Literature Review on the Effects of Heavy Metal Stress and Alleviating Possibilities through Exogenously Applied Agents in Alfalfa (Medicago sativa L.)
Abstract
:1. Introduction
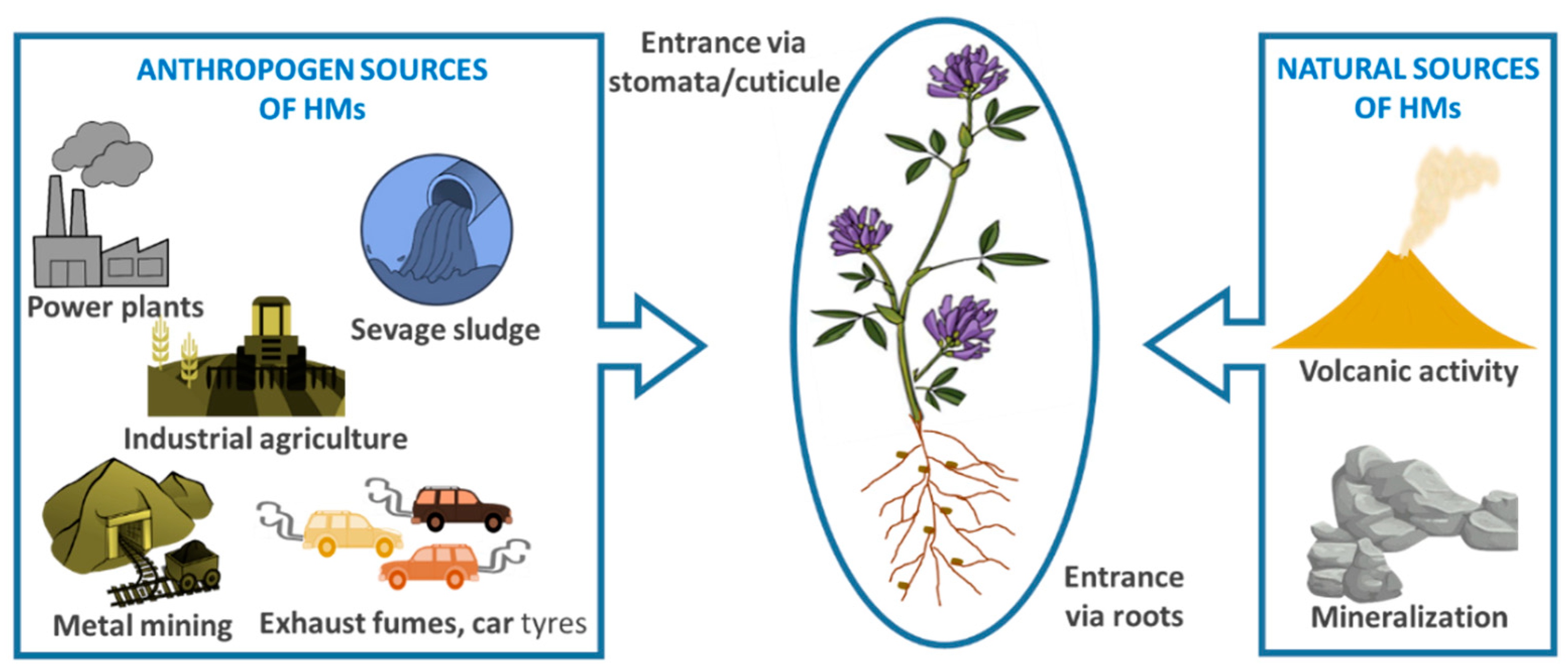
2. Definition of Heavy Metals and Their Sources
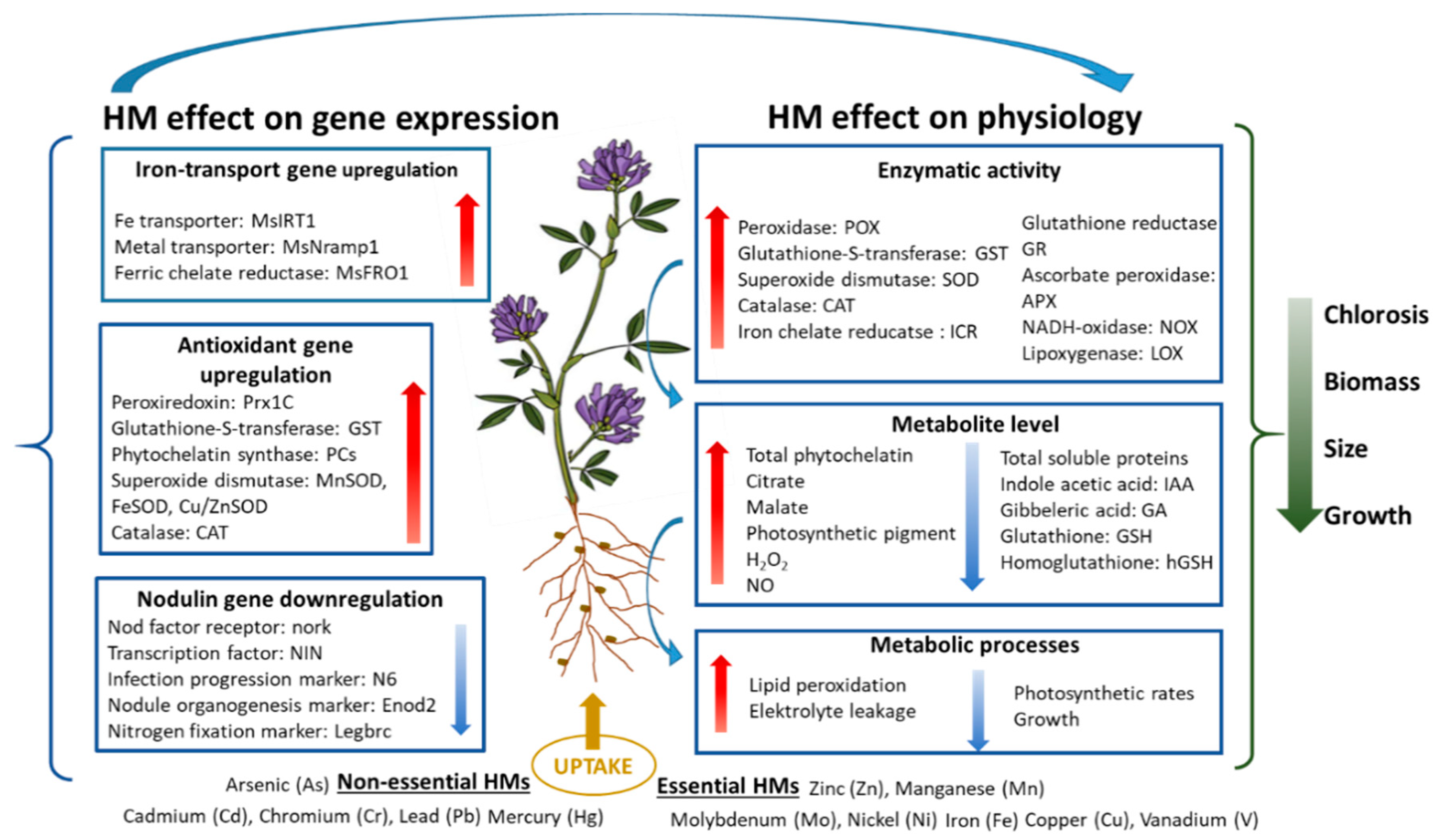
3. Effects of Essential Heavy Metals on the Growth and Development of Alfalfa
4. Effects of Non-Essential Heavy Metals on the Growth and Development of Alfalfa
| Plant Part | Applied Heavy Metal and Concentration | Affected Physiological Processes | Accumulation in Plant | Duration of Treatment | Reference |
|---|---|---|---|---|---|
| Whole plant | Mo |
| 346 ppm | 21 days | [29] |
| Whole plant | Mn: 60 mg L−1 |
| n.a. | 36 days | [26] |
| Seed | Zn: 1.5–24 mM Pb: 1.5–24 mM |
| Zn: root: 490 mg kg−1; shoot: 180 mg kg−1 Pb: root: 1330 mg/kg, shoot: 300 mg kg−1 | 24 h | [25] |
| Roots | Zn: 0.038–50 µM Cd:0.45–141.2 µM |
| Zn: 2700 mg kg−1 DW Cd: 1000 mg kg−1 DW | 14 days | [24] |
| Roots | Mn |
| 30–500 mg kg−1 DW | n.a. | [28] |
| Roots | Hg:10 μM |
| n.a. | 0; 6; 12; 24; 48; 72 h | [55] |
| Roots | Mn: 500 µg g−1 |
| 1822 µg g−1 DW | 49 days | [27] |
| Roots | Ni: 0; 50; 150; 250; 500 mg kg−1 |
| 0.61; 1.96; 9.97; 11.68; 23.65 mg kg−1 DW respectively | 60 days | [35] |
| Roots | Cu |
| n.a. | 2 years | [36] |
| Roots | Cd: 3 and 5 mg kg−1 |
| 600; 850 mg kg−1 DW respectively | 7 days | [44] |
| Roots | As: 25–35 μM |
| n.a. | 3; 6; 10; 28 days | [43] |
| Root | Cd: 1 mM |
| root: 10 mg kg−1 DW | 7 days | [47] |
| Root | Cd: 0–40 μM |
| 600–1700 mg kg−1 DW in tolerant cultivars and 600–1450 mg kg−1 DW in non-tolerant cultivars | 48; 72; 96 h | [48] |
| Stem | Cu: at high availability in soil |
| n.a. | [37] | |
| Root, Shoots | Pb: 0; 10; 100 |
| root: 766.66 mg kg−1 DW shoot: 385.67 mg kg−1 DW | 2 and 7 days | [53] |
| Shoots | Ni: 50; 150; 250; 500 mg kg−1 |
| 1.58; 8.92; 22.64; 32.84; 75.2 mg kg−1 DW respectively | 60 days | [35] |
| Shoots | Mn: 500 µg g−1 |
| 753 µg g−1 DW | 49 days | [27] |
| Shoots | Cd: 3 and 5 mg kg−1 |
| 600; 110 mg kg−1 DW respectively | 7 days | [44] |
| Shoots | Cd: 1 mM |
| 1.4 mg kg−1 DW | 7 days | [47] |
| Shoots | Cd: 0–40 μM |
| 25–31 mg kg−1 DW in tolerant and non-tolerant cultivars | 48; 72; 96 h | [48] |
| Leaves | Cr: 0.05; 0.5; 1; 5; 10 mg L−1 |
| 2.5; 2.8; 5; 8; 16 mg kg−1 DW | 59 days | [50] |
| Root Cotyledon Leaves | Pb |
| Root: 25,500 mg L−1 DW Cotyledon: 300 mg L−1 DW Leaves: 29 mg L−1 DW | 50 days | [51] |
| Leaves | Pb: 40 mg L−1 |
| n.a. | 10 days | [52] |
| Leaves | Hg: 1; 5; 10; 20; 40 µM |
| n.a. | 0; 6; 12; 24; 48; 72 h | [54] |
| Leaves | Zn: 4–7.3 mM |
| Zn: shoot: 300 mg kg−1 DW Cd: shoot: 40 mg kg−1 DW | 14 days | [24] |
| Leaves | Mn |
| n.a. | n.a. | [28] |
5. Phytoremediation
6. Heavy Metal Alleviation Possibilities
6.1. Role of Fungi in Mitigating Heavy Metal Stress
6.2. Combination of Organic Substances with Fungi
6.3. Plant Growth-Promoting Rhizobacteria (PGPR) Were Applied to Alfalfa Plants
6.4. Combining Fungi and Bacteria
6.5. The Application of Salicylic Acid
6.6. Organic Acids
6.7. Combined Use of Citric Acid and AM Fungal Strains
6.8. Further Applications
7. Potential Directions of HM Stress Research in Alfalfa
7.1. Element Effects
7.2. Cell Wall Processes
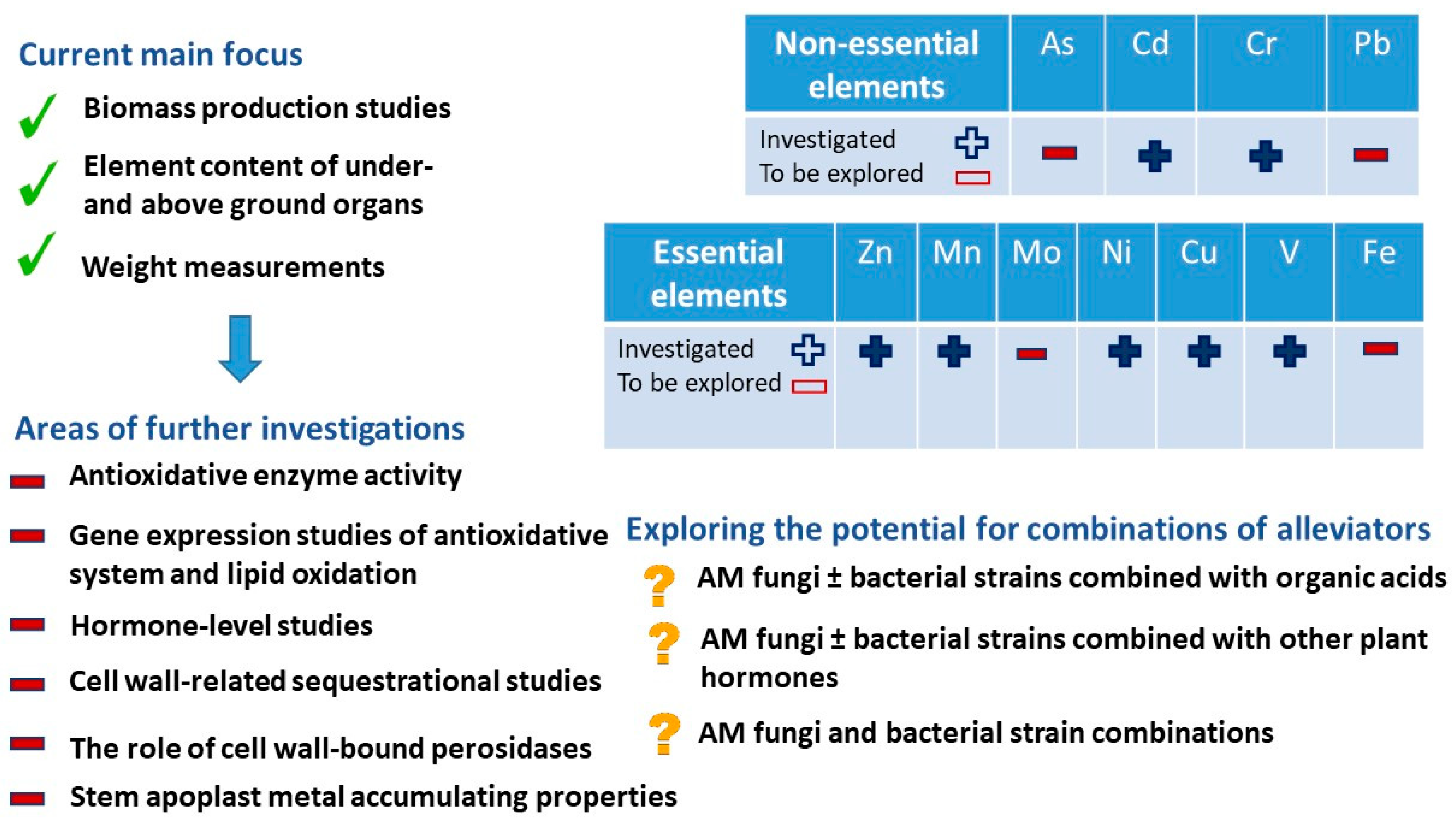
7.3. Complexity of Research Works
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alloway, B.J. Sources of heavy metals and metalloids in soils. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Bandyopadhyay, S.; Plascencia-Villa, G.; Mukherjee, A.; Rico, C.M.; José-Yacamán, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci. Total Environ. 2015, 515–516, 60–69. [Google Scholar] [CrossRef]
- Agnello, A.C.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G. Citric acid-and Tween® 80-assisted phytoremediation of a co-contaminated soil: Alfalfa (Medicago sativa L.) performance and remediation potential. Environ. Sci. Pollut. Res. 2016, 23, 9215–9226. [Google Scholar] [CrossRef]
- El-Kherbawy, M.; Angle, J.S.; Heggo, A.; Chaney, R.L. Soil pH, rhizobia, and vesicular-arbuscular mycorrhizae inoculation effects on growth and heavy metal uptake of alfalfa (Medicago sativa L.). Biol. Fertil. Soils 1989, 8, 61–65. [Google Scholar] [CrossRef]
- Gan, C.-D.; Chen, T.; Yang, J.-Y. Remediation of vanadium contaminated soil by alfalfa (Medicago sativa L.) combined with vanadium-resistant bacterial strain. Environ. Technol. Innov. 2020, 20, 101090. [Google Scholar] [CrossRef]
- Song, Y.; Lv, J.; Ma, Z.; Dong, W. The mechanism of alfalfa (Medicago sativa L.) response to abiotic stress. Plant Growth Regul. 2019, 89, 239–249. [Google Scholar] [CrossRef]
- Ghnaya, T.; Mnassri, M.; Ghabriche, R.; Wali, M.; Poschenrieder, C.; Lutts, S.; Abdelly, C. Nodulation by Sinorhizobium meliloti originated from a mining soil alleviates Cd toxicity and increases Cd-phytoextraction in Medicago sativa L. Front. Plant Sci. 2015, 6, 863. [Google Scholar] [CrossRef] [Green Version]
- Tabande, L.; Sepehri, M.; Yasrebi, J.; Zarei, M.; Ghasemi-Fasaei, R.; Khatabi, B. A comparison between the function of Serendipita indica and Sinorhizobium meliloti in modulating the toxicity of zinc oxide nanoparticles in alfalfa (Medicago sativa L.). Environ. Sci. Pollut. Res. 2022, 29, 8790–8803. [Google Scholar] [CrossRef]
- Wang, F.; Wang, E.T.; Wu, L.J.; Sui, X.H.; Li, Y., Jr.; Chen, W.X. Rhizobium vallis sp. nov., isolated from nodules of three leguminous species. Int. J. Syst. Evol. Microbiol. 2011, 61, 2582–2588. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; Hao, X.; Rensing, C.; Wei, G. Genes Conferring Copper Resistance inSinorhizobium melilotiCCNWSX0020 Also Promote the Growth of Medicago lupulinain Copper-Contaminated Soil. Appl. Environ. Microbiol. 2014, 80, 1961–1971. [Google Scholar] [CrossRef] [Green Version]
- Lindström, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2019, 13, 1314–1335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef]
- Bouton, J.H. Alfalfa; University of Georgia: Athens, GA, USA, 2021; p. 1. Available online: http://www.jstor.org/stable/42933830 (accessed on 26 June 2022).
- Jha, P.K.; Nair, S.; Gopinathan, M.C.; Babu, C.R. Suitability of rhizobia-inoculated wild legumes Argyrolobium flaccidum, Astragalus graveolens, Indigofera gangetica and Lespedeza stenocarpa in providing a vegetational cover in an unreclaimed limestone quarry. Plant Soil 1995, 177, 139–149. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef] [Green Version]
- Pourret, O.; Bollinger, J.-C. “Heavy metal”—What to do now: To use or not to use? Sci. Total Environ. 2018, 610–611, 419–420. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’—Proposal of a comprehensive definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Zafar-Ul-Hye, M.; Naeem, M.; Danish, S.; Fahad, S.; Datta, R.; Abbas, M.; Rahi, A.A.; Brtnicky, M.; Holátko, J.; Tarar, Z.H.; et al. Alleviation of Cadmium Adverse Effects by Improving Nutrients Uptake in Bitter Gourd through Cadmium Tolerant Rhizobacteria. Environments 2020, 7, 54. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, S.; Mishra, S.; Chauhan, D.K.; Dubey, N.K. Micronutrients and their diverse role in agricultural crops: Advances and future prospective. Acta Physiol. Plant. 2015, 37, 139. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S. Fortification of micronutrients for efficient agronomic production: A review. Agron. Sustain. Dev. 2016, 36, 7. [Google Scholar] [CrossRef] [Green Version]
- Selye, H. Confusion and Controversy in the Stress Field. J. Hum. Stress 1975, 1, 37–44. [Google Scholar] [CrossRef]
- Al-Helal, A.A. Effect of cadmium and mercury on seed germination and early seedling growth of rice and alfalfa. J. Univ. Kuwait Sci. 1995, 22, 76. [Google Scholar]
- Ibekwe, A.M.; Angle, J.S.; Chaney, R.L.; Van Berkum, P. Zinc and Cadmium Toxicity to Alfalfa and Its Microsymbiont. J. Environ. Qual. 1996, 25, 1032–1040. [Google Scholar] [CrossRef]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; Pueyo, J.J. Uptake and effects of lead and zinc on alfalfa (Medicago sativa L.) seed germination and seedling growth: Role of plant growth promoting bacteria. S. Afr. J. Bot. 2019, 124, 573–582. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef] [Green Version]
- Sale, P.W.G.; Couper, D.I.; Cachia, P.L.; Larkin, P.J. Tolerance to manganese toxicity among cultivars of lucerne (Medicago sativa L.). Plant Soil 1992, 146, 31–38. [Google Scholar] [CrossRef]
- Gherardi, M.J.; Rengel, Z. Genotypes of lucerne (Medicago sativa L.) show differential tolerance to manganese deficiency and toxicity when grown in bauxite residue sand. Plant Soil 2003, 249, 287–296. [Google Scholar] [CrossRef]
- Jensen, E.H.; Lesperance, A.L. Molybdenum Accumulation by Forage Plants. Agron. J. 1971, 63, 201–204. [Google Scholar] [CrossRef]
- Dixon, N.E.; Gazzola, C.; Blakeley, R.L.; Zerner, B. Jack bean urease (EC 3.5.1.5). Metalloenzyme. Simple biological role for nickel. J. Am. Chem. Soc. 1975, 97, 4131–4133. [Google Scholar] [CrossRef]
- Myrach, T.; Zhu, A.; Witte, C.-P. The assembly of the plant urease activation complex and the essential role of the urease accessory protein G (UreG) in delivery of nickel to urease. J. Biol. Chem. 2017, 292, 14556–14565. [Google Scholar] [CrossRef] [Green Version]
- Witte, C.-P.; Rosso, M.G.; Romeis, T. Identification of Three Urease Accessory Proteins That Are Required for Urease Activation in Arabidopsis. Plant Physiol. 2005, 139, 1155–1162. [Google Scholar] [CrossRef] [Green Version]
- Follmer, C. Insights into the role and structure of plant ureases. Phytochemistry 2008, 69, 18–28. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Ponnuraj, K. Crystal Structure of the First Plant Urease from Jack Bean: 83 Years of Journey from Its First Crystal to Molecular Structure. J. Mol. Biol. 2010, 400, 274–283. [Google Scholar] [CrossRef]
- Helaoui, S.; Boughattas, I.; Hattab, S.; Mkhinini, M.; Alphonse, V.; Livet, A.; Bousserrhine, N.; Banni, M. Physiological, biochemical and transcriptomic responses of Medicago sativa to nickel exposure. Chemosphere 2020, 249, 126121. [Google Scholar] [CrossRef]
- Leland, H.V.; Carter, J.L. Effects of copper on production of periphyton, nitrogen fixation and processing of leaf litter in a Sierra Nevada, California, stream. Freshw. Biol. 1985, 15, 155–173. [Google Scholar] [CrossRef]
- Strozycki, P.M.; Szymanski, M.; Szczurek, A.; Barciszewski, J.; Figlerowicz, M. A New Family of Ferritin Genes from Lupinus luteus—Comparative Analysis of Plant Ferritins, Their Gene Structure, and Evolution. Mol. Biol. Evol. 2010, 27, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Printz, B.; Guerriero, G.; Sergeant, K.; Audinot, J.-N.; Guignard, C.; Renaut, J.; Lutts, S.; Hausman, J.-F. Combining -Omics to Unravel the Impact of Copper Nutrition on Alfalfa (Medicago sativa) Stem Metabolism. Plant Cell Physiol. 2016, 57, 407–422. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.-Q.; Yang, J.-Y. Oral bioaccessibility and health risk assessment of vanadium(IV) and vanadium(V) in a vanadium titanomagnetite mining region by a whole digestive system in-vitro method (WDSM). Chemosphere 2019, 215, 294–304. [Google Scholar] [CrossRef]
- Cao, X.; Diao, M.; Zhang, B.; Liu, H.; Wang, S.; Yang, M. Spatial distribution of vanadium and microbial community responses in surface soil of Panzhihua mining and smelting area, China. Chemosphere 2017, 183, 9–17. [Google Scholar] [CrossRef]
- Fageria, N.K.; Santos, A.B.; Filho, M.P.B.; Guimarães, C.M. Iron Toxicity in Lowland Rice. J. Plant Nutr. 2008, 31, 1676–1697. [Google Scholar] [CrossRef]
- Lafuente, A.; Pajuelo, E.; Caviedes, M.A.; Rodriguez-Llorente, I.D. Reduced nodulation in alfalfa induced by arsenic correlates with altered expression of early nodulins. J. Plant Physiol. 2010, 167, 286–291. [Google Scholar] [CrossRef]
- Pajuelo, E.; Rodríguez-Llorente, I.D.; Dary, M.; Palomares, A.J. Toxic effects of arsenic on Sinorhizobium–Medicago sativa symbiotic interaction. Environ. Pollut. 2008, 154, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Drazic, G.; Mihailovic, N.; Lojić, M. Cadmium accumulation in Medicago sativa seedlings treated with salicylic acid. Biol. Plant. 2006, 50, 239–244. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Küpper, H. Cadmium: From toxicity to essentiality. In Cadmium Toxicity in Plants; Springer: Berlin/Heidelberg, Germany, 2013; Volume 11, pp. 395–413. [Google Scholar]
- Kabir, A.H.; Hossain, M.M.; Khatun, M.A.; Mandal, A.; Haider, S.A. Role of Silicon Counteracting Cadmium Toxicity in Alfalfa (Medicago sativa L.). Front. Plant Sci. 2016, 7, 1117. [Google Scholar] [CrossRef] [PubMed]
- García de la Torre, V.S.; Coba de la Peña, T.; Pueyo, J.J.; Lucas, M.M. Cadmium-Tolerant and -Sensitive Cultivars Identified by Screening of Medicago truncatula Germplasm Display Contrasting Responses to Cadmium Stress. Front. Plant Sci. 2021, 12, 595001. [Google Scholar] [CrossRef]
- Salmani Abyaneh, A.; Fazaelipoor, M.H. Evaluation of rhamnolipid (RL) as a biosurfactant for the removal of chromium from aqueous solutions by precipitate flotation. J. Environ. Manag. 2016, 165, 184–187. [Google Scholar] [CrossRef]
- Christou, A.; Georgiadou, E.C.; Zissimos, A.M.; Christoforou, I.C.; Christofi, C.; Neocleous, D.; Dalias, P.; Torrado, S.O.C.A.; Argyraki, A.; Fotopoulos, V. Hexavalent chromium leads to differential hormetic or damaging effects in alfalfa (Medicago sativa L.) plants in a concentration-dependent manner by regulating nitro-oxidative and proline metabolism. Environ. Pollut. 2020, 267, 115379. [Google Scholar] [CrossRef]
- Yan, Z.Z.; Ke, L.; Tam, N.F.Y. Lead stress in seedlings of Avicennia marina, a common mangrove species in South China, with and without cotyledons. Aquat. Bot. 2010, 92, 112–118. [Google Scholar] [CrossRef]
- López, M.L.; Peralta-Videa, J.R.; Castillo-Michel, H.; Martinez-Martinez, A.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. Lead toxicity in alfalfa plants exposed to phytohormones and ethylenediaminetetraacetic acid monitored by peroxidase, catalase, and amylase activities. Environ. Toxicol. Chem. 2007, 26, 2717–2723. [Google Scholar] [CrossRef]
- Hattab, S.; Hattab, S.; Flores-Casseres, M.L.; Boussetta, H.; Doumas, P.; Hernandez, L.E.; Banni, M. Characterisation of lead-induced stress molecular biomarkers in Medicago sativa plants. Environ. Exp. Bot. 2016, 123, 1–12. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Wang, S.J.; Yang, Z.M. Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 2008, 70, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Guo, K.; Elbaz, A.; Yang, Z. Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ. Exp. Bot. 2009, 65, 27–34. [Google Scholar] [CrossRef]
- McIntyre, T. Phytoremediation of heavy metals from soils. In Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2003; pp. 97–123. [Google Scholar]
- Saxena, P.K.; KrishnaRaj, S.; Dan, T.; Perras, M.R.; Vettakkorumakankav, N.N. Phytoremediation of Heavy Metal Contaminated and Polluted Soils. In Heavy Metal Stress in Plants; Prasad, M.N.V., Hagemeyer, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 305–329. [Google Scholar] [CrossRef]
- Jakucs, E. A föld alatti gombavilág titkai. Természet Világa 2009, 140, 413–415. [Google Scholar]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Raven, J.A.; Edwards, D. Roots: Evolutionary origins and biogeochemical significance. J. Exp. Bot. 2001, 52 (Suppl. S1), 381–401. [Google Scholar] [CrossRef] [PubMed]
- Püschel, D.; Janoušková, M.; Voříšková, A.; Gryndlerová, H.; Vosátka, M.; Jansa, J. Arbuscular Mycorrhiza Stimulates Biological Nitrogen Fixation in Two Medicago spp. through Improved Phosphorus Acquisition. Front. Plant Sci. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, S.; Sharif, F.; Khan, A.U. Effect of lead and cadmium on growth of Medicago sativa L. and their transfer to food chain. J. Anim. Plant Sci. 2015, 25, 472–477. [Google Scholar]
- Tirry, N.; Kouchou, A.; El Omari, B.; Ferioun, M.; El Ghachtouli, N. Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J. Genet. Eng. Biotechnol. 2021, 19, 149. [Google Scholar] [CrossRef]
- Al Agely, A.; Sylvia, D.M.; Ma, L.Q. Mycorrhizae Increase Arsenic Uptake by the Hyperaccumulator Chinese Brake Fern (Pteris vittata L.). J. Environ. Qual. 2005, 34, 2181–2186. [Google Scholar] [CrossRef] [Green Version]
- Citterio, S.; Prato, N.; Fumagalli, P.; Aina, R.; Massa, N.; Santagostino, A.; Sgorbati, S.; Berta, G. The arbuscular mycorrhizal fungus Glomus mosseae induces growth and metal accumulation changes in Cannabis sativa L. Chemosphere 2005, 59, 21–29. [Google Scholar] [CrossRef]
- Göhre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joner, E.J.; Briones, R.; Leyval, C. Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 2000, 226, 227–234. [Google Scholar] [CrossRef]
- Weissenhorn, I.; Leyval, C.; Berthelin, J. Cd-tolerant arbuscular mycorrhizal (AM) fungi from heavy-metal polluted soils. Plant Soil 1993, 157, 247–256. [Google Scholar] [CrossRef]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Gao, Y. Arbuscular Mycorrhizal Colonization Alters Subcellular Distribution and Chemical Forms of Cadmium in Medicago sativa L. and Resists Cadmium Toxicity. PLoS ONE 2012, 7, e48669. [Google Scholar] [CrossRef] [Green Version]
- Motaharpoor, Z.; Taheri, H.; Nadian, H. Rhizophagus irregularis modulates cadmium uptake, metal transporter, and chelator gene expression in Medicago sativa. Mycorrhiza 2019, 29, 389–395. [Google Scholar] [CrossRef]
- Bellini, E.; Varotto, C.; Borsò, M.; Rugnini, L.; Bruno, L.; Sanità Di Toppi, L. Eukaryotic and Prokaryotic Phytochelatin Synthases Differ Less in Functional Terms Than Previously Thought: A Comparative Analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407. Plants 2020, 9, 914. [Google Scholar] [CrossRef]
- Li, A.-M.; Yu, B.-Y.; Chen, F.-H.; Gan, H.-Y.; Yuan, J.-G.; Qiu, R.; Huang, J.-C.; Yang, Z.-Y.; Xu, Z.-F. Characterization of the Sesbania rostrata Phytochelatin Synthase Gene: Alternative Splicing and Function of Four Isoforms. Int. J. Mol. Sci. 2009, 10, 3269–3282. [Google Scholar] [CrossRef]
- Zaefarian, F.; Rezvani, M.; Rejali, F.; Ardakani, M.R.; Noormohammadi, G. Effect of Heavy Metals and Arbuscular Mycorrhizal Fungal on Growth and Nutrients (n, p, k, zn, cu, and fe) Accumulation of Alfalfa (Medicago Sativa L.). Am. Eurasian J. Agric. Environ. Sci. 2011, 11, 346–352. [Google Scholar]
- Jumpponen, A. Dark septate endophytes—Are they mycorrhizal? Mycorrhiza 2001, 11, 207–211. [Google Scholar] [CrossRef]
- Hou, L.; Yu, J.; Zhao, L.; He, X. Dark Septate Endophytes Improve the Growth and the Tolerance of Medicago sativa and Ammopiptanthus mongolicus under Cadmium Stress. Front. Microbiol. 2020, 10, 3061. [Google Scholar] [CrossRef]
- Kahromi, S.; Najafi, F. Growth and some physiological characteristics of alfalfa (Medicago sativa L.) in response to lead stress and Glomus intraradices symbiosis. J. Plant Process Funct. 2020, 9, 1–12. [Google Scholar]
- Savi, P.; Yasir, M.; Bartoli, M.; Giorcelli, M.; Longo, M. Electrical and Microwave Characterization of Thermal Annealed Sewage Sludge Derived Biochar Composites. Appl. Sci. 2020, 10, 1334. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Keitel, C.; Singh, B. Evaluation of the Influence of Individual Clay Minerals on Biochar Carbon Mineralization in Soils. Soil Syst. 2019, 3, 79. [Google Scholar] [CrossRef] [Green Version]
- Raklami, A.; El Gharmali, A.; Ait Rahou, Y.; Oufdou, K.; Meddich, A. Compost and mycorrhizae application as a technique to alleviate Cd and Zn stress in Medicago sativa. Int. J. Phytoremediation 2019, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. Toxicity and Carcinogenicity of Cr(VI) in Animal Models and Humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.-Q.; Yan, X.-W.; Wei, G.-H.; Zhang, J.-H.; Fang, L.-C. Rhizobium inoculation enhances copper tolerance by affecting copper uptake and regulating the ascorbate-glutathione cycle and phytochelatin biosynthesis-related gene expression in Medicago sativa seedlings. Ecotoxicol. Environ. Saf. 2018, 162, 312–323. [Google Scholar] [CrossRef]
- Gan, C.; Liu, M.; Lu, J.; Yang, J. Adsorption and Desorption Characteristics of Vanadium (V) on Silica. Water Air Soil Pollut. 2020, 231, 10. [Google Scholar] [CrossRef]
- Wang, X.; Fang, L.; Beiyuan, J.; Cui, Y.; Peng, Q.; Zhu, S.; Wang, M.; Zhang, X. Improvement of alfalfa resistance against Cd stress through rhizobia and arbuscular mycorrhiza fungi co-inoculation in Cd-contaminated soil. Environ. Pollut. 2021, 277, 116758. [Google Scholar] [CrossRef]
- Boullard, O.; Leblanc, H.; Besson, B. Salicylic Acid. Ullmann’s Encycl. Ind. Chem. 2000, 32, 127–128. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Kaur, R.; Kumar, V.; Khanna, K.; Bakshi, P.; Singh, R.; Arora, S.; Kaur, R.; Bhardwaj, R. Role of Salicylic Acid in Heavy Metal Stress Tolerance: Insight into Underlying Mechanism. In Salicylic Acid: A Multifaceted Hormone; Springer: Singapore, 2017; pp. 123–144. [Google Scholar] [CrossRef]
- Cheng, X.; Fang, T.; Zhao, E.; Zheng, B.; Huang, B.; An, Y.; Zhou, P. Protective roles of salicylic acid in maintaining integrity and functions of photosynthetic photosystems for alfalfa (Medicago sativa L.) tolerance to aluminum toxicity. Plant Physiol. Biochem. 2020, 155, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric Acid-Mediated Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, K.; Xie, Y.; Shen, W. Heme oxygenase-1 is involved in ascorbic acid-induced alleviation of cadmium toxicity in root tissues of Medicago sativa. Plant Soil 2013, 366, 605–616. [Google Scholar] [CrossRef]
- Lang, Q.; Wenlong, G.; Zhigang, W.; Baoqin, L.; Weimin, S.; Pin, G.; Xiaoxu, S.; Benru, S.; Yanxu, Z.; Tianle, K.; et al. Citric acid and AMF inoculation combination–assisted phytoextraction of vanadium (V) by Medicago sativa in V mining contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 67472–67486. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, L.; Cui, W.; Wang, Y.; Shen, W.; Ren, Y.; Zheng, T. Induction of heme oxygenase-1 with β-CD-hemin complex mitigates cadmium-induced oxidative damage in the roots of Medicago sativa. Plant Soil 2011, 345, 271–285. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, J.; Xuan, W.; Xie, Y. Up-regulation of heme oxygenase-1 contributes to the amelioration of aluminum-induced oxidative stress in Medicago sativa. J. Plant Physiol. 2013, 170, 1328–1336. [Google Scholar] [CrossRef]
- Shvaleva, A.; Coba De La Peña, T.; Rincón, A.; Morcillo, C.N.; García de la Torre, V.S.; Lucas, M.M.; Pueyo, J.J.; De La Torre, V.S.G. Flavodoxin overexpression reduces cadmium-induced damage in alfalfa root nodules. Plant Soil 2010, 326, 109–121. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Chen, X.; Gao, Z.; Xuan, W.; Xu, S.; Ding, X.; Shen, W. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol. 2008, 177, 155–166. [Google Scholar] [CrossRef]
- Dai, C.; Cui, W.; Pan, J.; Xie, Y.; Wang, J.; Shen, W. Proteomic analysis provides insights into the molecular bases of hydrogen gas-induced cadmium resistance in Medicago sativa. J. Proteom. 2017, 152, 109–120. [Google Scholar] [CrossRef]
- Samma, M.K.; Zhou, H.; Cui, W.; Zhu, K.; Zhang, J.; Shen, W. Methane alleviates copper-induced seed germination inhibition and oxidative stress in Medicago sativa. BioMetals 2017, 30, 97–111. [Google Scholar] [CrossRef]
- Shakya, M.; Sharma, P.; Meryem, S.S.; Mahmood, Q.; Kumar, A. Heavy Metal Removal from Industrial Wastewater Using Fungi: Uptake Mechanism and Biochemical Aspects. J. Environ. Eng. 2016, 142, C6015001. [Google Scholar] [CrossRef]
- Verma, K.; Shekhawat, G.S.; Sharma, A.; Mehta, S.K.; Sharma, V. Cadmium induced oxidative stress and changes in soluble and ionically bound cell wall peroxidase activities in roots of seedling and 3–4 leaf stage plants of Brassica juncea (L.) czern. Plant Cell Reprod. 2008, 27, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- García de la Torre, V.S.; Coba de la Peña, T.; Lucas, M.M.; Pueyo, J.J. Transgenic Medicago truncatula Plants That Accumulate Proline Display Enhanced Tolerance to Cadmium Stress. Front. Plant Sci. 2022, 13, 829069. [Google Scholar] [CrossRef] [PubMed]


| Plant Part | Applied Heavy Metal and Concentration | Applied Stress Alleviator | Affected Physiological Processes | Duration of Experiment | Reference |
|---|---|---|---|---|---|
| shoots and roots biomass | Cd 0.5, 5, and 20 mg kg−1 | Glomus intraradices AMF | growth, heavy metal uptake | 80 days | [9] |
| shoots and roots biomass | Cd 100 mg kg−1 | Rhizophagus irregularis AMF | shoots and roots dry weight, gene expression in roots | 5 weeks | [71] |
| shoots and roots | Co 51.91 mg kg−1, Cd 8.5 mg kg−1, Pb 436 mg kg−1 | Glomus mosseae AMF | plant growth and nutrients take up | until early flowering | [74] |
| shoots and roots | Cd 0, 5, 10, mg kg−1 | Acrocalymma vagum and Scytalidium lignicola DSE | plant growth, increase organic carbon level | 60 and 90 days | [76] |
| leaves, roots | Pb 0, 60, 120, 180 and 240 μm | Glomus intraradices AMF | plant growth, protein, carotenoid, pigments, proline and total phenol content, enzyme activities | 75 days | [77] |
| shoots and roots | Cd 20 mg kg−1 | four AMF species and biochar | growth, nutrient and cadmium uptake | 146 days | [79] |
| shoots and roots | Cd 300 mg kg−1 600 mg kg−1 Zn 300 mg kg−1 600 mg kg−1 | Rhizophagus irregularis AMF and compost | plant growth, Photosynthetic efficiency, water content and membrane permeability | two months | [80] |
| shoots and roots | Cu 2.3 and 1.5 mM Pb 0.35 and 0.18mM Zn 4,3 and 2.15 mM | Proteus sp. DSP1, Pseudomonas sp. DSP17, Ensifer meliloti RhOL6 and RhOL8 | plant growth, physiological state of the plants, nutrient composition of plants | two months | [80] |
| leaves, shoots and roots | Cr(VI) 100, 150, and 200 mg L−1 | Four PGP and Cr (IV) resistance bacterial isolates | chlorophyll, proline, hydrogen peroxide, malondialdehyde content | 5 weeks | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jócsák, I.; Knolmajer, B.; Szarvas, M.; Rabnecz, G.; Pál-Fám, F. Literature Review on the Effects of Heavy Metal Stress and Alleviating Possibilities through Exogenously Applied Agents in Alfalfa (Medicago sativa L.). Plants 2022, 11, 2161. https://doi.org/10.3390/plants11162161
Jócsák I, Knolmajer B, Szarvas M, Rabnecz G, Pál-Fám F. Literature Review on the Effects of Heavy Metal Stress and Alleviating Possibilities through Exogenously Applied Agents in Alfalfa (Medicago sativa L.). Plants. 2022; 11(16):2161. https://doi.org/10.3390/plants11162161
Chicago/Turabian StyleJócsák, Ildikó, Bence Knolmajer, Miklós Szarvas, Gyula Rabnecz, and Ferenc Pál-Fám. 2022. "Literature Review on the Effects of Heavy Metal Stress and Alleviating Possibilities through Exogenously Applied Agents in Alfalfa (Medicago sativa L.)" Plants 11, no. 16: 2161. https://doi.org/10.3390/plants11162161
APA StyleJócsák, I., Knolmajer, B., Szarvas, M., Rabnecz, G., & Pál-Fám, F. (2022). Literature Review on the Effects of Heavy Metal Stress and Alleviating Possibilities through Exogenously Applied Agents in Alfalfa (Medicago sativa L.). Plants, 11(16), 2161. https://doi.org/10.3390/plants11162161








