Non-Invasive Evaluation of Different Soil Tillage and Seed Treatment Effects on the Microbial Originating Physiological Reactions of Developing Juvenile Maize
Abstract
:1. Introduction
2. Results
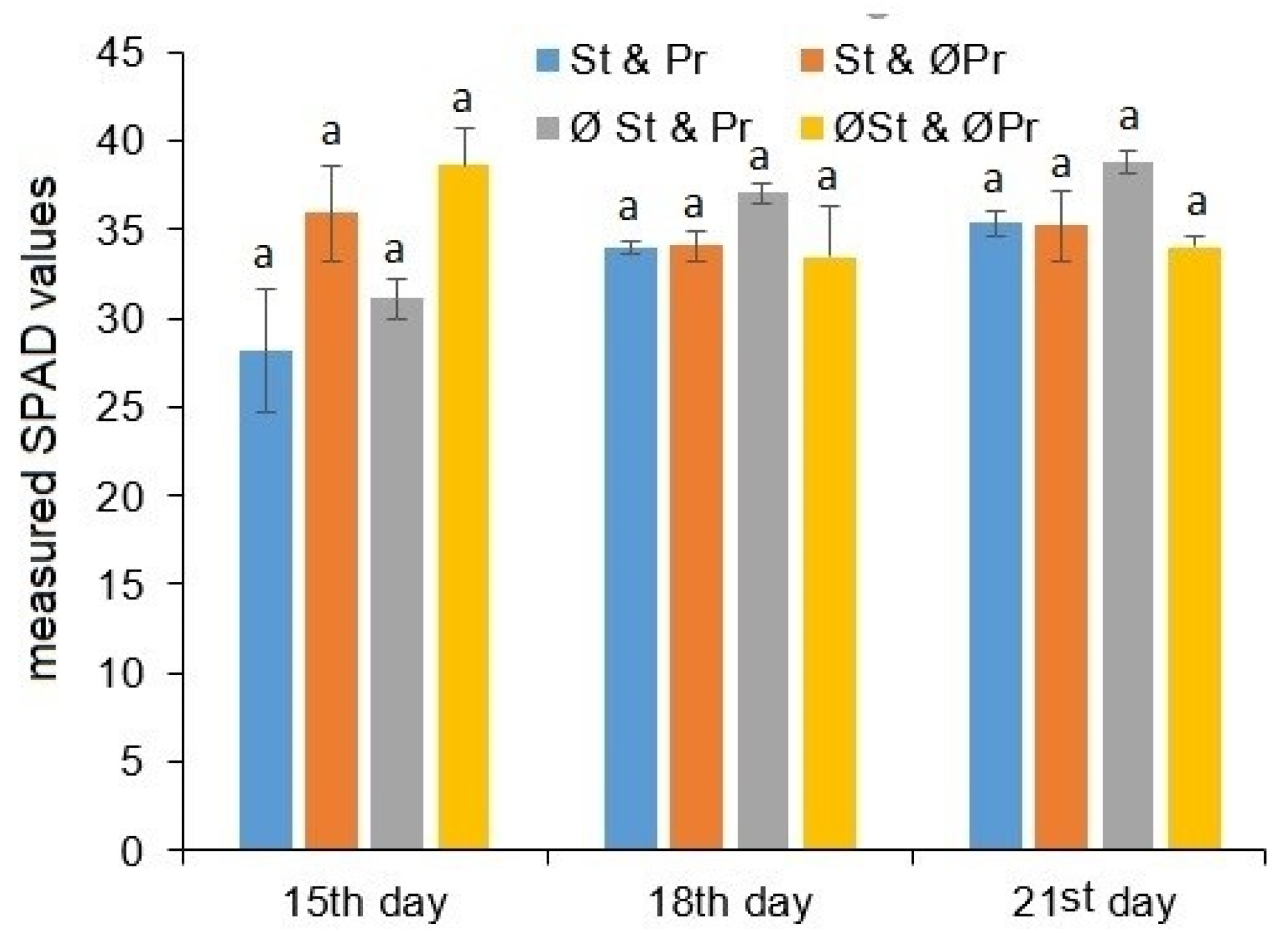
2.1. Plant Growth and Chlorophyll Content Estimation
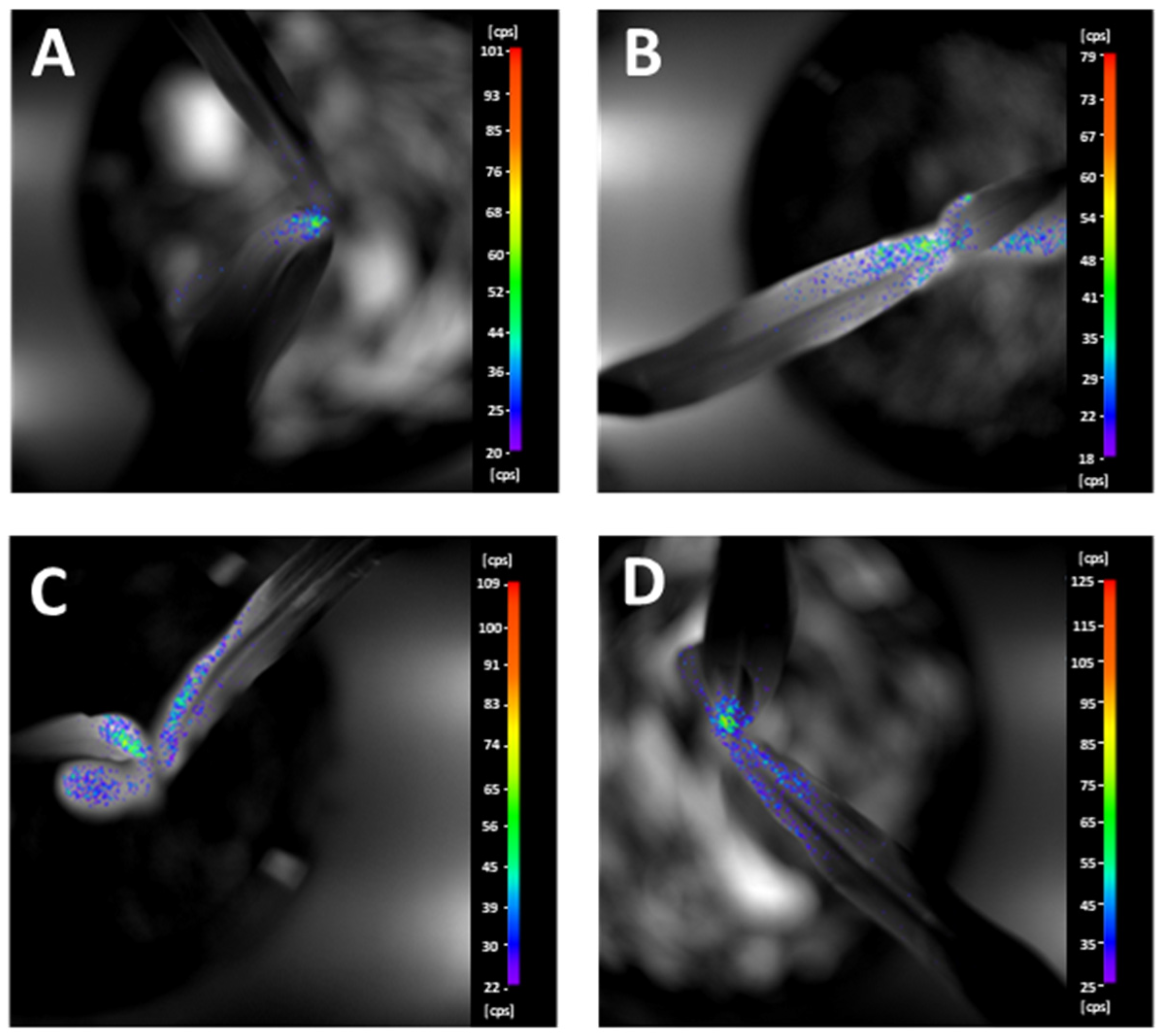
2.2. Imaging of Biophoton Emission
2.3. Microfungi Assortments Deriving from Plant Remains
2.4. Confirmation of the Microfungi Infestation
3. Discussion
4. Materials and Methods
4.1. Sampling and Experimental Settings
- Seeds treated by fungicides combined with plant remains left on the soil surface (St & Pr);
- Seeds treated by fungicides without plant remains (St & ØPr);
- Seeds without fungicide treatment combined with plant remains left on the soil surface (ØSt & Pr);
- Seeds without both fungicide treatment and plant remains (ØSt & ØPr).
4.2. Non-Invasive Imaging of Plant Stress by Means of Detection of Biophoton Emission
4.2.1. Chlorophyll Content Estimation (SPAD Index) Measurement
4.2.2. Biophoton Emission (BPE) Measurement
4.3. Microbial Cultivation of Potential Pathogens from the Plant Remains
- leaf remains (any part of dry and damaged leaves);
- maize stalks (longitudinal sections);
- husk remains;
- maize seeds and ear remains left on stubble;
- sown maize seeds.
4.4. Polymerase Chain Reaction Assay
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the global number and distribution of maize and wheat farms. Glob. Food Secur. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Jaidka, M.; Bathla, S.; Kaur, R. Improved technologies for higher maize production. In Maize-Production and Use; Hossain, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Memon, S.Q.; Mirjat, M.S.; Mughal, A.Q.; Amjad, N. Effect of conventional and non-conventional tillage practices on maize production. Pak. J. Agri. Agril. Eng. Vet. Sci. 2016, 29, 155–163. [Google Scholar]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Guo, R.; Sarwar, M.; Ren, X.; Krstic, D.; Aslam, Z.; Zulifqar, U.; Rauf, A.; Hano, C.; et al. Carbon Sequestration to Avoid Soil Degradation: A Review on the Role of Conservation Tillage. Plants 2021, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Landers, J.N.; de Freitas, P.L.; de Oliveira, M.C.; da Silva Neto, S.P.; Ralisch, R.; Kueneman, E.A. Next steps for conservation agriculture. Agronomy 2021, 11, 2496. [Google Scholar] [CrossRef]
- Selim, M. A review of advantages, disadvantages and challenges of crop rotations. Egypt. J. Agron. 2019, 41, 1–10. [Google Scholar] [CrossRef]
- Kassam, T.; Friedrich, R.; Derpsch, R.; Kienzle, J. Overview of the worldwide spread of conservation agriculture. Field Actions Sci Rep. 2015, 8. Available online: https://journals.openedition.org/factsreports/3966 (accessed on 28 January 2022).
- Akhtar, K.; Wang, W.; Khan, A.; Ren, G.; Afridi, M.Z.; Feng, Y.; Yang, G. Wheat straw mulching offset soil moisture deficient for improving physiological and growth performance of summer sown soybean. Agric. Water Manag. 2019, 211, 16–25. [Google Scholar] [CrossRef]
- Meltsaev, I.G.; Esedullaev, S.T. Effect of tillage methods on the phytosanitary condition and productivity of grain crops. Zas. Karantin Rast. 2019, 10, 13–16. [Google Scholar]
- Wang, H.; Li, X.; Li, X.; Wang, J.; Li, X.; Guo, Q.; Yu, Z.; Yang, T.; Zhang, H. Long-term no-tillage and different residue amounts alter soil microbial community composition and increase the risk of maize root rot in northeast China. Soil Till. Res. 2020, 196, 104452. [Google Scholar] [CrossRef]
- Pamphile, J.A.; Azevedo, J.L. Molecular characterization of endophytic strains of Fusarium verticillioides (=Fusarium moniliforme) from maize (Zea mays. L). World J. Microbiol. Biotechnol. 2002, 18, 391–396. [Google Scholar] [CrossRef]
- Hathout, A.S.; Abo-Sereih, N.A.; Sabry, B.A.; Sahab, A.F.; Aly, S.E. Molecular identification and control of some pathogenic Fusarium species isolated from maize in Egypt. Int. J. Chem Tech. Res. 2015, 7, 44–54. [Google Scholar]
- Taylor, F.W.; Wagner, F.G.; McMillin, C.W.; Morgan, I.L.; Hopkins, F.F. Locating knots by industrial tomography. A feasibility study. For. Prod. J. 1984, 34, 42–46. [Google Scholar]
- Wilson, T.; Hastings, J.W. Bioluminescence. Ann. Rev. Cell Develop. Biol. 1998, 14, 197–230. [Google Scholar] [CrossRef]
- Duran, N.; Cadenas, E. The role of singlet oxygen and triplet carbonyls in biological systems. Rev. Chem. Intermed. 1987, 8, 147–187. [Google Scholar] [CrossRef]
- Jócsák, I.; Malgwi, I.; Rabnecz, G.; Szegő, A.; Varga-Visi, É.; Végvári, G.; Pónya, Z. Effect of cadmium stress on certain physiological parameters, antioxidative enzyme activities and biophoton emission of leaves in barley (Hordeum vulgare L.) seedlings. PLoS ONE 2020, 15, e0240470. [Google Scholar] [CrossRef] [PubMed]
- Pónya, Z.; Jócsák, I.; Keszthelyi, S. Detection of ultra-weak photon emission in sunflower (Helianthus annuus L.) infested by two spotted-spider mite, Tetranychus urticae Koch-research note. Phytoparasitica 2022, 50, 43–50. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Dayal, D.; Bandyopadhyay, K.K.; Mohanty, M. Evaluation of straw and polythene mulch for enhancing productivity of irrigated summer groundnut. Field Crop. Res. 2006, 99, 76–86. [Google Scholar] [CrossRef]
- Ali, S.; Jan, A.; Manzoor; Sohail, A.; Khan, A.; Inamullah, M.I.; Zhang, J.; Daur, I. Soil amendments strategies to improve water-use efficiency and productivity of maize under different irrigation conditions. Agric. Water Manag. 2018, 210, 88–95. [Google Scholar] [CrossRef]
- Diallo, M.D.; Duponnois, R.; Guisse, A.; Sall, S.; Chotte, J.L.; Thioulouse, J. Biological effects of native and exotic plant residues on plant growth, microbial biomass and N availability under controlled conditions. Eur. J. Soil Biol. 2006, 42, 238–246. [Google Scholar] [CrossRef]
- Tian, G.; Kang, B.T.; Brussaard, L. Mulching effect of plant residues with chemically contrasting compositions on maize growth and nutrient accumulation. Plant Soil 1993, 153, 179–187. [Google Scholar] [CrossRef]
- Bradow, J.M.; Connick, W.J. Volatile seed germination inhibitors from plant residues. J. Chem. Ecol. 1990, 16, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Esper Neto, M.; Britt, D.W.; Lara, L.M.; Cartwright, A.; dos Santos, R.F.; Inoue, T.T.; Batista, M.A. Initial Development of Corn Seedlings after Seed Priming with Nanoscale Synthetic Zinc Oxide. Agronomy 2020, 10, 307. [Google Scholar] [CrossRef]
- Kimber, R.W.L. Phytotoxicity from plant residues: III. The relative effect of toxins and nitrogen immobilization on the germination and growth of wheat. Plant Soil 1973, 38, 543–555. [Google Scholar] [CrossRef]
- Munkvold, G.P. Cultural and genetic approaches to managing mycotoxins in maize. Ann. Rev. Phytopath. 2003, 41, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Gouripeddi, P.; Devireddy, H.R.N.; Ovsii, A.; Rachakonda, D.P.; Wijk, R.V.; Pospíšil, P. Spectral distribution of ultra-weak photon emission as a response to wounding in plants: An In Vivo Study. Biology 2020, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Sedlářová, M.; Balukova, A.; Rác, M.; Pospíšil, P. Reactive oxygen species as a response to wounding: In vivo imaging in Arabidopsis thaliana. Front. Plant Sci. 2020, 10, 1660. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, M.; Shi, Y.; Zhu, Z.; Van Wijk, E.; van Wij, R.; van Andel, T.R.; Wang, M. A comparative study of aged and contemporary Chinese herbal materials by using delayed luminescence technique. Chin. Med. 2020, 15, 6. [Google Scholar] [CrossRef]
- Jurado, M.; Vázquez, C.; Patiño, M.T.; González-Jaén, M.T. PCR detection assays for the trichothecene-producing species Fusarium graminearum, Fusarium culmorum, Fusarium poae, Fusarium equiseti and Fusarium sporotrichioides. Syst. Appl. Microbiol. 2005, 28, 562–568. [Google Scholar] [CrossRef]
- Jurado, M.; Vázquez, C.; Marin, S.; Sanchis, V.; González-Jaén, M.T. PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in maize. Syst. Appl. Microbiol. 2006, 29, 681–689. [Google Scholar] [CrossRef]



| Genera | Species | Plant Remains | Seed | |||
|---|---|---|---|---|---|---|
| Leaf | Stalk | Husk | Ear | |||
| Alternaria | A. alternata | 100 | 45 | 6 | - | - |
| A. sp. | ||||||
| Cladosporium | C. herbarum | 95 | 80 | 95 | 1 | - |
| C. cladosporioides | ||||||
| Rhizopus | R. stolonifer | - | 20 | - | 21 | 100 |
| Fusarium | F. verticillioides | 25 | 15 | 25 | 38 | 1 |
| F. spp. | ||||||
| Epicoccum | E. nigrum | 30 | 6 | 6 | - | - |
| Gonatobotrys | G. flava | 30 | - | 12 | - | - |
| Acremoniella | A. atra | 35 | 25 | - | 1 | - |
| Pithomyces | P. chartarum | 6 | - | - | - | - |
| Penicillium | P. spp. | - | 6 | 25 | 64 | 4 |
| Trichoderma | T. sp. | - | - | - | 1 | - |
| Verticillium | V. sp. | - | 6 | - | - | - |
| Aspergillus | A. flavus | - | 12 | - | - | - |
| A. clavatus | - | - | - | - | 1 | |
| Mucor | M. mucedo | - | 6 | - | - | - |
| Nigrospora | N. oryzae | - | 31 | - | - | - |
| Phoma | Ph. sp. | 12 | - | - | - | - |
| Puccinia | P. sorghi | 12 | - | - | - | - |
| Bipolaris | B. maydis | 12 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binder, A.; Jócsák, I.; Varga, Z.; Knolmajer, B.; Keszthelyi, S. Non-Invasive Evaluation of Different Soil Tillage and Seed Treatment Effects on the Microbial Originating Physiological Reactions of Developing Juvenile Maize. Plants 2022, 11, 2506. https://doi.org/10.3390/plants11192506
Binder A, Jócsák I, Varga Z, Knolmajer B, Keszthelyi S. Non-Invasive Evaluation of Different Soil Tillage and Seed Treatment Effects on the Microbial Originating Physiological Reactions of Developing Juvenile Maize. Plants. 2022; 11(19):2506. https://doi.org/10.3390/plants11192506
Chicago/Turabian StyleBinder, Antal, Ildikó Jócsák, Zsolt Varga, Bence Knolmajer, and Sándor Keszthelyi. 2022. "Non-Invasive Evaluation of Different Soil Tillage and Seed Treatment Effects on the Microbial Originating Physiological Reactions of Developing Juvenile Maize" Plants 11, no. 19: 2506. https://doi.org/10.3390/plants11192506
APA StyleBinder, A., Jócsák, I., Varga, Z., Knolmajer, B., & Keszthelyi, S. (2022). Non-Invasive Evaluation of Different Soil Tillage and Seed Treatment Effects on the Microbial Originating Physiological Reactions of Developing Juvenile Maize. Plants, 11(19), 2506. https://doi.org/10.3390/plants11192506








