Direct and Indirect Effects of Essential Oils for Sustainable Crop Protection
Abstract
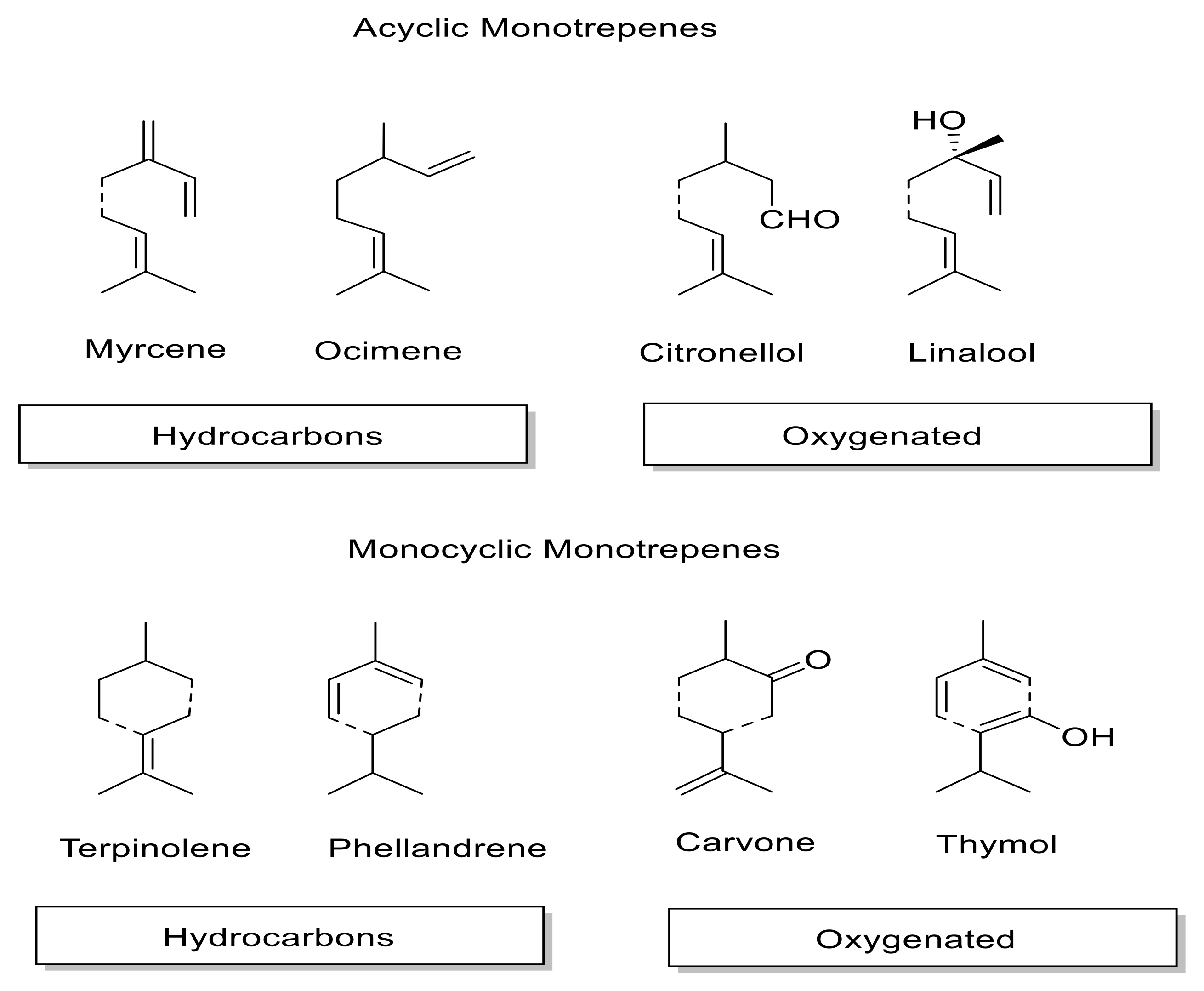
1. Introduction to Essential Oils
2. Cultivation and Domestication of MAPs to Produce EOs
3. EOs in Plant Protection: Direct Effects
3.1. Antifungal Activity
3.2. Nematicidal Activity
3.3. Insecticidal Activity
4. EOs in Plant Protection: Priming Effects
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Tamokou, J.D.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial activities of african medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Academic Press: New York, NY, USA, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Valerio, F.; Mezzapesa, G.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.; Perrino, E. Characterization and Antimicrobial Properties of Essential Oils from Four Wild Taxa of Lamiaceae Family Growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Impact of different storage conditions on the quality of selected essential oils. Food Res. Int. 2012, 46, 341–353. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Aït Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Buckle, J. Essential oils in practice. In Clinical Aromatherapy-E-Book; Churchill Livingstone: Edinburgh, UK, 2014; ISBN 9780702064869. [Google Scholar]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2019, 19, 235–241. [Google Scholar] [CrossRef]
- Hedden, P.; Harrewijn, P.; van Oosten, A.M.; Piron, P.G.M. Natural terpenoids as messengers. A multidisciplinary study of their production, biological functions and practical applications. Ann. Bot. 2002, 90, 299–300. [Google Scholar] [CrossRef]
- Isman, M.B. Bioinsecticides based on plant essential oils: A short overview. Z. Nat. C 2020, 75, 179–182. [Google Scholar] [CrossRef]
- Ravensberg, W.J. Commercialisation of Microbes: Present Situation and Future Prospects. In Principles of Plant-Microbe Interactions, Microbes for Sustainable Agriculture; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 309–317. [Google Scholar] [CrossRef]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Prabha, S.; Yadav, A.; Kumar, A.; Yadav, A.; Yadav, H.K.; Kumar, S.; Yadav, R.S.; Kumar, R. Biopesticides—An alternative and eco-friendly source for the control of pests in agricultural crops. Plant Arch. 2016, 16, 902–906. [Google Scholar]
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Arraiza, M.P.; González-Coloma, A.; Andres, M.F.; Berrocal-Lobo, M.; Domínguez-Núñez, J.A.; Da Costa, A.C.; Navarro-Rocha, J.; Guerrero, C.C. Antifungal Effect of Essential Oils. In Potential of Essential Oils; El-Shemy, H.E., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Sahraoui, A.L.-H. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Andrés, M.F.; González-Coloma, A.; Sanz, J.; Burillo, J.; Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef]
- Laghmouchi, Y.; Belmehdi, O.; Senhaji, N.; Abrini, J. Chemical composition and antibacterial activity of Origanum compactum Benth. essential oils from different areas at northern Morocco. S. Afr. J. Bot. 2018, 115, 120–125. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and Biological Properties of Satureja cuneifolia Ten. and Thymus spinulosus Ten.: Two Wild Officinal Species of Conservation Concern in Apulia (Italy). A Preliminary Survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Abbad, A.; Belaqziz, R.; Bekkouche, K.; Markouk, M. Influence of temperature and water potential on laboratory germination of two Moroccan endemic thymes: Thymus maroccanus Ball. and Thymus broussonetii Boiss. Afr. J. Agri. Res. 2011, 6, 4740–4745. [Google Scholar] [CrossRef]
- Navarro-Rocha, J.; Andrés, M.F.; Díaz, C.E.; Burillo, J.; González-Coloma, A. Composition and biocidal properties of essential oil from pre-domesticated Spanish Satureja montana. Ind. Crop. Prod. 2019, 145, 111958. [Google Scholar] [CrossRef]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crop. Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWRs) Threatened and Endemic to Italy: Urgent Actions for Protection and Use. Biology 2022, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Schippmann, U.; Leaman, D.; Cunningham, A. A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. In Medicinal and Aromatic Plants; Bogers, R.J., Craker, L.E., Lange, D., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 75–95. [Google Scholar]
- Franz, C.; Chizzola, R.; Novak, J.; Sponza, S. Botanical species being used for manufacturing plant food supplements (PFS) and related products in the EU member states and selected third countries. Food Funct. 2011, 2, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.; Novak, J. Sources of Essential Oils from: Handbook of Essential Oils, Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780815370963. [Google Scholar]
- Yousif, L.; Belmehdi, O.; Abdelhakim, B.; Senhaji, N.S.; Abrini, J. Does the domestication of Origanum compactum (Benth) affect its chemical composition and antibacterial activity? Flavour Fragr. J. 2020, 36, 264–271. [Google Scholar] [CrossRef]
- Kitiki, A. Status of cultivation and use of oregano in Turkey. In Proceedings of the IPGRI International Workshop on Oregano, Bari, Italy, 8–12 May 1996; pp. 122–132. [Google Scholar]
- Putievsky, E.; Dudai, N.; Ravid, U. Cultivation, selection, and conservation of oregano species in Israel. In Proceedings of the IPGRI International Workshop on Oregano, Bari, Italy, 8–12 May 1996; pp. 102–109. [Google Scholar]
- Ceylan, A.; Bayram, E.; Geren, H. Investigation on agronomic and quality characteristics of improved clones in Origanum (Origanum onites L.) breeding. Turk. J. Agric. For. 1999, 23, 1163–1168. [Google Scholar]
- Fischer, U.; Franz, C.; Lopez, R.; Pöll, E. Variability of the essential oils of Lippia graveolens HBK from Guatemala. In Essential Oils: Basic and Applied Research; Franz, C., Máthé, A., Buchbauer, A.G., Eds.; Allured Publishing: Carol Stream, IL, USA, 1997. [Google Scholar]
- Grassi, P. Botanical and Chemical Investigations in Hyptis spp. (Lamiaceae) in El Salvador. Ph.D. Thesis, Universität Wien, Vienna, Austria, 2003. [Google Scholar]
- Goehler, I. Domestikation von Medizinalpflanzen und Untersuchungen zur Inkulturnahme von Tagetes lucida Cav. Ph.D. Thesis, Universität für Bodenkultur Wien, Vienna, Austria, 2006. [Google Scholar]
- Julio, L.F.; Burillo, J.; Giménez, C.; Cabrera, R.; Díaz, C.E.; Sanz, J.; Gonzalez-Coloma, A. Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Ind. Crop. Prod. 2015, 76, 787–792. [Google Scholar] [CrossRef]
- Julio, L.F.; Barrero, A.F.; del Pino, M.M.H.; Arteaga, J.F.; Burillo, J.; Andres, M.F.; Díaz, C.E.; González-Coloma, A. Phytotoxic and Nematicidal Components of Lavandula luisieri. J. Nat. Prod. 2016, 79, 261–266. [Google Scholar] [CrossRef]
- Vining, K.J.; Hummer, K.E.; Bassil, N.V.; Lange, B.M.; Khoury, C.K.; Carver, D. Crop Wild Relatives as Germplasm Resource for Cultivar Improvement in Mint (Mentha L.). Front. Plant Sci. 2020, 11, 1217. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Perrino, E.V.; Musarella, C.M.; Magazzini, P. Management of grazing Italian river buffalo to preserve habitats defined by Directive 92/43/EEC in a protected wetland area on the Mediterranean coast: Palude Frattarolo, Apulia, Italy. Euro-Mediterr. J. Environ. Integr. 2021, 6, 32. [Google Scholar] [CrossRef]
- Duduk, N.; Markovic, T.; Vasic, M.; Duduk, B.; Vico, I.; Obradovic, A. Antifungal Activity of Three Essential Oils against Colltotrichum acutatum, the Causal Agent of Strawberry Anthracnose. J. Essent. Oil Bear. Plants 2015, 18, 529–537. [Google Scholar] [CrossRef]
- Hong, J.K.; Yang, H.J.; Jung, H.; Yoon, D.J.; Sang, M.K.; Jeun, Y.C. Application of Volatile Antifungal Plant Essential Oils for Controlling Pepper Fruit Anthracnose by Colletotrichum gloeosporioides. Plant Pathol. J. 2015, 31, 269–277. [Google Scholar] [CrossRef]
- Karimi, K.; Arzanlou, M.; Pertot, I. Antifungal activity of the dill (Anethum graveolens L.) seed essential oil against strawberry anthracnose under in vitro and in vivo conditions. Arch. Phytopathol. Plant Prot. 2016, 49, 554–566. [Google Scholar] [CrossRef]
- Amin, J.E.P.; Cuca, L.E.; González-Coloma, A. Antifungal and phytotoxic activity of benzoic acid derivatives from inflorescences of Piper cumanense. Nat. Prod. Res. 2021, 35, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Kalhoro, M.T.; Zhang, H.; Kalhoro, G.M.; Wang, F.; Chen, T.; Faqir, Y.; Nabi, F. Fungicidal properties of ginger (Zingiber officinale) essential oils against Phytophthora colocasiae. Sci. Rep. 2022, 12, 2191. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, M.; Huang, T.; Yang, K.; Zhou, S.; Li, Y.; Tian, J. Antifungal effect of nerol via transcriptome analysis and cell growth repression in sweet potato spoilage fungi Ceratocystis fimbriata. Postharvest Biol. Technol. 2020, 171, 111343. [Google Scholar] [CrossRef]
- de Billerbeck, V.G.; Roques, C.G.; Bessière, J.-M.; Fonvieille, J.-L.; Dargent, R. Effects of Cymbopogon nardus (L.) W. Watson essential oil on the growth and morphogenesis of Aspergillus niger. Can. J. Microbiol. 2001, 47, 9–17. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula, E.S.A.C.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Reuveni, M.; Sanches, E.; Barbier, M. Curative and Suppressive Activities of Essential Tea Tree Oil against Fungal Plant Pathogens. Agronomy 2020, 10, 609. [Google Scholar] [CrossRef]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Shahina, Z.; El-Ganiny, A.M.; Minion, J.; Whiteway, M.; Sultana, T.; Dahms, T.E.S. Cinnamomum zeylanicum bark essential oil induces cell wall remodelling and spindle defects in Candida albicans. Fungal Biol. Biotechnol. 2018, 5, 3. [Google Scholar] [CrossRef]
- Jensen-Pergakes, K.L.; Kennedy, M.A.; Lees, N.D.; Barbuch, R.; Koegel, C.; Bard, M. Sequencing, Disruption, and Characterization of the Candida albicans Sterol Methyltransferase (ERG6) Gene: Drug Susceptibility Studies in erg6 Mutants. Antimicrob. Agents Chemother. 1998, 42, 1160–1167. [Google Scholar] [CrossRef]
- Ouyang, Q.; Tao, N.; Jing, G. Transcriptional profiling analysis of Penicillium digitatum, the causal agent of citrus green mold, unravels an inhibited ergosterol biosynthesis pathway in response to citral. BMC Genom. 2016, 17, 599. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-J.; Stahl, T.; Hu, Y.; Kassie, F.; Mersch-Sundermann, V. The Production of Reactive Oxygen Species and the Mitochondrial Membrane Potential Are Modulated during Onion Oil–Induced Cell Cycle Arrest and Apoptosis in A549 Cells. J. Nutr. 2006, 136, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Keereedach, P.; Hrimpeng, K.; Boonbumrung, K. Antifungal Activity of Thai Cajuput Oil and Its Effect on Efflux-Pump Gene Expression in Fluconazole-Resistant Candida albicans Clinical Isolates. Int. J. Microbiol. 2020, 2020, 5989206. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.M.; Williamson, V.M.; Abad, P.; McCarter, J.; Danchin, E.G.; Castagnone-Sereno, P.; Opperman, C.H. The Genomes of Root-Knot Nematodes. Annu. Rev. Phytopathol. 2009, 47, 333–351. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Sena, I.; Ribeiro, B.; Rodrigues, A.M.; Maleita, C.M.N.; Abrantes, I.; Bennett, R.N.; Mota, M.; Figueiredo, A.C.D.S. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J. Pest Sci. 2015, 89, 207–217. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Ko, H.-R.; Kim, S.-J.; Lee, J.-K. Chemical Compositions and Nematicidal Activities of Essential Oils on Meloidogyne hapla (Nematoda: Tylenchida) Under Laboratory Conditions. Korean J. Pestic. Sci. 2016, 20, 30–34. [Google Scholar] [CrossRef]
- Patidar, R.K.; Sen, D.; Pathak, M.; Shakywar, R. Effect of essential oils on mortality, hatching and multiplication of root-knot nematode, Meloidogyne incognita and its Impact on plant growth parameters. Int. J. Agric. Environ. Biotechnol. 2016, 9, 887–895. [Google Scholar] [CrossRef]
- Ozdemir, E.; Gozel, U. Efficiency of Some Plant Essential Oils on Root-Knot Nematode Meloidogyne incognita. J. Agric. Sci. Technol. A 2017, 7, 178–183. [Google Scholar] [CrossRef]
- Ozdemir, E.; Gozel, U. Nematicidal activities of essential oils against Meloidogyne incognita on tomato plant. Fresenius Environ. Bull. 2018, 27, 4511–4517. [Google Scholar] [CrossRef]
- Barros, A.; Campos, V.; de Paula, L.; Oliveira, D.; de Silva, F.; Terra, W.; Silva, G.H.; Salimena, J.P. Nematicidal screening of essential oils and potent toxicity of Dysphania ambrosioides essential oil against Meloidogyne incognita in vitro and in vivo. J. Phytopathol. 2019, 167, 380–389. [Google Scholar] [CrossRef]
- Eloh, K.; Kpegba, K.; Sasanelli, N.; Koumaglo, H.K.; Caboni, P. Nematicidal activity of some essential plant oils from tropical West Africa. Int. J. Pest Manag. 2019, 66, 131–141. [Google Scholar] [CrossRef]
- Felek, A.; Ozcan, M.; Akyazi, F. Effects of essential oils distilled from some medicinal and aromatic plants against root knot nematode (Meloidogyne hapla). J. Appl. Sci. Environ. Manag. 2019, 23, 1425. [Google Scholar] [CrossRef]
- Keerthiraj, M.; Mandal, A.; Dutta, T.K.; Saha, S.; Dutta, A.; Singh, A.; Kundu, A. Nematicidal and Molecular Docking Investigation of Essential Oils from Pogostemon cablin Ecotypes against Meloidogyne incognita. Chem. Biodivers. 2021, 18, e2100320. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Dutta, A.; Mandal, A.; Negi, L.; Malik, M.; Puramchatwad, R.; Antil, J.; Singh, A.; Rao, U.; Saha, S.; et al. A Comprehensive in vitro and in silico Analysis of Nematicidal Action of Essential Oils. Front. Plant Sci. 2021, 11, 614143. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Isman, M.B.; Tak, J.H. Inhibition of acetylcholinesterase by essential oils and monoterpenoids: A relevant mode of action for insecticidal essential oils? Biopestic. Int. 2017, 13, 71–78. [Google Scholar] [CrossRef]
- Tak, J.-H.; Isman, M.B. Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crop. Prod. 2017, 108, 786–792. [Google Scholar] [CrossRef]
- Peter, R.; Josende, M.E.; da Silva Barreto, J.; da Costa Silva, D.G.; da Rosa, C.E.; Maciel, F.E. Effect of Illicium verum (Hook) essential oil on cholinesterase and locomotor activity of Alphitobius diaperinus (Panzer). Pestic. Biochem. Physiol. 2022, 181, 105027. [Google Scholar] [CrossRef]
- Oviedo-Sarmiento, J.S.; Cortes, J.J.B.; Ávila, W.A.D.; Suárez, L.E.C.; Daza, E.H.; Patiño-Ladino, O.J.; Prieto-Rodríguez, J.A. Fumigant toxicity and biochemical effects of selected essential oils toward the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Pestic. Biochem. Physiol. 2021, 179, 104941. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Price, D.N.; Berry, M.S. Comparison of effects of octopamine and insecticidal essential oils on activity in the nerve cord, foregut, and dorsal unpaired median neurons of cockroaches. J. Insect Physiol. 2006, 52, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABA(A) receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Coats, J.R. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl− uptake in American cockroach ventral nerve cord. Pestic. Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Tong, F.; Gross, A.D.; Dolan, M.C.; Coats, J.R. The phenolic monoterpenoid carvacrol inhibits the binding of nicotine to the housefly nicotinic acetylcholine receptor. Pest Manag. Sci. 2012, 69, 775–780. [Google Scholar] [CrossRef]
- Tak, J.-H.; Isman, M.B. Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Sci. Rep. 2015, 5, 12690. [Google Scholar] [CrossRef]
- Tak, J.-H.; Isman, M.B. Metabolism of citral, the major constituent of lemongrass oil, in the cabbage looper, Trichoplusia ni, and effects of enzyme inhibitors on toxicity and metabolism. Pestic. Biochem. Physiol. 2016, 133, 20–25. [Google Scholar] [CrossRef]
- Tak, J.-H.; Jovel, E.; Isman, M.B. Effects of rosemary, thyme and lemongrass oils and their major constituents on detoxifying enzyme activity and insecticidal activity in Trichoplusia ni. Pestic. Biochem. Physiol. 2017, 140, 9–16. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, J.; Tak, J.-H. Synergistic mechanism of insecticidal activity in basil and mandarin essential oils against the tobacco cutworm. J. Pest Sci. 2021, 94, 1119–1131. [Google Scholar] [CrossRef]
- Feng, R.; Isman, M.B. Selection for resistance to azadirachtin in the green peach aphid, Myzus persicae. Experientia 1995, 51, 831–833. [Google Scholar] [CrossRef]
- Akhtar, Y.; Isman, M.B. Binary mixtures of feeding deterrents mitigate the decrease in feeding deterrent response to antifeedants following prolonged exposure in the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Chemoecology 2003, 13, 177–182. [Google Scholar] [CrossRef]
- Akhtar, Y.; Pages, E.; Stevens, A.; Bradbury, R.; da Camara, C.A.G.; Isman, M.B. Effect of chemical complexity of essential oils on feeding deterrence in larvae of the cabbage looper. Physiol. Entomol. 2012, 37, 81–91. [Google Scholar] [CrossRef]
- Renoz, F.; Demeter, S.; Degand, H.; Nicolis, S.C.; Lebbe, O.; Martin, H.; Deneubourg, J.; Fauconnier, M.-L.; Morsomme, P.; Hance, T. The modes of action of Mentha arvensis essential oil on the granary weevil Sitophilus granarius revealed by a label-free quantitative proteomic analysis. J. Pest Sci. 2021, 95, 381–395. [Google Scholar] [CrossRef]
- Gaire, S.; Zheng, W.; Scharf, M.E.; Gondhalekar, A.D. Plant essential oil constituents enhance deltamethrin toxicity in a resistant population of bed bugs (Cimex lectularius L.) by inhibiting cytochrome P450 enzymes. Pestic. Biochem. Physiol. 2021, 175, 104829. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.; Gonzalez-Coloma, A.; Prigent-Combaret, C. Plant metabolomics to the benefit of crop protection and growth stimulation. In Advances in Botanical Research; Academic Press: New York, NY, USA, 2021; pp. 107–132. [Google Scholar] [CrossRef]
- Mustafa, G.; Masood, S.; Ahmed, N.; Saboor, A.; Ahmad, S.; Hussain, S.; Bilal, M.; Ali, M.A. Seed priming for disease resistance in plants. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Spadaro, D.; Herforth-Rahmé, J.; van der Wolf, J. Organic seed treatments of vegetables to prevent seedborne diseases. Acta Hortic. 2017, 1, 23–32. [Google Scholar] [CrossRef]
- Banani, H.; Olivieri, L.; Santoro, K.; Garibaldi, A.; Gullino, M.L.; Spadaro, D. Thyme and savory essential oil efficacy and induction of resistance against Botrytis Cinerea through priming of defense responses in apple. Foods 2018, 7, 11. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme essential oil as a defense inducer of tomato against gray mold and Fusarium wilt. Plant Physiol. Biochem. 2015, 94, 35–40. [Google Scholar] [CrossRef]
- Cueva, F.D.; Balendres, M.A. Efficacy of citronella essential oil for the management of chilli anthracnose. Eur. J. Plant Pathol. 2018, 152, 461–468. [Google Scholar] [CrossRef]
- Danh, L.T.; Giao, B.T.; Duong, C.T.; Nga, N.T.T.; Tien, D.T.K.; Tuan, N.T.; Cam-huong, B.T.; Nhan, T.C.; Trang, D.T.X. Use of essential oils for the control of anthracnose disease caused by Colletotrichum acutatum on post-harvest mangoes of cat hoa loc variety. Membranes 2021, 11, 719. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef]
- Gerefa, S.; Satheesh, N.; Berecha, G. Effect of essential oils treatment on anthracnose (Colletotrichum Gloeosporioides) disease development, quality and shelf life of mango fruits (Mangifera Indica L.). Am.-Eurasian J. Agric. Environ. Sci. 2015, 15, 2160–2169. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol. Agric. Hortic. 2017, 34, 18–26. [Google Scholar] [CrossRef]
- Vilaplana, R.; Pazmiño, L.; Valencia-Chamorro, S. Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biol. Technol. 2018, 138, 56–63. [Google Scholar] [CrossRef]
- Hosseini, S.; Amini, J.; Saba, M.K.; Karimi, K.; Pertot, I. Preharvest and Postharvest Application of Garlic and Rosemary Essential Oils for Controlling Anthracnose and Quality Assessment of Strawberry Fruit during Cold Storage. Front. Microbiol. 2020, 11, 1855. [Google Scholar] [CrossRef]
- Eke, P.; Adamou, S.; Fokom, R.; Dinango, V.N.; Tsouh Fokou, P.V.; Nana Wakam, L.; Nwaga, D.; Boyom, F.F. Arbuscular mycorrhizal fungi alter antifungal potential of lemongrass essential oil against Fusarium Solani, causing root rot in common bean (Phaseolus vulgaris L.). Heliyon 2020, 6, e05737. [Google Scholar] [CrossRef]
- Sukegawa, S.; Shiojiri, K.; Higami, T.; Suzuki, S.; Arimura, G.-I. Pest management using mint volatiles to elicit resistance in soy: Mechanism and application potential. Plant J. 2018, 96, 910–920. [Google Scholar] [CrossRef]
- Rienth, M.; Crovadore, J.; Ghaffari, S.; Lefort, F. Oregano essential oil vapour prevents Plasmopara viticola infection in grapevine (Vitis Vinifera) and primes plant immunity mechanisms. PLoS ONE 2019, 14, e0222854. [Google Scholar] [CrossRef]
- Soudani, S.; Poza-Carrión, C.; de la Cruz-Gómez, N.; González-Coloma, A.; Andrés, M.F.; Berrocal-labo, M. Essential Oils Prime Epigenetic and Metabolomic Changes in Tomato Defense Against Fusarium oxysporum. Front. Plant Sci. 2022, 13, 4104. [Google Scholar] [CrossRef]
| Phytopathogen | Plant Essential Oil | Primed Plant Crop | Reference |
|---|---|---|---|
| Botrytis cinerea | Satureja hortensis Thymus capitatus T. vulgaris, | Apple, Tomato | [90,91] |
| Colletotrichum acutatum | Cinnamomum verum, Citronella sp. Cymbopogon citratus Ocimum basilicum T. vulgaris, | Chili, Mango, Strawberry | [41,92,93] |
| C. gloeosporioides | C. verum S. hortensis T. vulgaris Zingiber officinale | Avocado, Mango, Papaya, Pepper fruit | [42,94,95,96] |
| C. musae | T. vulgaris | Bananas | [97] |
| C. nymphaeae | Allium sativum Anethum graveolens Rosmarinus officinalis | Strawberry | [43,98] |
| Fusarium wilt | T. capitatus | Tomato | [91] |
| F. solani | C. citratus | Bean | [99] |
| Mycosphaerella fijiensis | Melaleuca alternifolia | Bananas | [49] |
| Phakopsora pachyrhizi | Mentha piperita | Soybean | [100] |
| Plasmopara viticola | T. vulgaris Origanum vulgare | Grapevine | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesraoui, S.; Andrés, M.F.; Berrocal-Lobo, M.; Soudani, S.; Gonzalez-Coloma, A. Direct and Indirect Effects of Essential Oils for Sustainable Crop Protection. Plants 2022, 11, 2144. https://doi.org/10.3390/plants11162144
Kesraoui S, Andrés MF, Berrocal-Lobo M, Soudani S, Gonzalez-Coloma A. Direct and Indirect Effects of Essential Oils for Sustainable Crop Protection. Plants. 2022; 11(16):2144. https://doi.org/10.3390/plants11162144
Chicago/Turabian StyleKesraoui, Sabrina, Maria Fe Andrés, Marta Berrocal-Lobo, Serine Soudani, and Azucena Gonzalez-Coloma. 2022. "Direct and Indirect Effects of Essential Oils for Sustainable Crop Protection" Plants 11, no. 16: 2144. https://doi.org/10.3390/plants11162144
APA StyleKesraoui, S., Andrés, M. F., Berrocal-Lobo, M., Soudani, S., & Gonzalez-Coloma, A. (2022). Direct and Indirect Effects of Essential Oils for Sustainable Crop Protection. Plants, 11(16), 2144. https://doi.org/10.3390/plants11162144









