Valorization of Traditional Italian Walnut (Juglans regia L.) Production: Genetic, Nutritional and Sensory Characterization of Locally Grown Varieties in the Trentino Region
Abstract
:1. Introduction
- (i)
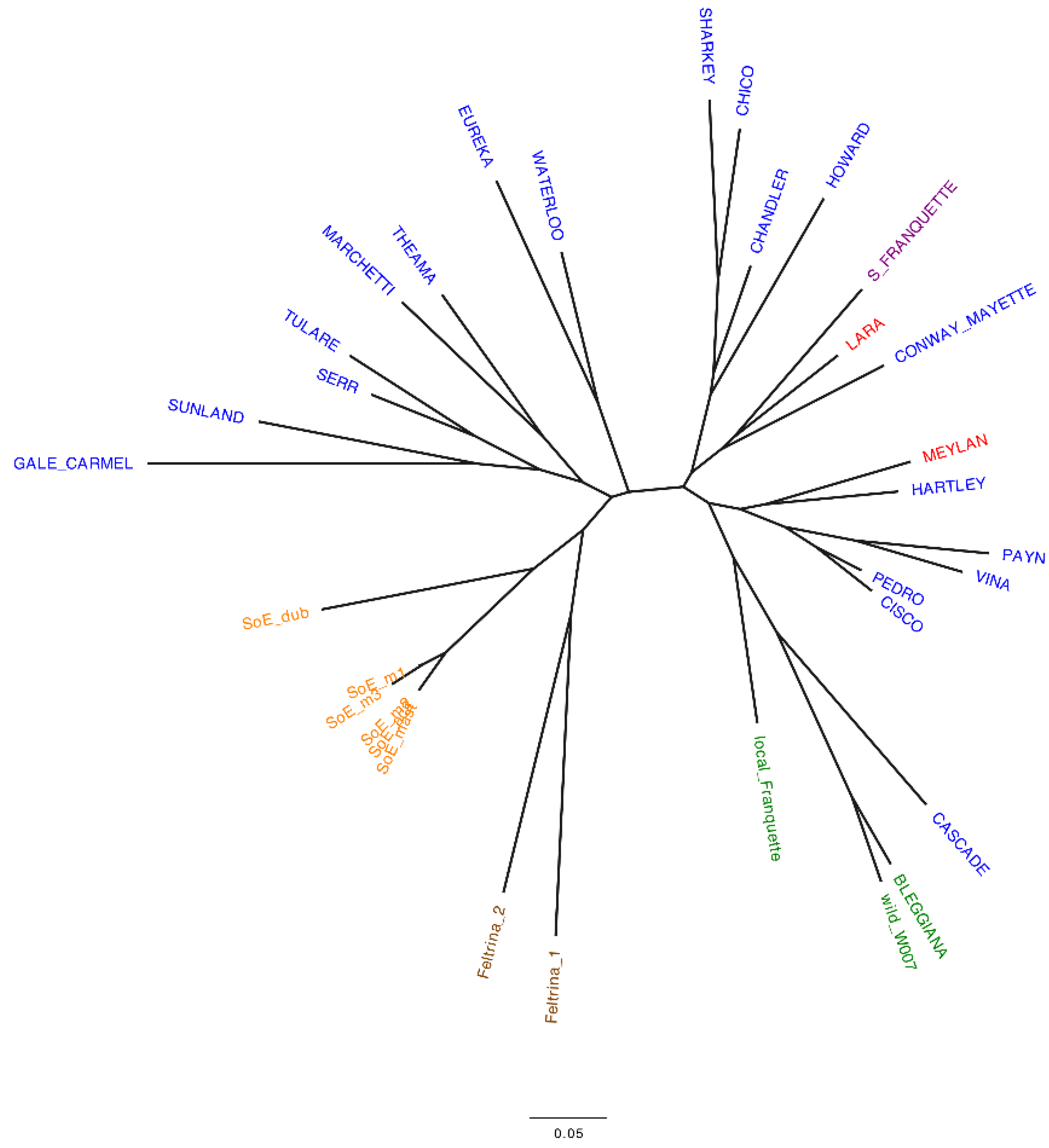
- genetically characterize the ‘Bleggiana’ and local Franquette accessions. Single sequence repeat (SSR) and single nucleotide polymorphism (SNP) markers were used to genotype the local accessions from Trentino and two popular Italian walnut landraces [19,39]. These latter ones are Feltrina, present only in the mountainous area of Feltre in the Veneto region, and Sorrento, spread across the Sorrento peninsula in the Campania region [40,41]. In order to understand how they relate to one another, both locally and globally, international commercial cultivars were also genotyped;
- (ii)
- highlight distinctive nutritional and volatile profiles that uniquely define the walnut ‘Bleggiana’ and local Franquette in comparison to ‘Lara’, a commercial cultivar recently introduced in the same cultivation area to enhance yield;
- (iii)
- explore consumers’ attitudes and preferences through a consumer acceptance test.
2. Results and Discussion
2.1. Italian Walnut Genetic Diversity and Cluster Analysis
2.2. Characterization of Local Walnuts by Metabolic Profiles
2.2.1. Phenolic Compounds and Ellagitannins
2.2.2. Lipid Profile
2.3. Walnut Volatilome Phenotyping by Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry (PTR-ToF-MS)
2.4. Consumer Study
3. Materials and Methods
3.1. Genetic Characterization
3.1.1. Plant Material
3.1.2. SSR and SNP Genotyping
3.1.3. Analysis of Genetic Diversity
3.2. Walnut Metabolite and Volatile Compounds Analysis
3.2.1. Plant Material
3.2.2. Analysis of Phenolic Compounds and Ellagitannins in Walnut Whole Kernels
3.2.3. Nuclear Magnetic Resonance (NMR) Analysis of Lipid Profiles
3.2.4. Metabolic Data Analysis
3.2.5. PTR-ToF-MS Analysis of VOC Profiles
3.3. Consumer Study
Statistical Analysis of Consumer Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGranahan, G.; Leslie, C. Walnuts (Juglans). Acta Hortic. 1991, 290, 907–974. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Ciudad, C.J.; Noé, V.; Izquierdo-Pulido, M. Health Benefits of Walnut Polyphenols: An Exploration beyond Their Lipid Profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hama, J.R.; Omer, R.A.; Rashid, R.S.M.; Mohammad, N.-E.-A.; Thoss, V. The Diversity of Phenolic Compounds along Defatted Kernel, Green Husk and Leaves of Walnut (Juglans regia L.). Anal. Chem. Lett. 2016, 6, 35–46. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive Identification of Walnut Polyphenols by Liquid Chromatography Coupled to Linear Ion Trap–Orbitrap Mass Spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and Quantification of Phenolic Compounds in Kernels, Oil and Bagasse Pellets of Common Walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, L.; Moore, J.; Wu, T.; Wang, Z. Antioxidant Phenolic Compounds from Walnut Kernels (Juglans regia L.). Food Chem. 2009, 113, 160–165. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Kikuchi, M.; Fukuda, T.; Ito, H.; Hatano, T.; Yoshida, T. Walnut Polyphenols Prevent Liver Damage Induced by Carbon Tetrachloride and D-Galactosamine: Hepatoprotective Hydrolyzable Tannins in the Kernel Pellicles of Walnut. J. Agric. Food Chem. 2008, 56, 4444–4449. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yin, R.; Zhang, Q.; Wang, X.; Hu, X.; Gao, Z.; Duan, Z. Chemometrics Analysis on the Content of Fatty Acid Compositions in Different Walnut (Juglans regia L.) Varieties. Eur. Food Res. Technol. 2017, 243, 2235–2242. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L. Bioactive Properties and Chemical Composition of Six Walnut (Juglans regia L.) Cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Maguire, L.S.; O’Sullivan, S.M.; Galvin, K.; O’Connor, T.P.; O’Brien, N.M. Fatty Acid Profile, Tocopherol, Squalene and Phytosterol Content of Walnuts, Almonds, Peanuts, Hazelnuts and the Macadamia Nut. Int. J. Food Sci. Nutr. 2004, 55, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. Alpha-Linolenic Acid. Circulation 2005, 111, 2872–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuccinardi, D.; Farr, O.M.; Upadhyay, J.; Oussaada, S.M.; Klapa, M.I.; Candela, M.; Rampelli, S.; Lehoux, S.; Lázaro, I.; Sala-Vila, A.; et al. Mechanisms Underlying the Cardiometabolic Protective Effect of Walnut Consumption in Obese People: A Cross-over, Randomized, Double-Blind, Controlled Inpatient Physiology Study. Diabetes Obes. Metab. 2019, 21, 2086–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglans regia) Chemical Composition and Research in Human Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef]
- Foroni, I.; Rao, R.; Woeste, K.; Gallitelli, M. Characterisation of Juglans regia L. with SSR Markers and Evaluation of Genetic Relationships among Cultivars and the ‘Sorrento’ Landrace. J. Hortic. Sci. Biotechnol. 2005, 80, 49–53. [Google Scholar] [CrossRef]
- Pollegioni, P.; Woeste, K.; Olimpieri, I.; Marandola, D.; Cannata, F.; Emilia Malvolti, M. Long-Term Human Impacts on Genetic Structure of Italian Walnut Inferred by SSR Markers. Tree Genet. Genomes 2011, 7, 707–723. [Google Scholar] [CrossRef]
- Reisner, Y.; de Filippi, R.; Herzog, F.; Palma, J. Target Regions for Silvoarable Agroforestry in Europe. Ecol. Eng. 2007, 29, 401–418. [Google Scholar] [CrossRef]
- Jarvis, D.I.; Brown, A.H.D.; Cuong, P.H.; Collado-Panduro, L.; Latournerie-Moreno, L.; Gyawali, S.; Tanto, T.; Sawadogo, M.; Mar, I.; Sadiki, M.; et al. A Global Perspective of the Richness and Evenness of Traditional Crop-Variety Diversity Maintained by Farming Communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5326–5331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Verma, R.P.S.; Singh, A.; Kumar Sharma, H.; Devi, G. Barley Landraces: Ecological Heritage for Edaphic Stress Adaptations and Sustainable Production. Environ. Sustain. Indic. 2020, 6, 100035. [Google Scholar] [CrossRef]
- Ficiciyan, A.; Loos, J.; Sievers-Glotzbach, S.; Tscharntke, T. More than Yield: Ecosystem Services of Traditional versus Modern Crop Varieties Revisited. Sustainability 2018, 10, 2834. [Google Scholar] [CrossRef] [Green Version]
- Berni, R.; Cantini, C.; Romi, M.; Hausman, J.-F.; Guerriero, G.; Cai, G. Agrobiotechnology Goes Wild: Ancient Local Varieties as Sources of Bioactives. Int. J. Mol. Sci. 2018, 19, 2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, K. Agrobiodiversity: The Living Library. Nature 2017, 544, S8–S10. [Google Scholar] [CrossRef]
- Borelli, T.; Hunter, D.; Padulosi, S.; Amaya, N.; Meldrum, G.; de Oliveira Beltrame, D.M.; Samarasinghe, G.; Wasike, V.W.; Güner, B.; Tan, A.; et al. Local Solutions for Sustainable Food Systems: The Contribution of Orphan Crops and Wild Edible Species. Agronomy 2020, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Lo Piccolo, E.; Landi, M.; Massai, R.; Remorini, D.; Conte, G.; Guidi, L. Ancient Apple Cultivars from Garfagnana (Tuscany, Italy): A Potential Source for ‘Nutrafruit’ Production. Food Chem. 2019, 294, 518–525. [Google Scholar] [CrossRef]
- Berni, R.; Romi, M.; Parrotta, L.; Cai, G.; Cantini, C. Ancient Tomato (Solanum lycopersicum L.) Varieties of Tuscany Have High Contents of Bioactive Compounds. Horticulturae 2018, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Iannarelli, R.; Caprioli, G.; Sut, S.; Dall’Acqua, S.; Fiorini, D.; Vittori, S.; Maggi, F. Valorizing Overlooked Local Crops in the Era of Globalization: The Case of Aniseed (Pimpinella anisum L.) from Castignano (Central Italy). Ind. Crops Prod. 2017, 104, 99–110. [Google Scholar] [CrossRef]
- Jarvis, D.I.; Hodgkin, T.; Sthapit, B.R.; Fadda, C.; Lopez-Noriega, I. An Heuristic Framework for Identifying Multiple Ways of Supporting the Conservation and Use of Traditional Crop Varieties within the Agricultural Production System. Crit. Rev. Plant Sci. 2011, 30, 125–176. [Google Scholar] [CrossRef] [Green Version]
- Mittempergher, L. La Situazione Varietale Del Noce in Italia. Riv. Ortoflorofruttic. Ital. 1966, 50, 358–369. [Google Scholar]
- Corrado, G.; Forlani, M.; Rao, R.; Basile, B. Diversity and Relationships among Neglected Apricot (Prunus armeniaca L.) Landraces Using Morphological Traits and SSR Markers: Implications for Agro-Biodiversity Conservation. Plants 2021, 10, 1341. [Google Scholar] [CrossRef] [PubMed]
- Manco, R.; Basile, B.; Capuozzo, C.; Scognamiglio, P.; Forlani, M.; Rao, R.; Corrado, G. Molecular and Phenotypic Diversity of Traditional European Plum (Prunus domestica L.) Germplasm of Southern Italy. Sustainability 2019, 11, 4112. [Google Scholar] [CrossRef] [Green Version]
- Perrings, C.; Jackson, L.; Bawa, K.; Brussaard, L.; Brush, S.; Gavin, T.; Papa, R.; Pascual, U.; De Ruiter, P. Biodiversity in Agricultural Landscapes: Saving Natural Capital without Losing Interest. Conserv. Biol. 2006, 20, 263–264. [Google Scholar] [CrossRef]
- Thrupp, L.A. Linking Agricultural Biodiversity and Food Security: The Valuable Role of Agrobiodiversity for Sustainable Agriculture. Int. Aff. 2000, 76, 265–281. [Google Scholar] [CrossRef]
- FAO. FAO Rome Declaration on World Food Security and World Food Summit Plan of Action. In Proceedings of the World Food Summit, Rome, Italy, 13–17 November 1996; Available online: http://www.fao.org/3/w3613e/w3613e00.htm (accessed on 17 September 2021).
- Kumar, A.; Anju, T.; Kumar, S.; Chhapekar, S.S.; Sreedharan, S.; Singh, S.; Choi, S.R.; Ramchiary, N.; Lim, Y.P. Integrating Omics and Gene Editing Tools for Rapid Improvement of Traditional Food Plants for Diversified and Sustainable Food Security. Int. J. Mol. Sci. 2021, 22, 8093. [Google Scholar] [CrossRef] [PubMed]
- Villa, T.C.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and Identifying Crop Landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Foroni, I.; Woeste, K.; Monti, L.M.; Rao, R. Identification of ‘Sorrento’ Walnut Using Simple Sequence Repeats (SSRs). Genet. Resour. Crop Evol. 2007, 54, 1081–1094. [Google Scholar] [CrossRef]
- Marrano, A.; Martínez-García, P.J.; Bianco, L.; Sideli, G.M.; Di Pierro, E.A.; Leslie, C.A.; Stevens, K.A.; Crepeau, M.W.; Troggio, M.; Langley, C.H.; et al. A New Genomic Tool for Walnut (Juglans regia L.): Development and Validation of the High-Density AxiomTM J. regia 700K SNP Genotyping Array. Plant Biotechnol. J. 2018, 17, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Dangl, G.S.; Woeste, K.; Aradhya, M.K.; Koehmstedt, A.; Simon, C.; Potter, D.; Leslie, C.A.; McGranahan, G. Characterization of 14 Microsatellite Markers for Genetic Analysis and Cultivar Identification of Walnut. J. Am. Soc. Hortic. Sci. 2005, 130, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Bernard, A.; Barreneche, T.; Lheureux, F.; Dirlewanger, E. Analysis of Genetic Diversity and Structure in a Worldwide Walnut (Juglans regia L.) Germplasm Using SSR Markers. PLoS ONE 2018, 13, e0208021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, A.; Barreneche, T.; Donkpegan, A.; Lheureux, F.; Dirlewanger, E. Comparison of Structure Analyses and Core Collections for Themanagement of Walnut Genetic Resources. Tree Genet. Genomes 2020, 16, 76. [Google Scholar] [CrossRef]
- Tulecke, W.; McGranahan, G. The Walnut Germplasm Collection of the University of California, Davis; Genetic Resources Conservation Program, Division of Agriculture and Natural Resources, University of California: Davis, CA, USA, 1994. [Google Scholar]
- Germain, E. Inheritance of late leafing and lateral bud fruitfulness in walnut (Juglans regia L.), phenotypic correlations among some traits of the trees. Acta Hortic. 1990, 284, 125–134. [Google Scholar] [CrossRef]
- Pavarini, D.P.; Pavarini, S.P.; Niehues, M.; Lopes, N.P. Exogenous Influences on Plant Secondary Metabolite Levels. Anim. Feed Sci. Technol. 2012, 176, 5–16. [Google Scholar] [CrossRef]
- Salcedo, C.L.; Nazareno, M.A. Effect of Phenolic Compounds on the Oxidative Stability of Ground Walnuts and Almonds. RSC Adv. 2015, 5, 45878–45887. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic Acids, Syringaldehyde, and Juglone in Fruits of Different Cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef] [PubMed]
- Labuckas, D.O.; Maestri, D.M.; Perelló, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from Walnut (Juglans regia L.) Kernels: Antioxidant Activity and Interactions with Proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Segura-Carretero, A.; Caboni, M.F.; Fernández-Gutiérrez, A. Development of a Rapid Method to Determine Phenolic and Other Polar Compounds in Walnut by Capillary Electrophoresis–Electrospray Ionization Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2008, 1209, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, K.; Lyu, S.; Huang, J.; Huang, C.; Xing, Y.; Wang, Y.; Xu, Y.; Li, P.; Hong, J.; et al. Genome-Wide Identification of Tannase Genes and Their Function of Wound Response and Astringent Substances Accumulation in Juglandaceae. Front. Plant Sci. 2021, 12, 664470. [Google Scholar] [CrossRef]
- Germain, E.; Prunet, J.P.; Garcin, A.; Urefv; Centre Technique Interprofessionnel Des Fruits Et Légumes. Le Noyer; CTIFL: Paris, French, 1999; p. 279. ISBN 978-2-87911-104-9. [Google Scholar]
- Colarič, M.; Štampar, F.; Hudina, M.; Solar, A. Sensory Evaluation of Different Walnut Cultivars (Juglans regia L.). Acta Agric. Slov. 2006, 87, 403–414. [Google Scholar]
- Sheng, F.; Hu, B.; Jin, Q.; Wang, J.; Wu, C.; Luo, Z. The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules 2021, 26, 3013. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.; Marhuenda, J.; Zafrilla, P.; Villaño, D.; Martínez-Cachá, A.; Tejada, L.; Cerdá, B.; Mulero, J. High-Performance Liquid Chromatography-Diode Array Detector Determination and Availability of Phenolic Compounds in 10 Genotypes of Walnuts. Int. J. Food Prop. 2017, 20, 1074–1084. [Google Scholar] [CrossRef] [Green Version]
- Joët, T.; Salmona, J.; Laffargue, A.; Descroix, F.; Dussert, S. Use of the Growing Environment as a Source of Variation to Identify the Quantitative Trait Transcripts and Modules of Co-Expressed Genes That Determine Chlorogenic Acid Accumulation. Plant Cell Environ. 2010, 33, 1220–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, K.R. Chlorogenic Acid Biosynthesis. Chemical Synthesis and Properties of the Mono-O-Cinnamoylquinic Acids. Biochemistry 1965, 4, 2719–2731. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic Resources, Chemistry, by-Products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, L.; Rovira, M.; Tronci, C.; Aramini, M.; Drogoudi, P.; Silva, A.P.; Solar, A.; Avanzato, D.; Botta, R.; Valentini, N.; et al. A Multidisciplinary Approach to Enhance the Conservation and Use of Hazelnut Corylus Avellana L. Genetic Resources. Genet. Resour. Crop Evol. 2015, 62, 649–663. [Google Scholar] [CrossRef]
- Čolić, S.D.; Fotirić Akšić, M.M.; Lazarević, K.B.; Zec, G.N.; Gašić, U.M.; Dabić Zagorac, D.Č.; Natić, M.M. Fatty Acid and Phenolic Profiles of Almond Grown in Serbia. Food Chem. 2017, 234, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Di Guardo, M.; Farneti, B.; Khomenko, I.; Modica, G.; Mosca, A.; Distefano, G.; Bianco, L.; Troggio, M.; Sottile, F.; La Malfa, S.; et al. Genetic Characterization of an Almond Germplasm Collection and Volatilome Profiling of Raw and Roasted Kernels. Hortic. Res. 2021, 8, 27. [Google Scholar] [CrossRef]
- Hao, J.; Xu, X.-L.; Jin, F.; Regenstein, J.M.; Wang, F.-J. HS-SPME GC–MS Characterization of Volatiles in Processed Walnuts and Their Oxidative Stability. J. Food Sci. Technol. 2020, 57, 2693–2704. [Google Scholar] [CrossRef]
- Pedrotti, M.; Khomenko, I.; Genova, G.; Castello, G.; Spigolon, N.; Fogliano, V.; Biasioli, F. Quality Control of Raw Hazelnuts by Rapid and Non-Invasive Fingerprinting of Volatile Compound Release. LWT 2021, 143, 111089. [Google Scholar] [CrossRef]
- Xie, C.; Ma, Z.F.; Li, F.; Zhang, H.; Kong, L.; Yang, Z.; Xie, W. Storage Quality of Walnut Oil Containing Lycopene during Accelerated Oxidation. J. Food Sci. Technol. 2018, 55, 1387–1395. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, W.; Chu, F.; Pei, D. Improvement of the Flavor and Oxidative Stability of Walnut Oil by Microwave Pretreatment. J. Am. Oil Chem. Soc. 2016, 93, 1563–1572. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, W.; Chu, F.; Wang, C.; Pei, D. Identification of Volatile Oxidation Compounds as Potential Markers of Walnut Oil Quality. J. Food Sci. 2018, 83, 2745–2752. [Google Scholar] [CrossRef]
- Fischer, M.; Wohlfahrt, S.; Varga, J.; Matuschek, G.; Saraji-Bozorgzad, M.R.; Walte, A.; Denner, T.; Zimmermann, R. Evolution of Volatile Flavor Compounds During Roasting of Nut Seeds by Thermogravimetry Coupled to Fast-Cycling Optical Heating Gas Chromatography-Mass Spectrometry with Electron and Photoionization. Food Anal. Methods 2017, 10, 49–62. [Google Scholar] [CrossRef]
- Elmore, J.S.; Nisyrios, I.; Mottram, D.S. Analysis of the Headspace Aroma Compounds of Walnuts (Juglans regia L.). Flavour Fragr. J. 2005, 20, 501–506. [Google Scholar] [CrossRef]
- Crowe, T.D.; Crowe, T.W.; Johnson, L.A.; White, P.J. Impact of Extraction Method on Yield of Lipid Oxidation Products from Oxidized and Unoxidized Walnuts. J. Am. Oil Chem. Soc. 2002, 79, 453–456. [Google Scholar] [CrossRef]
- Lytou, A.E.; Panagou, E.Z.; Nychas, G.-J.E. Volatilomics for Food Quality and Authentication. Curr. Opin. Food Sci. 2019, 28, 88–95. [Google Scholar] [CrossRef]
- Sinesio, F.; Moneta, E. Sensory Evaluation of Walnut Fruit. Food Qual. Prefer. 1997, 8, 35–43. [Google Scholar] [CrossRef]
- Sinesio, F.; Guerrero, L.; Romero, A.; Moneta, E.; Lombard, J.C. Sensory Evaluation of Walnut: An Interlaboratory Study. Food Sci. Technol. Int. 2001, 7, 37–47. [Google Scholar] [CrossRef]
- Sorrenti, G. Calcagni Giuseppe Situazione internazionale, prospettive e potenzialità della nocicoltura. In Proceedings of the Atti Delle Giornate Tecniche Nazionali SOI NOCE 2019, Bologna, Italy, 20–21 September 2019; pp. 9–14. [Google Scholar] [CrossRef]
- McNeil, D.L.; Smith, B.E.; Gardner, L. Consumer Variety Preferences among New Zealand Walnut Lines. In Proceedings of the Nutrition Society of New Zealand, Canterbury, New Zealand, 1994; Savage, G.P., Ed.; Nutrition Society of New Zealand: Canterbury, New Zealand, 1994; Volume 19, pp. 87–92. [Google Scholar]
- Ruiz-Garcia, L.; Lopez-Ortega, G.; Denia, A.F.; Tomas, D.F. Identification of a Walnut (Juglans regia L.) Germplasm Collection and Evaluation of Their Genetic Variability by Microsatellite Markers. Span. J. Agric. Res. 2011, 9, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R Package for Genetic Analysis of Populations with Clonal, Partially Clonal, and/or Sexual Reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [Green Version]
- Kamvar, Z.N.; Brooks, J.C.; Grünwald, N.J. Novel R Tools for Analysis of Genome-Wide Population Genetic Data with Emphasis on Clonality. Front. Genet. 2015, 6, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradis, E.; Schliep, K. Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Estimation of Average Heterozygosity and Genetic Distance from a Small Number of Individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Bruvo, R.; Michiels, N.K.; D’souza, T.G.; Schulenburg, H. A Simple Method for the Calculation of Microsatellite Genotype Distances Irrespective of Ploidy Level. Mol. Ecol. 2004, 13, 2101–2106. [Google Scholar] [CrossRef]
- Prevosti, A.; Ocaña, J.; Alonso, G. Distances between Populations Of Drosophila Subobscura, Based on Chromosome Arrangement Frequencies. Theor. Appl. Genet. 1975, 45, 231–241. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.3.1. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 19 June 2022).
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A Versatile Targeted Metabolomics Method for the Rapid Quantification of Multiple Classes of Phenolics in Fruits and Beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Vrhovsek, U.; Guella, G.; Mattivi, F. Profiling and Accurate Quantification of Rubus Ellagitannins and Ellagic Acid Conjugates Using Direct UPLC-Q-TOF HDMS and HPLC-DAD Analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kuhn, M.; Wickham, H. Tidymodels: A Collection of Packages for Modeling and Machine Learning Using Tidyverse Principles. 2020. Available online: https://www.tidymodels.org/ (accessed on 3 March 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Package Version 1.0.5. 2017. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 3 March 2022).
- Martin, M.; Legat, B.; Leenders, J.; Vanwinsberghe, J.; Rousseau, R.; Boulanger, B.; Eilers, P.H.C.; De Tullio, P.; Govaerts, B. PepsNMR for 1H NMR Metabolomic Data Pre-Processing. Anal. Chim. Acta 2018, 1019, 1–13. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Grisenti, M.; Ajelli, M.; Betta, E.; Algarra, A.A.; Cappellin, L.; Aprea, E.; Gasperi, F.; Biasioli, F.; et al. Exploring Blueberry Aroma Complexity by Chromatographic and Direct-Injection Spectrometric Techniques. Front. Plant Sci. 2017, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Lê Cao, K.-A.; González, I.; Déjean, S. IntegrOmics: An R Package to Unravel Relationships between Two Omics Datasets. Bioinformatics 2009, 25, 2855–2856. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Guo, Y.; Sheng, Q.; Shyr, Y. Heatmap3: An Improved Heatmap Package with More Powerful and Convenient Features. BMC Bioinform. 2014, 15, P16. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- ISO 8589:2007(En), Sensory Analysis—General Guidance for the Design of Test Rooms. Available online: https://www.iso.org/obp/ui/#iso:std:iso:8589:ed-2:v1:en (accessed on 21 July 2022).
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Food Science Texts Series; Springer: New York, NY, USA, 2013; ISBN 978-1-4615-7843-7. [Google Scholar]
- Peryam, D.R.; Pilgrim, F.J. Hedonic Scale Method of Measuring Food Preferences. Food Technol. 1957, 11, 9–14. [Google Scholar]
- Fisher, R.A. Statistical Methods for Research Workers. In Breakthroughs in Statistics: Methodology and Distribution; Kotz, S., Johnson, N.L., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1992; pp. 66–70. ISBN 978-1-4612-4380-9. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Publishing Company: Reading, MA, USA, 1977; Volume 2. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]








| SSR Locus | N | Ho | Hexp | H | Evenness | Private Alleles 1 |
|---|---|---|---|---|---|---|
| WGA005 | 10 | 0.69 | 0.82 | 1.85 | 0.79 | 267 bp: ‘Bleggiana’ 235 bp: Feltrina_1 271 bp: ‘Howard’ |
| WGA032 | 6 | 0.62 | 0.51 | 0.97 | 0.62 | 172 bp: ‘Lara’ |
| WGA069 | 6 | 0.60 | 0.70 | 1.33 | 0.79 | 163 bp: Feltrina_2 |
| WGA089 | 4 | 0.29 | 0.58 | 0.97 | 0.82 | |
| WGA118 | 3 | 0.71 | 0.60 | 1.00 | 0.86 | |
| WGA202 | 4 | 0.88 | 0.67 | 1.22 | 0.83 | |
| WGA276 | 7 | 0.76 | 0.71 | 1.45 | 0.71 | 181 bp: Feltrina_1 |
| WGA321 | 6 | 0.80 | 0.72 | 1.49 | 0.73 | |
| WGA331 | 4 | 0.40 | 0.59 | 0.98 | 0.83 | 269 bp: SoE_dub |
| WGA332 | 4 | 0.32 | 0.41 | 0.80 | 0.56 | |
| WGA376 | 3 | 0.59 | 0.48 | 0.71 | 0.86 | 244 bp: ‘Cascade’ |
| mean | 5.18 | 0.61 | 0.62 | 1.16 | 0.76 |
| Variety | Mean Acceptability (SD *) | Visual Mean Rank |
|---|---|---|
| Bleggiana | 6.55 (1.70) ab | 408.5 b |
| Chandler | 6.38 (1.72) b | 263.5 |
| Blegette | 6.95 (1.47) a | 321.5 a |
| Lara | 6.84 (1.59) a | 512.5 c |
| SSR | References | Size Range (bp) | Dye | Multiplex |
|---|---|---|---|---|
| WGA005 | [19,76] | 235–273 | FAM | M1 |
| WGA032 | [19,76] | 166–198 | FAM | M2A |
| WGA069 | [40,42,76] | 159–181 | FAM | M3 |
| WGA089 | [40,42,76] | 212–222 | HEX | M2B |
| WGA118 | [40,42,76] | 185–199 | HEX | M3 |
| WGA202 | [40,42,76] | 261–277 | FAM | M3 |
| WGA276 | [40,42,76] | 167–193 | HEX | M2A |
| WGA321 | [40,42,76] | 223–248 | FAM | M2B |
| WGA331 | [42,76] | 269–277 | HEX | M5 |
| WGA332 | [42,76] | 215–227 | FAM | M5 |
| WGA376 | [42,76] | 244–256 | HEX | M5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pierro, E.A.; Franceschi, P.; Endrizzi, I.; Farneti, B.; Poles, L.; Masuero, D.; Khomenko, I.; Trenti, F.; Marrano, A.; Vrhovsek, U.; et al. Valorization of Traditional Italian Walnut (Juglans regia L.) Production: Genetic, Nutritional and Sensory Characterization of Locally Grown Varieties in the Trentino Region. Plants 2022, 11, 1986. https://doi.org/10.3390/plants11151986
Di Pierro EA, Franceschi P, Endrizzi I, Farneti B, Poles L, Masuero D, Khomenko I, Trenti F, Marrano A, Vrhovsek U, et al. Valorization of Traditional Italian Walnut (Juglans regia L.) Production: Genetic, Nutritional and Sensory Characterization of Locally Grown Varieties in the Trentino Region. Plants. 2022; 11(15):1986. https://doi.org/10.3390/plants11151986
Chicago/Turabian StyleDi Pierro, Erica A., Pietro Franceschi, Isabella Endrizzi, Brian Farneti, Lara Poles, Domenico Masuero, Iuliia Khomenko, Francesco Trenti, Annarita Marrano, Urska Vrhovsek, and et al. 2022. "Valorization of Traditional Italian Walnut (Juglans regia L.) Production: Genetic, Nutritional and Sensory Characterization of Locally Grown Varieties in the Trentino Region" Plants 11, no. 15: 1986. https://doi.org/10.3390/plants11151986
APA StyleDi Pierro, E. A., Franceschi, P., Endrizzi, I., Farneti, B., Poles, L., Masuero, D., Khomenko, I., Trenti, F., Marrano, A., Vrhovsek, U., Gasperi, F., Biasioli, F., Guella, G., Bianco, L., & Troggio, M. (2022). Valorization of Traditional Italian Walnut (Juglans regia L.) Production: Genetic, Nutritional and Sensory Characterization of Locally Grown Varieties in the Trentino Region. Plants, 11(15), 1986. https://doi.org/10.3390/plants11151986










