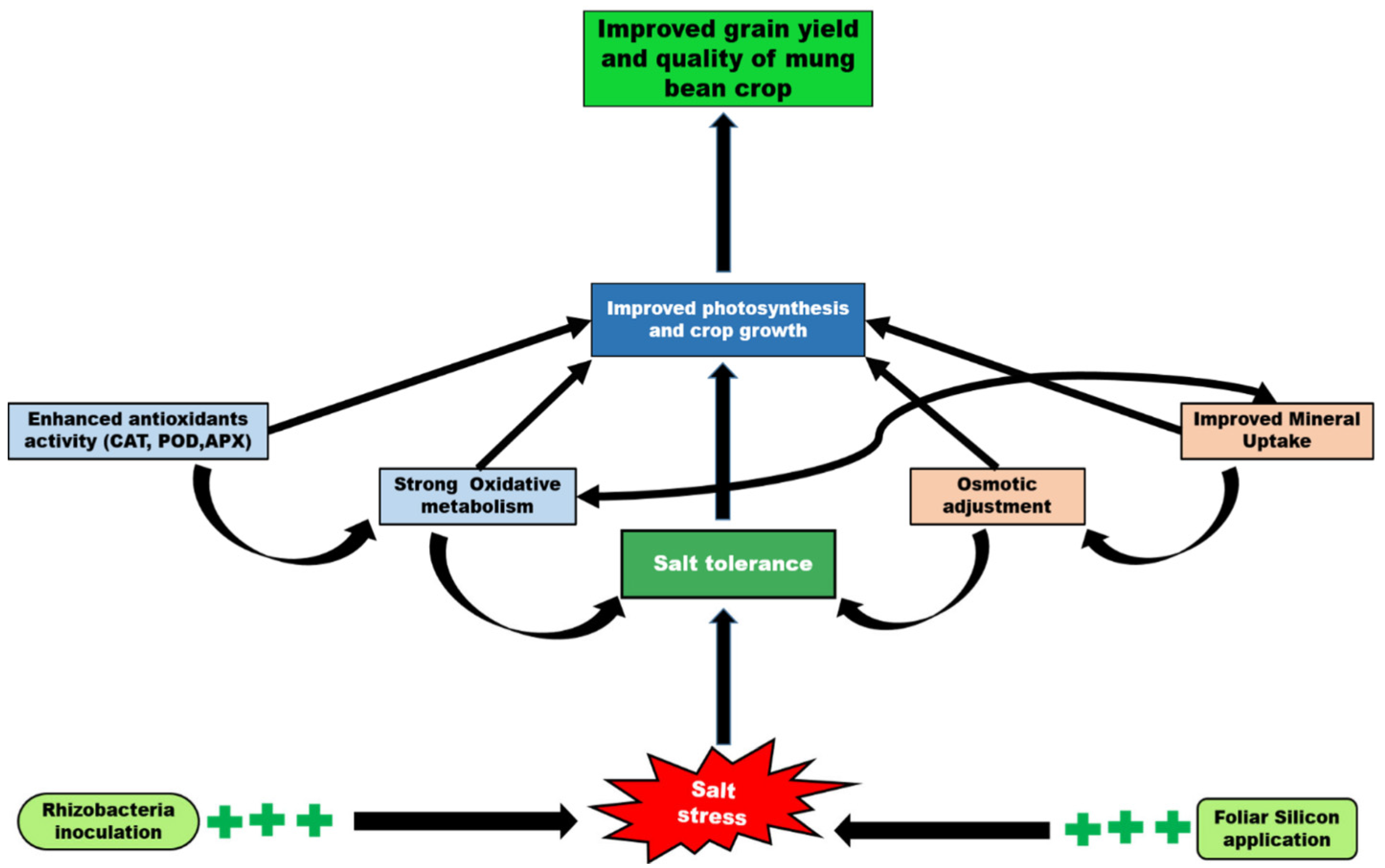
3. Discussion
4. Materials and Methods
4.1. Rhizobacterial Strains and Growth Conditions
4.2. Location and Characteristics of Site
4.3. Details of Field Trials and Treatments
4.4. Measurement of the Agronomic Traits
4.5. Antioxidants
4.6. Determination of Mineral Content
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects, Highlights; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Motos, J.R.; Diaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sanchez-Blanco, M.J.; Hernández, J.A. Physiological and biochemical mechanisms of the ornamental Eugenia myrtifolia L. plants for coping with NaCl stress and recovery. Planta 2015, 242, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wang, Y.; Flowers, T.J.; Gong, H. Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. J. Plant Physiol. 2013, 170, 847–853. [Google Scholar] [CrossRef]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; Rehman, H.; Iqbal, S.; Mahmood, S. Soil drenching of paclobutrazol: An efficient way to improve quinoa performance under salinity. Physiol. Plant 2019, 165, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Bosnic, P.; Bosnic, D.; Jasnic, J.; Nikolic, M. Silicon mediates sodium transport and partitioning in maize under moderate salt stress. Environ. Exp. Bot. 2018, 155, 681–687. [Google Scholar] [CrossRef]
- Suarez, C.; Cardinale, M.; Ratering, S.; Steffens, D.; Jung, S.; Montoya, A.M.Z.; Geissler-Plaum, R.; Schnell, S. Plant growth-promoting effects of Hartmannibacter diazotrophicus on summer barley (Hordeum vulgare L.) under salt stress. Appl. Soil Ecol. 2015, 95, 23–30. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maillard, A.; Ali, N.; Schwarzenberg, A.; Jamois, F.; Yvin, J.; Hosseini, S.A. Silicon transcriptionally regulates sulfur and ABA metabolism and delays leaf senescence in barley under combined sulfur deficiency and osmotic stress. Environ. Exp. Bot. 2018, 155, 394–410. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yu, X.; Li, M.; Lang, D.; Zhang, X.; Xie, Z. Silicon promotes growth and root yield of Glycyrrhiza uralensis under salt and drought stresses through enhancing osmotic adjustment and regulating antioxidant metabolism. Crop Prot. 2018, 107, 1–11. [Google Scholar] [CrossRef]
- Martínez, R.; Espejo, A.; Sierra, M.; Ortiz-Bernad, I.; Correa, D.; Bedmar, E.; López-Jurado, M.; Porres, J.M. Co-inoculation of Halomonas maura and Ensifer meliloti to improve alfalfa yield in saline soils. Appl. Soil Ecol. 2015, 87, 81–86. [Google Scholar] [CrossRef]
- Li, H.; Lei, P.; Pang, X.; Li, S.; Xu, H.; Xu, Z.; Feng, X. Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl. Soil Ecol. 2017, 119, 26–34. [Google Scholar] [CrossRef]
- Pereira, S.I.; Castro, P.M. Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecol. Eng. 2014, 73, 526–535. [Google Scholar] [CrossRef]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016, 7, 876. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, S.; Daur, I.; Hussain, M.B.; Nazir, Q.; Al-Solaimani, S.G.; Ahmad, S.; Bakhashwain, A.A.; Elsafor, A.K. Silicon application and rhizobacterial inoculation regulate mung bean response to saline water irrigation. Clean Soil Air Water 2017, 45, 1600436. [Google Scholar] [CrossRef]
- Chen, H.; Qiao, L.; Wang, L.; Wang, S.; Blair, M.W.; Cheng, X. Assessment of Genetic Diversity and Population Structure of Mung Bean (Vigna radiata) Germplasm Using EST-Based and Genomic SSR Markers. Gene 2015, 566, 175–183. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J. Plant Physiol. 2014, 171, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Arshad, M. The Combined Application of Rhizobial Strains and Plant Growth Promoting Rhizobacteria Improves Growth and Productivity of Mung Bean (Vigna radiata L.) Under Salt-Stressed Conditions. Ann. Microbiol. 2012, 62, 1321–1330. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Lotfi, R. The Impact of Salicylic Acid and Silicon on Chlorophyll a Fluorescence in Mung Bean under Salt Stress. Russ. J. Plant Physiol. 2015, 62, 611–616. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef]
- Hashemi, A.; Abdolzadeh, A.; Sadeghipour, H.R. Beneficial effects of silicon nutrition in alleviating salinity stress in hydroponically grown canola, Brassica napus L., plants. Soil Sci. Plant Nutr. 2010, 56, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Wang, S.; Li, J.; Tanaka, K.; Oka, M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol. Plant 2013, 35, 3099–3107. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.; Jha, P.N. The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015, 184, 57–67. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Zahir, Z.; Mahmood, S.; Nadeem, S.M. Use of plant growth-promoting rhizobacteria to ameliorate the performance of lentil under salinity. J. Appl. Bot. Food Qual. 2019, 92, 179–186. [Google Scholar]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Mateos-Naranjo, E.; Andrades-Moreno, L.; Davy, A.J. Silicon alleviates deleterious effects of high salinity on the halophytic grass Spartina densiflora. Plant Physiol. Biochem. 2013, 63, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Hu, Y.; Han, W.; Gong, H. Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiol. Plant 2015, 37, 71. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Ortuño, M.F.; Nortes, P.A.; Bernavé, A.; Fernández, F.; Sánchez-Blanco, M.J. Effectiveness of bacterial inoculation in alleviation of salinity on water status, mineral content, gas exchange and photosynthetic parameters of Viburnum tinus L. plants. Sci. Hortic. 2018, 237, 303–310. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Yuvakkumar, R.; Rajendran, V.; Prabu, P.; Kannan, N. Effect of nanosilica and silicon sources on plant growth promoting rhizobacteria, soil nutrients and maize seed germination. IET Nanobiotechnol. 2013, 7, 70–77. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Wang, G.; Wang, X.; Guo, J. Germination, osmotic adjustment, and antioxidant enzyme activities of gibberellin-pretreated Picea asperata seeds under water stress. New For. 2010, 39, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Chen, K.; Zhao, Z.; Chen, G.; Zhou, W. Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol. Plant 2008, 52, 592–596. [Google Scholar] [CrossRef]
- Farshidi, M.; Abdolzadeh, A.; Sadeghipour, H.R. Silicon nutrition alleviates physiological disorders imposed by salinity in hydroponically grown canola (Brassica napus L.) plants. Acta Physiol. Plant 2012, 34, 1779–1788. [Google Scholar] [CrossRef]
- Chatterjee, P.; Samaddar, S.; Niinemets, Ü.; Sa, T.M. Brevibacterium linens RS16 confers salt tolerance to Oryza sativa genotypes by regulating antioxidant defense and H+ ATPase activity. Microbiol. Res. 2018, 215, 89–101. [Google Scholar] [CrossRef]
- Gong, Q.; Li, P.; Ma, S.; Indu Rupassara, S.; Bohnert, H.J. Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J. 2005, 44, 826–839. [Google Scholar] [CrossRef]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; ur Rehman, H.; Bilal, H.M. Synergistic consequences of salinity and potassium deficiency in quinoa: Linking with stomatal patterning, ionic relations and oxidative metabolism. Plant Physiol. Biochem. 2021, 159, 17–27. [Google Scholar] [CrossRef]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual; ICARDA: Aleppo, Syria, 2001. [Google Scholar]
- Aebi, H. Catalase in vitro. Meth. Ezymol. 1984, 105, 121–126. [Google Scholar]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Elsevier: Amsterdam, The Netherlands, 1974; pp. 685–690. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill, Inc. Book Co.: New York, NY, USA, 1997; pp. 352–358. [Google Scholar]

| Fresh Biomass t ha−1 | No of Branches Plant−1 | ||||
|---|---|---|---|---|---|
| Si (kg ha−1) | Salinity/PGPR | S1 | S2 | S1 | S2 |
| 0 | Un-inoculated | 18.25 d | 2.25 d | 9.08 d | 6.83 e |
| E. cloacae | 20.13 cd | 4.13 c | 9.59 cd | 9.25 cd | |
| B. drentensis | 22.38 bc | 5.63 b | 10.83 b | 9.75 bc | |
| 1 | Un-inoculated | 18.63 d | 3.75 c | 9.08 d | 7.00 e |
| E. cloacae | 23.25 b | 5.38 b | 9.67 cd | 9.25 cd | |
| B. drentensis | 24.75 b | 5.93 b | 11.08 b | 10.50 ab | |
| 2 | Un-inoculated | 19.38 d | 4.00 c | 9.42 cd | 8.42 d |
| E. cloacae | 24.25 b | 6.13 b | 10.42 bc | 9.25 cd | |
| B. drentensis | 28.00 a | 7.75 a | 12.25 a | 11.08 a | |
| LSD | 2.68 | 0.75 | 1.03 | 0.99 | |
| No of Pods Plant−1 | 1000 Seed Weight (g) | ||||
| 0 | Un-inoculated | 16.83 c | 9.58 e | 55.73 f | 40.13 e |
| E. cloacae | 18.23 bc | 12.06 d | 71.38 cd | 43.65 de | |
| B. drentensis | 19.53 b | 14.87 ab | 73.99 bcd | 51.00 bcd | |
| 1 | Un-inoculated | 18.08 bc | 10.22 e | 59.83 ef | 45.18 cde |
| E. cloacae | 18.44 bc | 12.73 cd | 75.65 bcd | 50.39 bcd | |
| B. drentensis | 20.59 b | 15.31 ab | 79.20 abc | 57.11 ab | |
| 2 | Un-inoculated | 18.18 bc | 10.27 e | 68.70 de | 47.78 cde |
| E. cloacae | 20.25 b | 13.96 bc | 82.38 ab | 52.88 bc | |
| B. drentensis | 24.42 a | 16.02 a | 87.39 a | 60.95 a | |
| LSD | 2.68 | 1.53 | 8.93 | 7.93 | |
| Leaf Mg (mg g−1) | Leaf Zn (mg kg−1) | ||||
|---|---|---|---|---|---|
| Si (kg ha−1) | Salinity/PGPR | S1 | S2 | S1 | S2 |
| 0 | Un-inoculated | 4.86 d | 2.58 c | 100.35 f | 68.86 g |
| E. cloacae | 5.03 b–d | 2.92 abc | 95.75 f | 83.74 e | |
| B. drentensis | 5.15 a–d | 3.03 ab | 128.38 d | 100.76 c | |
| 1 | Un-inoculated | 4.99 cd | 2.88 bc | 116.50 e | 74.92 f |
| E. cloacae | 5.14 abcd | 3.01 ab | 143.08 c | 88.52 d | |
| B. drentensis | 5.22 abc | 3.16 ab | 155.95 b | 105.50 b | |
| 2 | Un-inoculated | 5.12 abcd | 3.07 ab | 145.63 c | 88.34 d |
| E. cloacae | 5.35 ab | 3.19 ab | 155.50 b | 91.40 d | |
| B. drentensis | 5.42 a | 3.27 a | 184.75 a | 119.90 a | |
| LSD | 0.35 | 0.36 | 9.44 | 4.43 | |
| Leaf Fe (mg kg−1) | Leaf Cu (mg kg−1) | ||||
| 0 | Un-inoculated | 66.07 h | 57.17 g | 27.74 f | 23.19 d |
| E. cloacae | 108.70 f | 96.54 d | 31.90 def | 25.77 cd | |
| B. drentensis | 166.24 d | 113.74 c | 34.24 cde | 32.42 ab | |
| 1 | Un-inoculated | 79.80 g | 64.18 f | 30.34 ef | 24.16 d |
| E. cloacae | 159.95 d | 98.50 d | 36.14 cd | 28.81 bc | |
| B. drentensis | 230.54 b | 134.17 b | 36.62 c | 34.05 a | |
| 2 | Un-inoculated | 127.96 e | 90.26 e | 33.05 cde | 27.62 cd |
| E. cloacae | 175.41 c | 118.58 c | 42.93 b | 33.78 a | |
| B. drentensis | 356.07 a | 310.79 a | 49.40 a | 36.38 a | |
| LSD | 8.88 | 5.89 | 4.27 | 4.54 | |
| Seed Mg (mg g−1) | Seed Zn (mg kg−1) | ||||
|---|---|---|---|---|---|
| Si (kg ha−1) | Salinity/PGPR | S1 | S2 | S1 | S2 |
| 0 | Un-inoculated | 1.53 c | 1.34 b | 34.99 h | 25.06 f |
| E. cloacae | 1.74 bc | 1.60 ab | 44.46 ef | 28.20 ef | |
| B. drentensis | 1.86 ab | 1.78 a | 47.02 de | 34.36 d | |
| 1 | Un-inoculated | 1.70 bc | 1.55 ab | 39.97 g | 29.55 e |
| E. cloacae | 1.80 b | 1.77 a | 48.20 d | 42.73 c | |
| B. drentensis | 1.90 ab | 1.85 a | 62.92 b | 47.45 ab | |
| 2 | Un-inoculated | 1.81 ab | 1.64 ab | 41.40 fg | 35.38 d |
| E. cloacae | 1.88 ab | 1.81 a | 55.73 c | 44.21 bc | |
| B. drentensis | 2.08 a | 1.85 a | 69.36 a | 49.66 a | |
| LSD | 0.27 | 0.35 | 3.15 | 4.40 | |
| Seed Fe (mg kg−1) | Seed Cu (mg kg−1) | ||||
| 0 | Un-inoculated | 3.47 e | 0.96 e | 9.31 d | 3.14 f |
| E. cloacae | 3.69 e | 2.21 cd | 14.64 c | 5.55 e | |
| B. drentensis | 5.82 cd | 2.52 c | 15.29 bc | 9.44 c | |
| 1 | Un-inoculated | 5.55 d | 1.76 d | 9.57 d | 5.48 e |
| E. cloacae | 7.09 bc | 2.50 c | 14.87 c | 6.11 e | |
| B. drentensis | 7.93 b | 3.86 b | 18.73 a | 11.40 b | |
| 2 | Un-inoculated | 6.82 bcd | 2.05 cd | 14.01 c | 8.02 d |
| E. cloacae | 7.38 b | 3.69 b | 16.96 b | 11.17 b | |
| B. drentensis | 12.82 a | 7.00 a | 19.28 a | 12.92 a | |
| LSD | 1.45 | 0.48 | 1.69 | 1.15 | |
| Soil | Water | ||
|---|---|---|---|
| Type I | Type II | ||
| Soil texture | Sandy loam | -- | -- |
| Organic matter (%) | 0.63 | -- | -- |
| Total N (%) | 0.037 | -- | -- |
| EC (dS m−1) | 2.78 | 3.12 | 7.81 |
| pH | 7.73 | 7.90 | 7.93 |
| SAR | -- | 6.94 | 16.1 |
| mg kg−1 | mg L−1 | ||
| Na+ | -- | 288.5 | 740.2 |
| Cl− | -- | 74.1 | 165.6 |
| P | 5.8 | 0.14 | 0.13 |
| K+ | 95 | 0.21 | 0.27 |
| Ca2+ | -- | 66.64 | 48.84 |
| Mg2+ | -- | 38.01 | 66.48 |
| TDS | -- | 1983 | 6247 |
| Zn2+ | 2.97 | -- | -- |
| Fe2+ | 1.85 | -- | -- |
| Cu2+ | 1.33 | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, S.; Daur, I.; Yasir, M.; Waqas, M.; Hirt, H. Synergistic Practicing of Rhizobacteria and Silicon Improve Salt Tolerance: Implications from Boosted Oxidative Metabolism, Nutrient Uptake, Growth and Grain Yield in Mung Bean. Plants 2022, 11, 1980. https://doi.org/10.3390/plants11151980
Mahmood S, Daur I, Yasir M, Waqas M, Hirt H. Synergistic Practicing of Rhizobacteria and Silicon Improve Salt Tolerance: Implications from Boosted Oxidative Metabolism, Nutrient Uptake, Growth and Grain Yield in Mung Bean. Plants. 2022; 11(15):1980. https://doi.org/10.3390/plants11151980
Chicago/Turabian StyleMahmood, Sajid, Ihsanullah Daur, Muhammad Yasir, Muhammad Waqas, and Heribert Hirt. 2022. "Synergistic Practicing of Rhizobacteria and Silicon Improve Salt Tolerance: Implications from Boosted Oxidative Metabolism, Nutrient Uptake, Growth and Grain Yield in Mung Bean" Plants 11, no. 15: 1980. https://doi.org/10.3390/plants11151980
APA StyleMahmood, S., Daur, I., Yasir, M., Waqas, M., & Hirt, H. (2022). Synergistic Practicing of Rhizobacteria and Silicon Improve Salt Tolerance: Implications from Boosted Oxidative Metabolism, Nutrient Uptake, Growth and Grain Yield in Mung Bean. Plants, 11(15), 1980. https://doi.org/10.3390/plants11151980







