Monoterpenoids Evolution and MEP Pathway Gene Expression Profiles in Seven Table Grape Varieties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Monoterpenoids Content, Flavour Contribution, Variety and Season Effect
2.2. Evolution of Monoterpenoids during Berry Development
2.3. Transcript Level of MEP Pathway Key Genes during Development
2.4. Correlation of Genes Expression and Monoterpenoids Accumulation
2.5. The Correlation of the Genotype snp1822 in VvDXS1 to the Composition of Monoterpenoids
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Sample Collection
3.3. Extraction of Volatiles
3.4. Identification and Quantitation of Volatiles
3.5. Extraction of Total RNA and Analysis by Real-Time qPCR
3.6. Genotyping of the snp1822
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, M.-X.; Jin, X.-Q.; Yao, H.; Zhu, T.-Y.; Guo, S.-H.; Li, S.; Lei, Y.-L.; Xing, Z.-G.; Zhao, X.-H.; Xu, T.-F.; et al. Evolution of volatile profile and aroma potential of ‘Gold Finger’ table grapes during berry ripening. J. Sci. Food Agric. 2022, 102, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, J.; Xu, X.; Perl, A.; Chen, S.; Ma, H. Adoption of table grape cultivars: An attribute preference study on Chinese grape growers. Sci. Hortic. 2017, 216, 66–75. [Google Scholar] [CrossRef]
- Lin, J.; Massonnet, M.; Cantu, D. The genetic basis of grape and wine aroma. Hortic. Res. 2019, 6, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, W.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Evolution of volatile compounds during the development of Muscat grape ‘Shine Muscat’ (Vitis labrusca × V. vinifera). Food Chem. 2020, 309, 125778. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669. [Google Scholar] [CrossRef]
- Magnard, J.-L.; Roccia, A.; Caissard, J.-C.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Hibrand-Saint Oyant, L.; Jullien, F.; Nicolè, F.; et al. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef]
- Luan, F.; Wüst, M. Differential incorporation of 1-deoxy-d-xylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp. Phytochemistry 2002, 60, 451–459. [Google Scholar] [CrossRef]
- Liu, B.; Xu, X.-Q.; Cai, J.; Lan, Y.-B.; Zhu, B.-Q.; Wang, J. The free and enzyme-released volatile compounds of distinctive Vitis amurensis var. Zuoshanyi grapes in China. Eur. Food Res. Technol. 2015, 240, 985–997. [Google Scholar] [CrossRef]
- Zhang, P.; Fuentes, S.; Siebert, T.; Krstic, M.; Herderich, M.; Barlow, E.W.R.; Howell, K. Terpene evolution during the development of Vitis vinifera L. cv. Shiraz grapes. Food Chem. 2016, 204, 463–474. [Google Scholar] [CrossRef]
- He, L.; Xu, X.-Q.; Wang, Y.; Chen, W.-K.; Sun, R.-Z.; Cheng, G.; Liu, B.; Chen, W.; Duan, C.-Q.; Wang, J.; et al. Modulation of volatile compound metabolome and transcriptome in grape berries exposed to sunlight under dry-hot climate. BMC Plant Biol. 2020, 20, 59–76. [Google Scholar] [CrossRef]
- Xi, X.; Zha, Q.; He, Y.; Tian, Y.; Jiang, A. Influence of cluster thinning and girdling on aroma composition in ‘Jumeigui’ table grape. Sci. Rep. 2020, 10, 6877–6879. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Q.; Liu, B.; Zhu, B.-Q.; Lan, Y.-B.; Gao, Y.; Wang, D.; Reeves, M.J.; Duan, C.-Q. Differences in volatile profiles of cabernet sauvignon grapes grown in two distinct regions of china and their responses to weather conditions. Plant Physiol. Biochem. 2015, 89, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hjelmeland, A.K.; Ebeler, S.E. Glycosidically bound volatile aroma compounds in grapes and wine: A review. Am. J. Enol. Vitic. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Voirin, S.G.; Baumes, R.L.; Bitteur, S.M.; Gunata, Z.Y.; Bayonove, C.L. Novel monoterpene disaccharide glycosides of Vitis vinifera grapes. J. Agric. Food Chem. 1990, 38, 1373–1378. [Google Scholar] [CrossRef]
- Wilson, B.; Strauss, C.R.; Williams, P.J. The distribution of free and glycosidically-bound monoterpenes among skin, juice, and pulp fractions of some white grape varieties. Am. J. Enol. Vitic. 1986, 37, 107–111. [Google Scholar]
- Matarese, F.; Scalabrelli, G.; D’Onofrio, C. Analysis of the expression of terpene synthase genes in relation to aroma content in two aromatic Vitis vinifera varieties. Funct. Plant Biol. 2013, 40, 552–565. [Google Scholar] [CrossRef]
- Chappell, J. The biochemistry and molecular biology of isoprenoid metabolism. Plant Physiol. 1995, 107, 1–6. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Battilana, J.; Costantini, L.; Emanuelli, F.; Sevini, F.; Segala, C.; Moser, S.; Velasco, R.; Versini, G.; Grando, M.S. The 1-deoxy-d-xylulose 5-phosphate synthase gene co-localizes with a major QTL affecting monoterpene content in grapevine. Theor. Appl. Genet. 2009, 118, 653–669. [Google Scholar] [CrossRef]
- Emanuelli, F.; Sordo, M.; Lorenzi, S.; Battilana, J.; Grando, M.S. Development of user-friendly functional molecular markers for VvDXS gene conferring muscat flavor in grapevine. Mol. Breed. 2014, 33, 235–241. [Google Scholar] [CrossRef]
- Costantini, L.; Kappel, C.D.; Trenti, M.; Battilana, J.; Emanuelli, F.; Sordo, M.; Moretto, M.; Camps, C.; Larcher, R.; Delrot, S.; et al. Drawing Links from transcriptome to metabolites: The evolution of aroma in the ripening berry of moscato bianco (Vitis vinifera L.). Acta Hortic. 2017, 1046, 493–498. [Google Scholar] [CrossRef]
- Wen, Y.-Q.; Zhong, G.-Y.; Gao, Y.; Lan, Y.-B.; Duan, C.-Q.; Pan, Q.-H. Using the combined analysis of transcripts and metabolites to propose key genes for differential terpene accumulation across two regions. BMC Plant Biol. 2015, 15, 240–251. [Google Scholar] [CrossRef]
- Wang, W.; Feng, J.; Wei, L.; Khalil-Ur-Rehman, M.; Nieuwenhuizen, N.J.; Yang, L.; Zheng, H.; Tao, J. Transcriptomics Integrated with free and bound terpenoid aroma profiling during “Shine Muscat” (Vitis labrusca × V. vinifera) grape berry development reveals coordinate regulation of MEP pathway and terpene synthase gene expression. J. Agric. Food Chem. 2021, 69, 1413–1429. [Google Scholar] [CrossRef]
- Ji, X.-H.; Wang, B.-L.; Wang, X.-D.; Wang, X.-L.; Liu, F.-Z.; Wang, H.-B. Differences of aroma development and metabolic pathway gene expression between Kyoho and 87-1 grapes. J. Integr. Agric. 2021, 20, 1525–1539. [Google Scholar] [CrossRef]
- Ruiz-García, L.; Hellín, P.; Flores, P.; Fenoll, J. Prediction of muscat aroma in table grape by analysis of rose oxide. Food Chem. 2014, 154, 151–157. [Google Scholar] [CrossRef]
- Yue, X.; Ju, Y.; Fang, Y.; Zhang, Z. Transcriptomics integrated with metabolomics reveals the effect of cluster thinning on monoterpene biosynthesis in ‘Muscat Hamburg’ grape. Foods 2021, 10, 2718. [Google Scholar] [CrossRef]
- Wang, W.; Khalil-Ur-Rehman, M.; Wei, L.-L.; Nieuwenhuizen, N.J.; Zheng, H.; Tao, J.-M. Effect of thidiazuron on terpene volatile constituents and terpenoid biosynthesis pathway gene expression of Shine Muscat (Vitis labrusca × V. vinifera) grape berries. Molecules 2020, 25, 2578. [Google Scholar] [CrossRef]
- Martin, D.M.; Chiang, A.; Lund, S.T.; Bohlmann, J. Biosynthesis of wine aroma: Transcript profiles of hydroxymethylbutenyl diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewürztraminer grapes. Planta 2012, 236, 919–929. [Google Scholar] [CrossRef]
- Río Segade, S.; Vilanova, M.; Giacosa, S.; Perrone, I.; Chitarra, W.; Pollon, M.; Torchio, F.; Boccacci, P.; Gambino, G.; Gerbi, V.; et al. Ozone improves the aromatic fingerprint of white grapes. Sci. Rep. 2017, 7, 16301–16316. [Google Scholar] [CrossRef]
- Emanuelli, F.; Battilana, J.; Costantini, L.; Le Cunff, L.; Boursiquot, J.-M.; This, P.; Grando, M.S. A candidate gene association study on muscat flavor in grapevine (Vitis vinifera L.). BMC Plant Biol. 2010, 10, 241–257. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, B.; Zhang, X.; Wang, H.; Yan, A.; Zhang, G.; Wang, X.; Xu, H. The accumulation profiles of terpene metabolites in three muscat table grape cultivars through HS-SPME-GCMS. Sci. Data 2020, 7, 5–10. [Google Scholar] [CrossRef]
- Qian, X.; Sun, L.; Xu, X.-Q.; Zhu, B.-Q.; Xu, H.-Y. Differential expression of VvLOXA diversifies C6 volatile profiles in some Vitis vinifera table grape cultivars. Int. J. Mol. Sci. 2017, 18, 2705. [Google Scholar] [CrossRef]
- Liu, S.; Shan, B.; Zhou, X.; Gao, W.; Liu, Y.; Zhu, B.; Sun, L. Transcriptome and Metabolomics Integrated Analysis Reveals Terpene Synthesis Genes Controlling Linalool Synthesis in Grape Berries. J. Agric. Food Chem. 2022, 70, 9084–9094. [Google Scholar] [CrossRef]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 27–37. [Google Scholar] [CrossRef]
- Qian, X.; Xu, X.-Q.; Yu, K.-J.; Zhu, B.-Q.; Lan, Y.-B.; Duan, C.-Q.; Pan, Q.-H. Varietal dependence of GLVs accumulation and LOX-HPL pathway gene expression in four Vitis vinifera wine grapes. Int. J. Mol. Sci. 2016, 17, 1924. [Google Scholar] [CrossRef]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Edwards, K.; Johnstone, C.; Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991, 19, 1349. [Google Scholar] [CrossRef]
- Tamari, F.; Hinkley, C.S.; Ramprashad, N. A comparison of DNA extraction methods using Petunia hybrida tissues. J. Biomol. Tech. 2013, 24, 113–118. [Google Scholar] [CrossRef]
- Battilana, J.; Emanuelli, F.; Gambino, G.; Gribaudo, I.; Gasperi, F.; Boss, P.K.; Grando, M.S. Functional effect of grapevine 1-deoxy-D-xylulose 5-phosphate synthase substitution K284N on muscat flavour formation. J. Exp. Bot. 2011, 62, 5497–5508. [Google Scholar] [CrossRef]
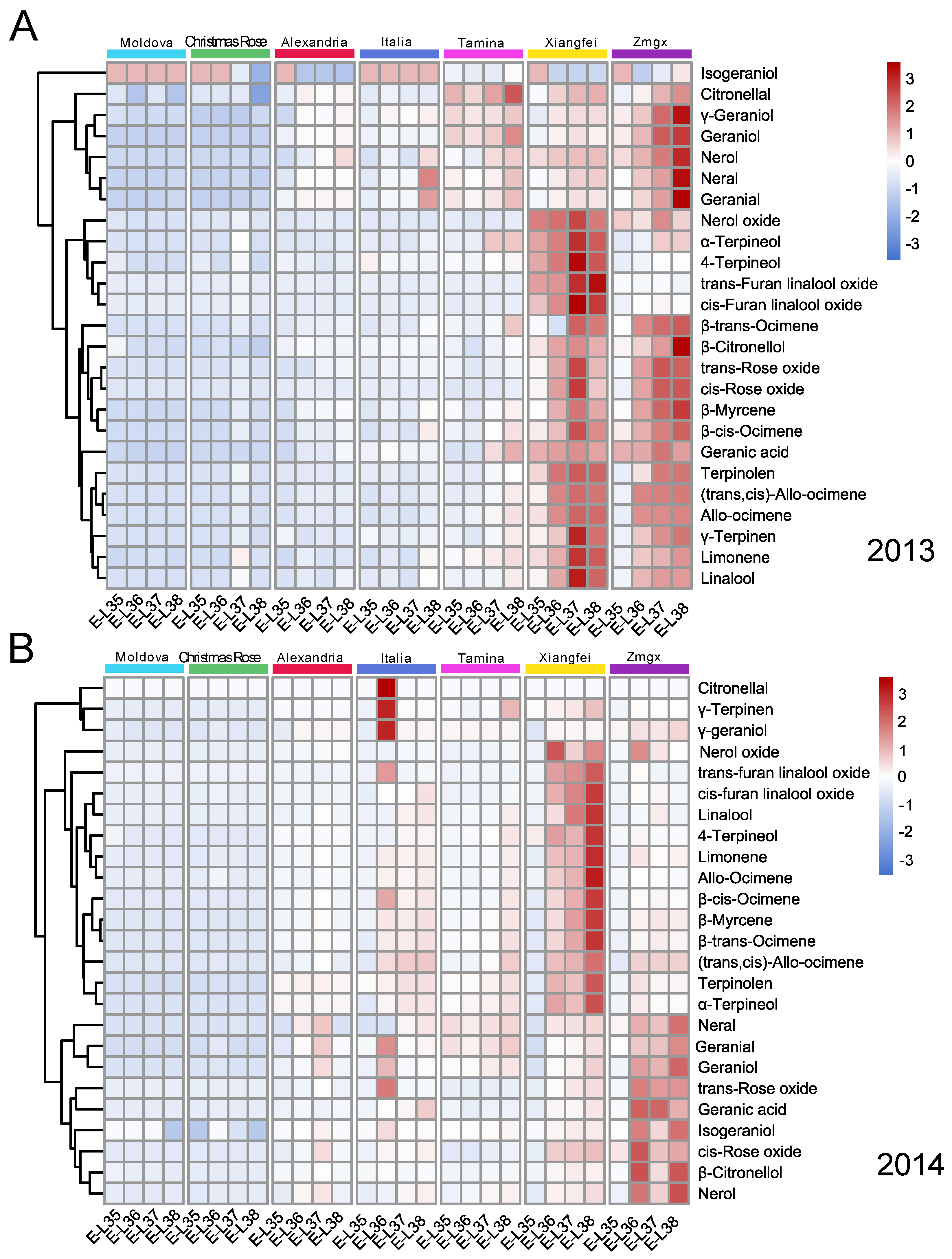

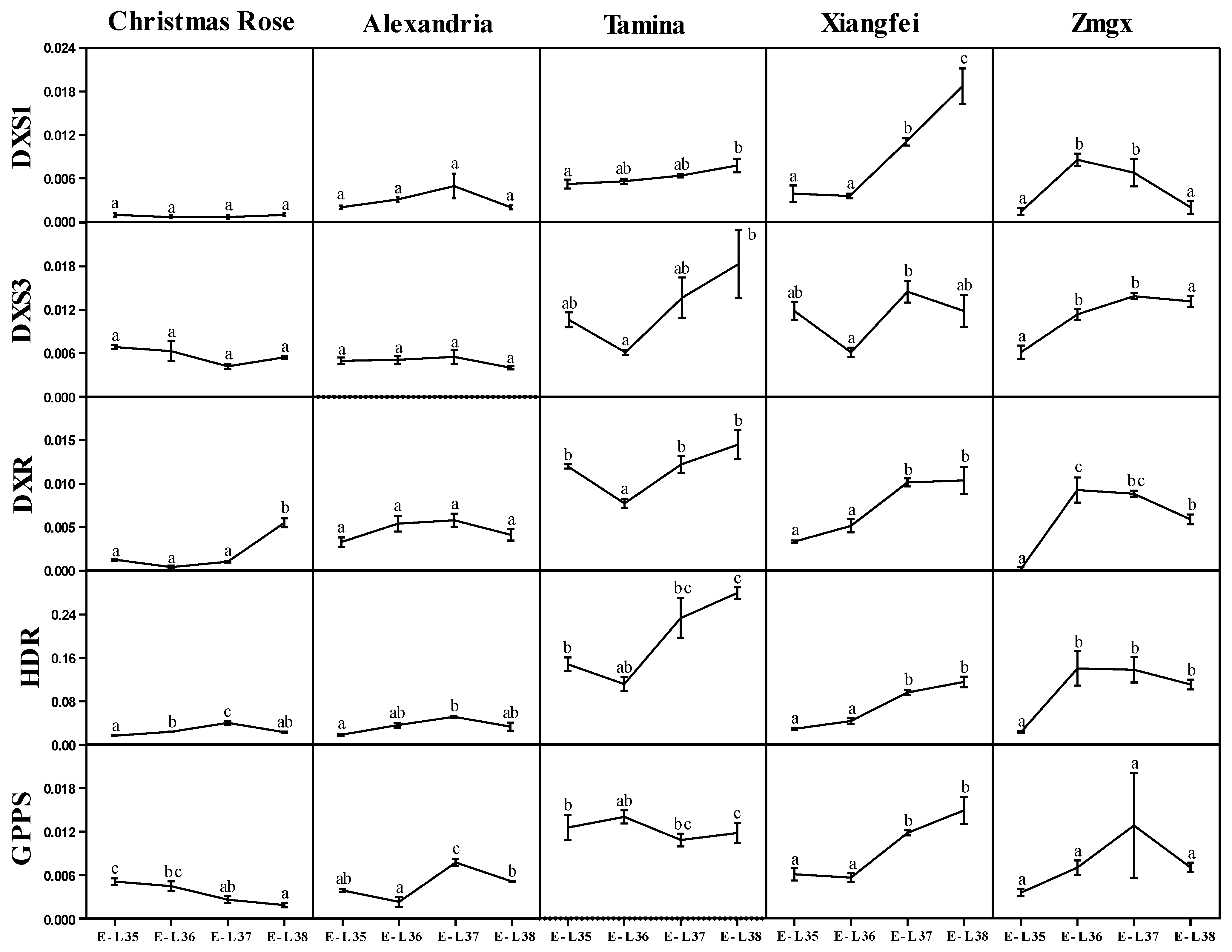
| Compounds | Moldova | Christmas Rose | Alexandria | Italia | Tamina | Xiangfei | Zmgx |
|---|---|---|---|---|---|---|---|
| β-Myrcene | tr | 0.09 ± 0.01 d | 0.86 ± 0.01 c | 0.97 ± 0.01 c | 1.13 ± 0.04 c | 2.08 ± 0.59 b | 3.59 ± 0.22 a |
| Limonene | 0.10 ± 0.03 d | 0.30 ± 0.18 d | 0.33 ± 0.00 d | 0.94 ± 0.00 cd | 1.21 ± 0.06 bc | 2.75 ± 0.97 a | 2.06 ± 0.04 ab |
| β-trans-Ocimene | nd | nd | 0.21 ± 0.00 c | 0.31 ± 0.07 c | 0.73 ± 0.26 b | 1.29 ± 0.34 a | 1.51 ± 0.07 a |
| β-cis-Ocimene | nd | 0.05 ± 0.01 c | 0.52 ± 0.01 bc | 0.94 ± 0.00 b | 1.07 ± 0.03 b | 2.14 ± 0.82 a | 2.63 ± 0.19 a |
| γ-Terpinen | tr | nd | 0.04 ± 0.00 b | 0.07 ± 0.00 b | 0.15 ± 0.01 b | 0.42 ± 0.21 a | 0.41 ± 0.07 a |
| Terpinolene | 0.02 ± 0.00 f | nd | 0.16 ± 0.01 e | 0.24 ± 0.01 d | 0.47 ± 0.03 c | 1.86 ± 0.06 a | 1.74 ± 0.01 b |
| trans-Rose oxide | nd | nd | 0.05 ± 0.00 c | 0.05 ± 0.00 c | 0.06 ± 0.00 bc | 0.21 ± 0.17 b | 0.36 ± 0.02 a |
| cis-Rose oxide | nd | nd | 0.06 ± 0.00 c | 0.06 ± 0.00 c | 0.08 ± 0.00 c | 0.59 ± 0.54 b | 1.26 ± 0.04 a |
| (trans,cis)-Allo-ocimene | 0.02 ± 0.00 d | nd | 0.35 ± 0.01 c | 0.39 ± 0.08 c | 0.94 ± 0.07 b | 2.53 ± 0.29 a | 2.42 ± 0.04 a |
| Allo-ocimene | 0.02 ± 0.00 c | nd | 0.24 ± 0.01 c | 0.21 ± 0.06 c | 0.84 ± 0.06 b | 2.03 ± 0.29 a | 1.78 ± 0.05 a |
| trans-furan linalool oxide | 2.69 ± 0.01 b | 1.44 ± 2.03 b | 4.01 ± 1.43 b | 5.19 ± 0.32 b | 6.64 ± 0.87 b | 283.97 ± 17.99 a | 17.96 ± 3.47 b |
| cis-Furan linalool oxide | 2.72 ± 0.01 c | 1.62 ± 2.29 c | 3.44 ± 0.01 c | 9.79 ± 0.73 c | 8.99 ± 1.36 c | 315.83 ± 23.07 a | 50.62 ± 9.76 b |
| Citronellal | 1.34 ± 1.89 de | nd | 3.85 ± 0.00 bc | 2.97 ± 0.12 cd | 7.46 ± 0.69 a | 5.43 ± 0.98 ab | 6.19 ± 0.93 a |
| Nerol oxide | 1.35 ± 1.90 c | 3.01 ± 0.18 c | 3.83 ± 1.19 c | 3.31 ± 0.71 c | 4.87 ± 0.80 c | 47.62 ± 3.22 a | 25.85 ± 8.85 b |
| Linalool | 0.14 ± 0.00 c | 0.14 ± 0.01 c | 7.17 ± 0.01 c | 11.87 ± 0.88 c | 16.39 ± 0.13 bc | 46.93 ± 24.60 a | 35.38 ± 2.49 ab |
| 4-Terpineol | tr | nd | 0.05 ± 0.00 d | 0.06 ± 0.01 c | 0.10 ± 0.00 b | 0.31 ± 0.01 a | 0.09 ± 0.01 b |
| Neral | 0.02 ± 0.00 d | nd | 0.19 ± 0.00 cd | 0.46 ± 0.06 b | 0.32 ± 0.01 bc | 0.29 ± 0.10 bc | 0.73 ± 0.25 a |
| α-Terpineol | 0.07 ± 0.00 d | 0.03 ± 0.03 d | 0.89 ± 0.00 cd | 1.41 ± 0.25 c | 5.54 ± 0.18 b | 10.93 ± 1.34 a | 5.15 ± 0.11 b |
| Geranial | 0.15 ± 0.00 c | 0.16 ± 0.02 c | 1.28 ± 0.02 bc | 2.56 ± 0.33 b | 2.18 ± 0.12 bc | 1.51 ± 0.31 bc | 4.97 ± 2.30 a |
| β-Citronellol | 1.19 ± 0.04 cd | nd | 4.08 ± 0.02 cd | 4.17 ± 0.10 cd | 4.87 ± 0.10 c | 10.47 ± 3.96 b | 20.98 ± 2.79 a |
| γ-Geraniol | 0.99 ± 0.04 d | 0.89 ± 0.13 d | 3.31 ± 0.01 c | 3.43 ± 0.12 bc | 4.76 ± 0.25 b | 4.09 ± 0.80 bc | 10.36 ± 1.32 a |
| Nerol | tr | tr | 0.17 ± 0.00 b | 0.17 ± 0.00 b | 0.20 ± 0.01 b | 0.22 ± 0.03 b | 0.47 ± 0.06 a |
| Isogeraniol | tr | nd | nd | tr | tr | nd | tr |
| Geraniol | 1.48 ± 0.07 d | 2.30 ± 0.31 d | 25.10 ± 0.27 c | 18.34 ± 0.88 c | 54.76 ± 4.44 b | 26.37 ± 3.35 c | 74.75 ± 9.71 a |
| Geranic acid | tr | 0.42 ± 0.07 c | 5.73 ± 0.14 b | 5.68 ± 3.33 b | 15.19 ± 0.96 a | 15.95 ± 0.16 a | 16.44 ± 1.64 a |
| Compounds | Moldova | Christmas Rose | Alexandria | Italia | Tamina | Xiangfei | Zmgx |
|---|---|---|---|---|---|---|---|
| β-Myrcene | 0.01 ± 0.01 e | 0.09 ± 0.00 e | 2.00 ± 0.02 d | 5.29 ± 0.03 b | 4.98 ± 0.16 b | 17.18 ± 0.32 a | 4.45 ± 0.03 c |
| Limonene | nd | nd | 1.35 ± 0.00 e | 3.48 ± 0.01 b | 3.26 ± 0.01 c | 14.94 ± 0.10 a | 3.07 ± 0.03 d |
| β-trans-Ocimene | 0.02 ± 0.01 e | nd | 1.46 ± 0.00 d | 3.35 ± 0.00 b | 3.21 ± 0.01 b | 11.04 ± 0.20 a | 2.29 ± 0.01 c |
| β-cis-Ocimene | nd | nd | 1.68 ± 0.01 d | 4.83 ± 0.00 b | 4.80 ± 0.21 b | 17.77 ± 0.33 a | 3.21 ± 0.00 c |
| γ-Terpinen | nd | nd | 0.93 ± 0.01 a | 1.36 ± 0.02 a | 3.67 ± 3.31 a | 3.38 ± 0.05 a | 1.17 ± 0.00 a |
| Terpinolene | nd | nd | 2.48 ± 0.00 c | 3.27 ± 0.04 b | 3.19 ± 0.16 b | 8.49 ± 0.03 a | 2.44 ± 0.02 c |
| trans-Rose oxide | nd | nd | 0.10 ± 0.00 d | 0.25 ± 0.01 c | nd | 0.80 ± 0.05 b | 1.80 ± 0.01 a |
| cis-Rose oxide | nd | nd | 0.51 ± 0.02 d | 0.95 ± 0.01 c | 0.26 ± 0.02 e | 1.93 ± 0.04 b | 2.30 ± 0.03 a |
| (trans,cis)-Allo-ocimene | nd | nd | 0.27 ± 0.00 d | 1.42 ± 0.01 b | 1.40 ± 0.01 b | 2.73 ± 0.00 a | 1.32 ± 0.00 c |
| Allo-Ocimene | nd | nd | 1.64 ± 0.00 e | 4.62 ± 0.08 b | 4.34 ± 0.16 c | 22.12 ± 0.23 a | 2.38 ± 0.03 d |
| trans-furan linalool oxide | nd | nd | 4.77 ± 0.01 d | 12.19 ± 0.39 b | 9.48 ± 0.22 c | 207.16 ± 1.16 a | 4.08 ± 0.19 d |
| cis-furan linalool oxide | nd | nd | 11.72 ± 0.01 e | 59.05 ± 1.35 b | 15.41 ± 0.01 c | 227.66 ± 0.82 a | 13.31 ± 0.13 d |
| Citronellal | nd | nd | nd | nd | 4.70 ± 0.66 b | 13.57 ± 0.14 a | 5.24 ± 0.02 b |
| nerol oxide | nd | nd | 41.87 ± 0.16 b | 22.94 ± 0.76 d | 24.16 ± 0.57 d | 201.86 ± 4.84 a | 35.04 ± 2.09 c |
| Linalool | 0.16 ± 0.00 e | 0.14 ± 0.01 e | 9.14 ± 0.06 d | 74.85 ± 0.16 b | 61.33 ± 1.36 c | 329.59 ± 7.56 a | 14.32 ± 0.26 d |
| 4-Terpineol | nd | nd | 0.13 ± 0.00 d | 0.21 ± 0.00 c | 0.27 ± 0.00 b | 1.06 ± 0.02 a | 0.13 ± 0.01 d |
| Neral | nd | nd | nd | 0.20 ± 0.00 b | 0.23 ± 0.04 b | 0.23 ± 0.01 b | 0.51 ± 0.00 a |
| α-Terpineol | 0.02 ± 0.03 d | nd | 6.68 ± 0.00 c | 8.61 ± 0.21 b | 9.29 ± 0.14 b | 26.02 ± 0.82 a | 6.21 ± 0.06 c |
| Geranial | nd | nd | 0.77 ± 0.00 d | 1.00 ± 0.00 cd | 1.55 ± 0.38 b | 1.28 ± 0.09 bc | 2.53 ± 0.03 a |
| β-Citronellol | nd | nd | 4.53 ± 0.00 d | 5.99 ± 0.04 c | 4.64 ± 0.10 d | 10.00 ± 0.13 b | 43.62 ± 1.41 a |
| γ-geraniol | nd | nd | 7.48 ± 0.01 c | 7.99 ± 0.02 b | 7.91 ± 0.05 b | 7.87 ± 0.05 b | 12.29 ± 0.13 a |
| Nerol | tr | tr | 0.06 ± 0.00 d | 0.22 ± 0.00 c | 0.21 ± 0.00 c | 0.30 ± 0.00 b | 0.93 ± 0.03 a |
| Isogeraniol | nd | nd | 0.10 ± 0.00 d | 0.11 ± 0.00 c | 0.12 ± 0.01 c | 0.13 ± 0.00 b | 0.27 ± 0.01 a |
| Geraniol | 0.41 ± 0.00 f | 1.62 ± 0.05 f | 16.40 ± 0.14 e | 26.35 ± 0.24 d | 37.46 ± 0.80 c | 48.19 ± 0.34 b | 102.37 ± 3.06 a |
| Geranic acid | 0.14 ± 0.19 e | nd | 13.20 ± 0.41 d | 60.30 ± 7.50 b | 6.61 ± 0.93 de | 41.08 ± 5.74 c | 84.75 ± 2.84 a |
| Compounds | Variety | Season | Variety × Season | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F Value | p | Sig. | F Value | p | Sig. | F Value | p | Sig. | |
| β-Myrcene | 12.99 | 9.30 × 10−11 | *** | 34.67 | 5.48 × 10−8 | *** | 5.09 | 1.38 × 10−4 | *** |
| Limonene | 14.81 | 5.52 × 10−12 | *** | 22.44 | 7.00 × 10−6 | *** | 4.81 | 2.41 × 10−4 | *** |
| β-trans-Ocimene | 10.81 | 3.31 × 10−9 | *** | 43.93 | 1.86 × 10−9 | *** | 6.11 | 1.80 × 10−5 | *** |
| β-cis-Ocimene | 8.85 | 1.04 × 10−7 | *** | 25.30 | 2.00 × 10−6 | *** | 4.20 | 8.39 × 10−4 | *** |
| γ-Terpinen | 2.03 | 6.83 × 10−2 | 13.81 | 3.36 × 10−4 | *** | 1.67 | 1.36 × 10−1 | ||
| Terpinolen | 24.46 | 1.57 × 10−17 | *** | 104.28 | 4.21 × 10−17 | *** | 8.80 | 1.13 × 10−7 | *** |
| trans-Rose oxide | 7.90 | 5.87 × 10−7 | *** | 12.57 | 6.03 × 10−4 | *** | 3.99 | 1.30 × 10−3 | ** |
| cis-Rose oxide | 31.77 | 5.88 × 10−21 | *** | 33.93 | 7.27 × 10−8 | *** | 5.62 | 4.77 × 10−5 | *** |
| (trans,cis)-Allo-ocimene | 29.67 | 4.99 × 10−20 | *** | 0.33 | 5.67 × 10−1 | 4.37 | 5.90 × 10−4 | *** | |
| Allo-ocimene | 12.25 | 3.04 × 10−10 | *** | 30.52 | 2.72 × 10−7 | *** | 6.67 | 6.12 × 10−6 | *** |
| trans-furan linalool oxide | 37.79 | 2.03 × 10−23 | *** | 1.39 | 2.41 × 10−1 | 3.36 | 4.67 × 10−3 | ** | |
| cis-furan linalool oxide | 50.36 | 8.49 × 10−28 | *** | 6.85 | 1.03 × 10−2 | 7.64 | 9.66 × 10−7 | *** | |
| Citronellal | 1.00 | 4.31 × 10−1 | 0.99 | 3.22 × 10−1 | 1.00 | 4.30 × 10−1 | |||
| Nerol oxide | 17.42 | 1.27 × 10−13 | *** | 16.78 | 8.65 × 10−5 | *** | 4.04 | 1.15 × 10−3 | ** |
| Linalool | 12.68 | 1.53 × 10−10 | *** | 12.49 | 6.27 × 10−4 | *** | 5.26 | 9.76 × 10−5 | *** |
| 4-Terpineol | 41.38 | 9.18 × 10−25 | *** | 25.31 | 2.21 × 10−6 | *** | 5.20 | 1.12 × 10−4 | *** |
| Neral | 24.33 | 1.81 × 10−17 | *** | 7.26 | 8.31 × 10−3 | ** | 0.82 | 5.55 × 10−1 | |
| α-Terpineol | 28.37 | 1.98 × 10−19 | *** | 29.30 | 4.42 × 10−7 | *** | 2.92 | 1.16 × 10−2 | |
| Geranial | 19.35 | 9.12 × 10−15 | *** | 7.09 | 9.04 × 10−3 | ** | 0.99 | 4.38 × 10−1 | |
| β-Citronellol | 22.74 | 1.23 × 10−16 | *** | 4.44 | 3.77 × 10−2 | 5.08 | 1.42 × 10−4 | *** | |
| γ-Geraniol | 4.04 | 1.16 × 10−3 | ** | 7.51 | 7.30 × 10−3 | ** | 1.47 | 1.97 × 10−1 | |
| Nerol | 22.19 | 2.40 × 10−16 | *** | 3.64 | 5.90 × 10−2 | 1.98 | 7.50 × 10−2 | ||
| Isogeraniol | 12.74 | 1.39 × 10−10 | *** | 394.71 | 3.85 × 10−36 | *** | 11.50 | 1.05 × 10−9 | *** |
| Geraniol | 28.95 | 1.06 × 10−19 | *** | 1.01 | 3.19 × 10−1 | 0.94 | 4.72 × 10−1 | ||
| Geranic acid | 22.40 | 1.84 × 10−16 | *** | 30.37 | 2.88 × 10−7 | *** | 12.11 | 3.80 × 10−10 | *** |
| VvDXS1 | VvDXS3 | VvDXR | VvHDR | VvGPPS | |
|---|---|---|---|---|---|
| β-Myrcene | 0.482 * | 0.609 ** | 0.410 | 0.351 | 0.426 |
| Limonene | 0.737 ** | 0.652 ** | 0.511 * | 0.391 | 0.576 ** |
| β-trans-Ocimene | 0.643 ** | 0.728 ** | 0.498 * | 0.457 * | 0.534 * |
| β-cis-Ocimene | 0.599 ** | 0.668 ** | 0.430 | 0.360 | 0.473 * |
| γ-Terpinen | 0.661 ** | 0.651 ** | 0.441 | 0.314 | 0.505 * |
| Terpinolen | 0.587 ** | 0.556 * | 0.346 | 0.232 | 0.443 |
| trans-Rose oxide | 0.467 * | 0.573 ** | 0.336 | 0.244 | 0.390 |
| cis-Rose oxide | 0.411 | 0.518 * | 0.270 | 0.170 | 0.316 |
| (trans,cis)-Allo-ocimene | 0.636 ** | 0.598 ** | 0.427 | 0.326 | 0.455 * |
| Allo-Ocimene | 0.682 ** | 0.644 ** | 0.408 | 0.379 | 0.509 * |
| trans-furan linalool oxide | 0.713 ** | 0.323 | 0.223 | −0.006 | 0.370 |
| cis-furan linalool oxide | 0.684 ** | 0.365 | 0.264 | 0.029 | 0.378 |
| Citronellal | 0.550 * | 0.749 ** | 0.715 ** | 0.786 ** | 0.733 ** |
| Nerol oxide | 0.518 * | 0.426 | 0.150 | 0.004 | 0.309 |
| Linalool | 0.693 ** | 0.598 ** | 0.441 | 0.301 | 0.503 * |
| 4-Terpineol | 0.666 ** | 0.434 | 0.338 | 0.132 | 0.432 |
| Neral | 0.359 | 0.626 ** | 0.472 * | 0.489* | 0.447 * |
| α-Terpineol | 0.676 ** | 0.619 ** | 0.449 * | 0.330 | 0.506 * |
| Geranial | 0.252 | 0.589 ** | 0.449 * | 0.490 * | 0.402 |
| β-Citronellol | 0.366 | 0.528 * | 0.264 | 0.215 | 0.300 |
| γ-Geraniol | 0.329 | 0.609 ** | 0.510 * | 0.533 * | 0.494 * |
| Geraniol | 0.392 | 0.648 ** | 0.424 | 0.449 * | 0.429 |
| Nerol | 0.397 | 0.686 ** | 0.774 ** | 0.819 ** | 0.716 ** |
| Isogeraniol | 0.334 | 0.693 ** | 0.599 ** | 0.666 ** | 0.540 * |
| Geranic acid | 0.543 * | 0.715 ** | 0.425 | 0.434 | 0.435 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Liu, S.; Gao, W.; Hu, B.; Zhu, B.; Sun, L. Monoterpenoids Evolution and MEP Pathway Gene Expression Profiles in Seven Table Grape Varieties. Plants 2022, 11, 2143. https://doi.org/10.3390/plants11162143
Zhou X, Liu S, Gao W, Hu B, Zhu B, Sun L. Monoterpenoids Evolution and MEP Pathway Gene Expression Profiles in Seven Table Grape Varieties. Plants. 2022; 11(16):2143. https://doi.org/10.3390/plants11162143
Chicago/Turabian StyleZhou, Xiaomiao, Songyu Liu, Wengping Gao, Binfang Hu, Baoqing Zhu, and Lei Sun. 2022. "Monoterpenoids Evolution and MEP Pathway Gene Expression Profiles in Seven Table Grape Varieties" Plants 11, no. 16: 2143. https://doi.org/10.3390/plants11162143
APA StyleZhou, X., Liu, S., Gao, W., Hu, B., Zhu, B., & Sun, L. (2022). Monoterpenoids Evolution and MEP Pathway Gene Expression Profiles in Seven Table Grape Varieties. Plants, 11(16), 2143. https://doi.org/10.3390/plants11162143







