Transpirational Leaf Cooling Effect Did Not Contribute Equally to Biomass Retention in Wheat Genotypes under High Temperature
Abstract
1. Introduction
2. Results
2.1. Morpho–Physiological Responses under Elevated Temperature
2.1.1. Daily Tr
2.1.2. Leaf Glaucousness
2.1.3. Pubescence
2.1.4. Stomatal Density
Total Stomatal Density (Abaxial and Adaxial Combined)
Abaxial and Adaxial Stomatal Densities
2.2. Growth Parameters
2.2.1. Green Leaf Area
2.2.2. Specific Leaf Weight
2.2.3. Shoot Dry Weight
2.2.4. Root Dry Weight and Shoot: Root Ratio
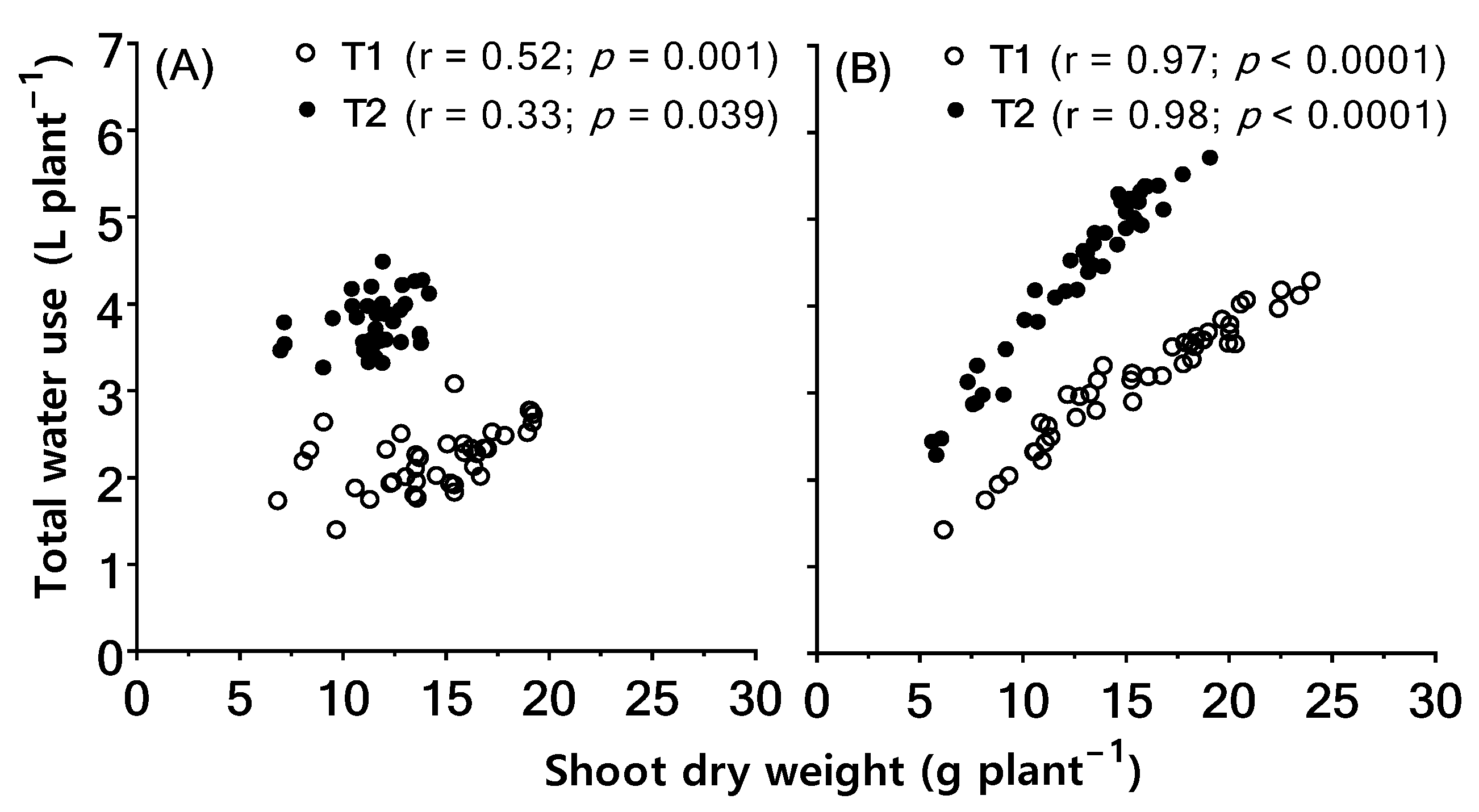
2.3. Associations between Biomass Retention under High Temperature and Total Water Use Per Plant and Leaf-to-Air Temperature Differential (Tair–Tleaf)
2.4. Genotypic Variability in Tleaf Response to Tair
2.5. Relationship between Instantaneous Tr Response to VPD and Tleaf Regulation
3. Discussion
3.1. Greater Water Use through Tr Increases Shoot Biomass Retention under High Temperature
- (1)
- Environmental factors that may confound the relationship between Tair–Tleaf and Tr, such as VPD, Tair, net radiation, and boundary layer resistance [68]. For instance, Tair–Tleaf may not be constant and may increase with Tair. Ideally, Tleaf measurements in each genotype should be made at the same Tair to prevent differences in Tair confounding the results.
- (2)
- Biomass retention under high temperature may be related to heat tolerance, independent of Tair–Tleaf or Tr. The internal tolerance mechanisms of some genotypes could enable them to withstand the heat, despite high Tleaf, without reducing biomass. For instance, the production of heat shock proteins that have a protective role against heat stress can be induced in some wheat genotypes exposed to temperatures around 32 °C [69]. In this study, Tair sometimes exceeded 35 °C, suggesting that some genotypes acquired heat tolerance. However, the reverse can also happen, where some genotypes become more heat-sensitive, even with relatively low Tleaf.
- (3)
- Tair–Tleaf measured once or at different times during the experimental period may not relate to biomass retention or water use during the whole period. The ability to maintain greater Tair–Tleaf throughout the plant’s growing cycle, regardless of growth stage or changes in environmental conditions, is more important (stability) and translates into biomass retention under warmer conditions. This trait may be more prominent in some genotypes than others.
3.2. Water Use through Tr and Associated Morpho-Physiological and Growth Characteristics as Affected by Temperature
3.3. Different Responses between the Two Batches of Genotypes as Explained by Differential Adaptations to Warm and Dry Conditions
3.4. Association of Instantaneous Tr Response to VPD and Tleaf Regulation
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Temperature Treatment
4.3. Experimental Design
4.4. Measurements
4.4.1. Growth and Morpho–Physiological Parameters
4.4.2. Environmental Parameters
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wollenweber, B.; Porter, J.R.; Schellberg, J. Lack of Interaction between Extreme High-Temperature Events at Vegetative and Reproductive Growth Stages in Wheat. J. Agron. Crop Sci. 2003, 189, 142–150. [Google Scholar] [CrossRef]
- Ullah, A.; Nadeem, F.; Nawaz, A.; Siddique, K.H.M.; Farooq, M. Heat stress effects on the reproductive physiology and yield of wheat. J. Agron. Crop Sci. 2021, 208, 1–17. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Asseng, S.; Foster, I.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Shpiler, L.; Blum, A. Differential reaction of wheat cultivars to hot environments. Euphytica 1986, 35, 483–492. [Google Scholar] [CrossRef]
- Zhong-Hu, H.; Rajaram, S. Differential responses of bread wheat characters to high temperature. Euphytica 1993, 72, 197–203. [Google Scholar] [CrossRef]
- Rahman, M.A.; Chikushi, J.; Yoshida, S.; Karim, A. Growth and yield components of wheat genotypes exposed to high temperature stress under control environment. Bangladesh J. Agric. Res. 1970, 34, 360–372. [Google Scholar] [CrossRef]
- Hawker, J.; Jenner, C. High Temperature Affects the Activity of Enzymes in the Committed Pathway of Starch Synthesis in Developing Wheat Endosperm. Funct. Plant Biol. 1993, 20, 197–209. [Google Scholar] [CrossRef]
- Nuttall, J.G.; Barlow, K.M.; Delahunty, A.J.; Christy, B.P.; O’Leary, G.J. Acute High Temperature Response in Wheat. Agron. J. 2018, 110, 1296–1308. [Google Scholar] [CrossRef]
- Paulsen, G.M. High temperature response of crop plants. In Physiology and Determination of Crop Yield; Boote, K.J., Bennet, I.M., Sinclair, T.R., Paulsen, G.M., Eds.; American Society of Agronomy: Madison, WI, USA, 1994; pp. 365–389. [Google Scholar]
- Stone, P. The effects of heat stress on cereal yield and quality of hexaploid wheat. Euphytica. 2001, 126, 275–282. [Google Scholar]
- Dolferus, R.; Ji, X.; Richards, R.A. Abiotic stress and control of grain number in cereals. Plant Sci. 2011, 181, 331–341. [Google Scholar] [CrossRef]
- De Oliveira, E.D.; Palta, J.A.; Bramley, H.; Stefanova, K.; Siddique, K. Elevated CO2 Reduced Floret Death in Wheat Under Warmer Average Temperatures and Terminal Drought. Front. Plant Sci. 2015, 6, 1010. [Google Scholar] [CrossRef] [PubMed]
- Fábián, A.; Sáfrán, E.; Szabó-Eitel, G.; Barnabás, B.; Jäger, K. Stigma Functionality and Fertility Are Reduced by Heat and Drought Co-stress in Wheat. Front. Plant Sci. 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Bramley, H.; Mahmood, T.; Trethowan, R. A strategy of ideotype development for heat-tolerant wheat. J. Agron. Crop Sci. 2019, 206, 229–241. [Google Scholar] [CrossRef]
- Ullah, S.; Trethowan, R.; Bramley, H. The physiological basis of improved heat tolerance in selected emmer-derived hexa-ploid wheat genotypes. Front. Plant Sci. 2021, 12, 739246. [Google Scholar] [CrossRef] [PubMed]
- Coast, O.; Posch, B.C.; Bramley, H.; Gaju, O.; Richards, R.A.; Lu, M.; Ruan, Y.; Trethowan, R.; Atkin, O.K. Acclimation of leaf photosynthesis and respiration to warming in field-grown wheat. Plant, Cell Environ. 2020, 44, 2331–2346. [Google Scholar] [CrossRef]
- Posch, B.C.; Zhai, D.; Coast, O.; Scafaro, A.P.; Bramley, H.; Reich, P.; Ruan, Y.-L.; Trethowan, R.; A Way, D.; Atkin, O. Wheat respiratory O2 consumption falls with night warming alongside greater respiratory CO2 loss and reduced biomass. J. Exp. Bot. 2021, 73, 915–926. [Google Scholar] [CrossRef]
- Shirdelmoghanloo, H.; Cozzolino, D.; Lohraseb, I.; Collins, N.C. Truncation of grain filling in wheat (Triticum aestivum) trig-gered by brief heat stress during early grain filling: Association with senescence responses and reductions in stem re-serves. Funct. Plant Biol. 2016, 43, 919–930. [Google Scholar] [CrossRef]
- Sarkar, S.; Islam, A.; Barma, N.; Ahmed, J. Tolerance mechanisms for breeding wheat against heat stress: A review. South Afr. J. Bot. 2021, 138, 262–277. [Google Scholar] [CrossRef]
- Prasad, P.V.; Bheemanahalli, R.; Jagadish, S.K. Field crops and the fear of heat stress—opportunities, challenges and future directions. Field Crops Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Vilagrosa, A.; Alonso-Forn, D.; Ferrio, J.P.; Sancho-Knapik, D.; Gil-Pelegrín, E. Living in drylands: Func-tional adaptations of trees and shrubs to cope with high temperatures and water scarcity. Forests 2020, 11, 1028. [Google Scholar] [CrossRef]
- Matsumoto, J.; Muraoka, H.; Washitani, I. Ecophysiological Mechanisms used by Aster kantoensis, an Endangered Species, to Withstand High Light and Heat Stresses of its Gravelly Floodplain Habitat. Ann. Bot. 2000, 86, 777–785. [Google Scholar] [CrossRef]
- Yadav, S.K.; Tiwari, Y.K.; Kumar, D.P.; Shanker, A.; Lakshmi, N.J.; Vanaja, M.; Maheswari, M. Genotypic Variation in Physiological Traits Under High Temperature Stress in Maize. Agric. Res. 2015, 5, 119–126. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Murty, M.V.R.; Quick, W.P. Rice responses to rising temperatures–challenges, perspectives and future di-rections. Plant Cell Environ. 2015, 38, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- De Costa, W.A.J.M. Plant Water Relations: Principles and Applications; University of Peradeniya: Peradeniya, Sri Lanka, 2004. [Google Scholar]
- Rehman, H.; Tariq, A.; Ashraf, I.; Ahmed, M.; Muscolo, A.; Basra, S.; Reynolds, M. Evaluation of Physiological and Morphological Traits for Improving Spring Wheat Adaptation to Terminal Heat Stress. Plants 2021, 10, 455. [Google Scholar] [CrossRef]
- Gates. Transpiration and leaf temperature. Annu. Rev. Plant Physiol. 1968, 19, 211–238. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Zhang, H.; Fu, P.; Fan, Z. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 2017, 31, 2202–2211. [Google Scholar] [CrossRef]
- Clum, H.H. The effect of transpiration and environmental factors on leaf temperatures I. transpiration. Am. J. Bot. 1926, 13, 194–216. [Google Scholar] [CrossRef]
- Curtis, O.F. Transpiration and the cooling of leaves. Am. J. Bot. 1936, 23, 7–10. [Google Scholar] [CrossRef]
- Maricle, B.R.; Cobos, D.R.; Campbell, C.S. Biophysical and morphological leaf adaptations to drought and salinity in salt marsh grasses. Environ. Exp. Bot. 2007, 60, 458–467. [Google Scholar] [CrossRef]
- Shull, C.A. The mass factor in the energy relations of leaves. Plant Physiol. 1930, 5, 279–282. [Google Scholar] [CrossRef]
- Clements, H.F. SIGNIFICANCE OF TRANSPIRATION. Plant Physiol. 1934, 9, 165–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monteiro, M.V.; Blanuša, T.; Verhoef, A.; Hadley, P.; Cameron, R.W.F. Relative importance of transpiration rate and leaf morphological traits for the regulation of leaf temperature. Aust. J. Bot. 2016, 64, 32–44. [Google Scholar] [CrossRef]
- Hegde, V.S.; Yadav, S.S.; Kumar, J. Heterosis and combining ability for biomass and harvest index in chickpea under a drought-prone, short-duration environment. Euphytica 2007, 157, 223–230. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Bennett, J.M.; Muchow, R.C. Relative Sensitivity of Grain Yield and Biomass Accumulation to Drought in Field-Grown Maize. Crop Sci. 1990, 30, 690–693. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, P.; Wang, L.X.; Hu, Z.L.; Zhu, L.H.; Zhu, Y.G. Genetic dissection of the relationships of biomass production and partitioning with yield and yield related traits in rice. Plant Sci. 2004, 167, 1–8. [Google Scholar] [CrossRef]
- Sharma, R.C. Selection for biomass yield in wheat. Euphytica 1993, 70, 35–42. [Google Scholar] [CrossRef]
- Hughes, W.G.; Westcott, B.; Sharp, P.L. Joint Selection for High Yield and Low Sensitivity in Winter Wheat (Triticum aestivum L.). Plant Breed. 1987, 99, 107–117. [Google Scholar] [CrossRef]
- Damisch, W.; Wiberg, A. Biomass Yield—A Topical Issue in Modern Wheat Breeding Programmes. Plant Breed. 1991, 107, 11–17. [Google Scholar] [CrossRef]
- Boukerrou, L.; Rasmusson, D.D. Breeding for High Biomass Yield in Spring Barley. Crop Sci. 1990, 30, 31–35. [Google Scholar] [CrossRef]
- Laza, M.R.; Peng, S.; Akita, S.; Saka, H. Contribution of Biomass Partitioning and Translocation to Grain Yield under Sub-Optimum Growing Conditions in Irrigated Rice. Plant Prod. Sci. 2003, 6, 28–35. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Wang, A.; Wang, S.; Tang, L.; Cao, W.; Zhu, Y.; Liu, L. Modelling the effects of post-heading heat stress on biomass growth of winter wheat. Agric. For. Meteorol. 2017, 247, 476–490. [Google Scholar] [CrossRef]
- Richards, R.A.; Gómez-Macpherson, H.; Condon, A. Improving the efficiency of water use by plant breeding and molecular biology. Irrig. Sci. 1993, 14, 93–104. [Google Scholar] [CrossRef]
- López-Castañeda, C.; Richards, R. Variation in temperate cereals in rainfed environments III. Water use and water-use efficiency. Field Crop. Res. 1994, 39, 85–98. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E. On the conservative behavior of biomass water productivity. Irrig. Sci. 2007, 25, 189–207. [Google Scholar] [CrossRef]
- Xue, Q.; Stewart, B.A.; Lazar, M.D.; Piccinni, G.; Salisbury, C.D. Genotypic Variation of Osmotic Adjustment, Water-use and Transpiration Efficiency among Closely Related Wheat Lines. J. Crop Improv. 2012, 26, 258–281. [Google Scholar] [CrossRef]
- Musick, J.T.; Jones, O.R.; Stewart, B.A.; Dusek, D.A. Water-yield relationship for irrigated and dryland wheat in the U. S. Southern Plains. Agron. J. 1994, 86, 980–986. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crop. Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Trnka, M.; Feng, S.; Semenov, M.A.; Olesen, J.E.; Kersebaum, K.C.; Rötter, R.P.; Semerádová, D.; Klem, K.; Huang, W.; Ruiz-Ramos, M.; et al. Mitigation efforts will not fully alleviate the in-crease in water scarcity occurrence probability in wheat-producing areas. Sci. Adv. 2019, 5, eaau2406. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Nassiri, M.; Koocheki, A.; Kamali, G.A.; Shahandeh, H. Potential impact of climate change on rainfed wheat production in Iran. Arch. Agron. Soil Sci. 2006, 52, 113–124. [Google Scholar] [CrossRef]
- Amani, I.; Fischer, R.A.; Reynolds, M.P. Canopy temperature depression association with yield of irrigated spring wheat cul-tivars in a hot climate. J. Agron. Crop Sci. 1996, 176, 119–129. [Google Scholar] [CrossRef]
- Cossani, C.M.; Pietragalla, J.; Reynolds, M.P. Canopy temperature and plant water relations traits. In Physiological Breeding I: Interdisciplinary Approaches to Improve Crop Adaptation; Reynolds, M.P., Pask, A.J.D., Mullan, D.M., Eds.; CIMMYT: Texcoco, Mexico, 2012; pp. 60–68. [Google Scholar]
- Blum, A.; Mayer, J.; Gozlan, G. Infrared thermal sensing of plant canopies as a screening technique for dehydration avoidance in wheat. Field Crops Res. 1982, 5, 137–146. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P. Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct. Plant Biol. 2010, 37, 147–156. [Google Scholar] [CrossRef]
- Reynolds, M.; Balota, M.; Delgado, M.; Amani, I.; Fischer, R. Physiological and Morphological Traits Associated With Spring Wheat Yield Under Hot, Irrigated Conditions. Funct. Plant Biol. 1994, 21, 717–730. [Google Scholar] [CrossRef]
- Olivares-Villegas, J.J.; Reynolds, M.P.; McDonald, G.K. Drought-adaptive attributes in the Seri/Babax hexaploid wheat pop-ulation. Funct. Plant Biol. 2007, 34, 189–203. [Google Scholar]
- Pietragalla, J. Canopy temperature. In Physiological Breeding II: A Field Guide to Wheat Phenotyping; Pask, A.J.D., Pietragalla, J., Mullan, D.M., Reynolds, M.P., Eds.; CIMMYT: Texcoco, Mexico, 2012; pp. 10–14. [Google Scholar]
- Ghojogh, H.; Mohammadi, V.; Zeinali, H.; Alizadeh, H. Efficiency of canopy temperature and relative water content in screening wheat genotypes for heat tolerance. Iran. J. Field Crop Sci. 2019, 50, 195–207. [Google Scholar]
- Sinclair, T.R.; Tanner, C.B.; Bennett, J.M. Water-Use Efficiency in Crop Production. BioScience 1984, 34, 36–40. [Google Scholar] [CrossRef]
- Richards, R.; Rawson, H.; Johnson, D. Glaucousness in Wheat: Its Development and Effect on Water-use Efficiency, Gas Exchange and Photosynthetic Tissue Temperatures*. Funct. Plant Biol. 1986, 13, 465–473. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Levitt, J. Responses of Plants to Environmental Stresses: Chilling, Freezing, and High Temperature Stresses; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Bonos, S.A.; Murphy, J.A. Growth Responses and Performance of Kentucky Bluegrass under Summer Stress. Crop Sci. 1999, 39, 770–774. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, C.; Huang, B. Heat Shock Proteins in Association with Heat Tolerance in Grasses. Int. J. Proteom. 2011, 2011, 529648. [Google Scholar] [CrossRef]
- Smith, R.C.G.; Barrs, H.D.; Steiner, J.L. Alternative models for predicting the foliage–air temperature difference of well irri-gated wheat under variable meteorological conditions. I. derivation of parameters II. accuracy of predictions. Irrig. Sci. 1986, 7, 225–236. [Google Scholar] [CrossRef]
- Hendershot, K.L.; Weng, J.; Nguyen, H.T. Induction Temperature of Heat-Shock Protein Synthesis in Wheat. Crop Sci. 1992, 32, 256–261. [Google Scholar] [CrossRef]
- Ranawana, S.R.W.M.C.J.K.; Siddique, K.H.M.; Palta, J.A.; Stefanova, K.; Bramley, H. Stomata coordinate with plant hydraulics to regulate transpiration response to vapour pressure deficit in wheat. Funct. Plant Biol. 2021, 48, 839–850. [Google Scholar] [CrossRef]
- Frank, A.B.; Power, J.F.; Willis, W.O. Effect of Temperature and Plant Water Stress on Photosynthesis, Diffusion Resistance, and Leaf Water Potential in Spring Wheat. Agron. J. 1973, 65, 777–780. [Google Scholar] [CrossRef]
- Salisbury, E.J.I. On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora. Philos. Trans. R. Soc. Ser. B 1928, 216, 1–65. [Google Scholar] [CrossRef]
- Leuschner, C. Air humidity as an ecological factor for woodland herbs: Leaf water status, nutrient uptake, leaf anatomy, and productivity of eight species grown at low or high VPD levels. Flora Morphol. Distrib. Funct. Ecol. Plants 2002, 197, 262–274. [Google Scholar] [CrossRef]
- Murphy, M.R.C.; Jordan, G.J.; Brodribb, T.J. Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant Cell Environ. 2013, 37, 124–131. [Google Scholar] [CrossRef]
- Bakker, J. Effects of humidity on stomatal density and its relation to leaf conductance. Sci. Hortic. 1991, 48, 205–212. [Google Scholar] [CrossRef]
- Woodward, F.I.; Bazzaz, F.A. The Responses of Stomatal Density to CO2 Partial Pressure. J. Exp. Bot. 1988, 39, 1771–1781. [Google Scholar] [CrossRef]
- Ohsumi, A.; Kanemura, T.; Homma, K.; Horie, T.; Shiraiwa, T. Genotypic Variation of Stomatal Conductance in Relation to Stomatal Density and Length in Rice (Oryza sativa L.). Plant Prod. Sci. 2007, 10, 322–328. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Araus, J.L.; Febrero, A.; Vendrell, P. Epidermal conductance in different parts of durum wheat grown under Mediterranean conditions: The role of epicuticular waxes and stomata. Plant Cell Environ. 1991, 14, 545–558. [Google Scholar] [CrossRef]
- Wardlaw, I.; Sofield, I.; Cartwright, P. Factors Limiting the Rate of Dry Matter Accumulation in the Grain of Wheat Grown at High Temperature. Funct. Plant Biol. 1980, 7, 387–400. [Google Scholar] [CrossRef]
- Hotsonyame, G.K.; Hunt, L.A. Seeding date, photoperiod and nitrogen effects on specific leaf area of field-grown wheat. Can. J. Plant Sci. 1998, 78, 51–61. [Google Scholar] [CrossRef]
- Garnier, E.; Laurent, G. Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. N. Phytol. 1994, 128, 725–736. [Google Scholar] [CrossRef]
- Ehleringer, J.; Björkman, O.; Mooney, H.A. Leaf Pubescence: Effects on Absorptance and Photosynthesis in a Desert Shrub. Science 1976, 192, 376–377. [Google Scholar] [CrossRef]
- Johnson, J.D.; Ferrell, W.K. Stomatal response to vapour pressure deficit and the effect of plant water stress. Plant Cell Environ. 1983, 6, 451–456. [Google Scholar]
- Australian Grain Technologies. Espada-Fact Sheet; AGT: Roseworthy, Australia, 2010. [Google Scholar]
- Ferguson, H.; Cooper, C.S.; Brown, J.H.; Eslick, R.F. Effect of leaf color, chlorohyll concentration, and temperature on pho-tosynthetic rates of isogenic barley lines. Agron. J. 1972, 64, 671–673. [Google Scholar] [CrossRef]
- Lin, B.-S.; Lin, Y.-J. Cooling Effect of Shade Trees with Different Characteristics in a Subtropical Urban Park. HortScience 2010, 45, 83–86. [Google Scholar] [CrossRef]
- Izanloo, A.; Condon, A.G.; Langridge, P.; Tester, M.; Schnurbusch, T. Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. J. Exp. Bot. 2008, 59, 3327–3346. [Google Scholar] [CrossRef]
- Roberts, J.J.; Gallun, R.L.; Patterson, F.L.; Finney, R.E.; Ohm, H.W.; Shaner, G.E. Registration of Downy wheat (Reg. No. 641). Crop Sci. 1981, 21, 350. [Google Scholar] [CrossRef]
- Sharma, H.C.; Waines, J.G. Inheritance of Leaf Pubescence in Diploid Wheat. J. Hered. 1994, 85, 286–288. [Google Scholar] [CrossRef]
- Pinter Jr, P.J.; Jackson, R.D.; Ezra, C.E.; Gausman, H.W. Sun-angle and canopy-architecture effects on the spectral reflectance of six wheat cultivars. Int. J. Remote Sens. 1985, 6, 1813–1825. [Google Scholar] [CrossRef]
- InterGrain. Magenta: Product Profile; InterGrain Pty Ltd.: Bibra Lake, WA, USA, 2012. [Google Scholar]
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G.; van Herwaarden, A.F. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci. 2002, 42, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bourgault, M.; Dreccer, M.F.; James, A.T.; Chapman, S.C. Genotypic variability in the response to elevated CO2 of wheat lines differing in adaptive traits. Funct. Plant Biol. 2013, 40, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Dias de Oliveira, E.; Bramley, H.; Siddique, K.H.M.; Henty, S.; Berger, J.; Palta, J.A. Can elevated CO2 combined with high temperature ameliorate the effect of terminal drought in wheat? Funct. Plant Biol. 2013, 40, 160–171. [Google Scholar] [CrossRef]
- Akbari, M.; Wenzl, P.; Caig, V.; Carling, J.; Xia, L.; Yang, S.; Uszynski, G.; Mohler, V.; Lehmensiek, A.; Kuchel, H.; et al. Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor. Appl. Genet. 2006, 113, 1409–1420. [Google Scholar] [CrossRef]
- Richards, R.A. Physiological traits used in the breeding of new cultivars for water-scarce environments. Agric. Water Manag. 2006, 80, 197–211. [Google Scholar] [CrossRef]
- Hare, R.A. Characterization and Inheritance of Adult Plant Stem Rust Resistance in Durum Wheat. Crop Sci. 1997, 37, 1094–1098. [Google Scholar] [CrossRef]
- Ford, K.L.; Cassin, A.; Bacic, A.F. Quantitative Proteomic Analysis of Wheat Cultivars with Differing Drought Stress Tolerance. Front. Plant Sci. 2011, 2, 44. [Google Scholar] [CrossRef]
- InterGrain. Emu Rock: Product Profile; InterGrain Pty Ltd.: Bibra Lake, WA, USA, 2012. [Google Scholar]
- Australian Grain Technologies. Mace: Wheat Variety Fact Sheet for South Australia and Western Victoria; AGT: Roseworthy, Australia, 2013. [Google Scholar]
- Australian Grain Technologies. Gladius: Wheat Variety Fact Sheet for South Australia; AGT: Roseworthy, Australia, 2010. [Google Scholar]
- Fleury, D.; Jefferies, S.; Kuchel, H.; Langridge, P. Genetic and genomic tools to improve drought tolerance in wheat. J. Exp. Bot. 2010, 61, 3211–3222. [Google Scholar] [CrossRef] [PubMed]
- Bramley, H.; Turner, N.; Turner, D.; Tyerman, S. Roles of Morphology, Anatomy, and Aquaporins in Determining Contrasting Hydraulic Behavior of Roots. Plant Physiol. 2009, 150, 348–364. [Google Scholar] [CrossRef]
- Saradadevi, R.; Bramley, H.; Siddique, K.H.M.; Edwards, E.; Palta, J.A. Contrasting stomatal regulation and leaf ABA con-centrations in wheat genotypes when split root systems were exposed to terminal drought. Field Crops Res. 2014, 162, 77–86. [Google Scholar] [CrossRef]
- Turner, N.C.; Palta, J.A.; Shrestha, R.; Ludwig, C.; Siddique, K.H.M.; Turner, D.W. Carbon isotope discrimination is not cor-related with transpiration efficiency in three cool-season grain legumes (Pulses). J. Integr. Plant Biol. 2007, 49, 1478–1483. [Google Scholar] [CrossRef]
- Fletcher, A.; Chenu, K. Change in biomass partitioning and transpiration efficiency in Australian wheat varieties over the last decades. In Building Productive, Diverse and Sustainable Landscapes, Proceedings of the 17th Australian Agronomy Conference, Hobart, Australia, 20–24 September 2015; Acuna, T.B., Harrison, M., Moeller, C., Parsons, D., Eds.; Australian Society of Agronomy Inc.: Horsham, Australia, 2015; pp. 394–397. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Acevedo, E.; Silva, P.; Silva, H. Wheat growth and physiology. In Bread Wheat: Improvement and Production; Curtis, B.C., Rajaram, S., Macpherson, H.G., Eds.; FAO: Rome, Italy, 2002; pp. 39–70. [Google Scholar]
- Liao, M.; Palta, J.A.; Fillery, I.R.P. Root characteristics of vigorous wheat improve early nitrogen uptake. Aust. J. Agric. Res. 2006, 57, 1097–1107. [Google Scholar] [CrossRef]
- Palta, J.A.; Fillery, I.R.P.; Rebetzke, G.J. Restricted-tillering wheat does not lead to greater investment in roots and early nitrogen uptake. Field Crops Res. 2007, 104, 52–59. [Google Scholar] [CrossRef]
- Monteith, J.L.; Unsworth, M.H. Principles of Environmental Physics, 2nd ed.; Edward Arnold: London, UK, 1990. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 11 August 2021).
- Gilmour, A.R.; Gogel, B.; Cullis, B.; Thompson, R. ASReml User Guide Release 3.0; VSN International Ltd.: Hemel Hempstead, UK, 2009. [Google Scholar]



| Batch 01 | Batch 02 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype (G) | Treatment (T) | Stomatal Density (Number of Stomata per mm−2) | Genotype (G) | Treatment (T) | Stomatal Density (Number of Stomata per mm−2) | ||||
| Abaxial | Adaxial | Total | Abaxial | Adaxial | Total | ||||
| Excalibur | T1 | 58.7 ± 1.7 | 59.1 ± 1.1 | 117.9 ± 2.0 | Drysdale | T1 | 49.1 ± 1.4 | 61.1 ± 1.5 | 110.2 ± 2.5 |
| T2 | 71.2 ± 1.2 | 70.7 ± 5.2 | 141.8 ± 6.3 | T2 | 58.5 ± 1.1 | 67.0 ± 1.3 | 125.5 ± 1.7 | ||
| Glennson 81 | T1 | 52.5 ± 2.4 | 66.7 ± 3.5 | 119.2 ± 1.3 | LongReach-Envoy | T1 | 60.7 ± 1.8 | 61.3 ± 2.6 | 122.1 ± 2.4 |
| T2 | 53.0 ± 3.5 | 79.2 ± 4.4 | 132.2 ± 6.9 | T2 | 63.7 ± 2.2 | 70.1 ± 2.5 | 133.9 ± 2.4 | ||
| Sonora 64 | T1 | 55.7 ± 2.8 | 64.0 ± 3.6 | 119.7 ± 5.3 | Hartog | T1 | 57.7 ± 0.6 | 64.2 ± 1.7 | 122.0 ± 1.3 |
| T2 | 55.9 ± 2.5 | 63.0 ± 2.4 | 118.9 ± 1.3 | T2 | 66.9 ± 0.9 | 78.3 ± 1.6 | 145.2 ± 2.4 | ||
| Downey | T1 | 36.5 ± 2.4 | 46.8 ± 6.1 | 83.3 ± 8.3 | Glossy-Huguenot | T1 | 71.9 ± 1.3 | 80.0 ± 1.0 | 151.9 ± 2.1 |
| T2 | 43.0 ± 1.0 | 52.5 ± 4.3 | 95.5 ± 5.0 | T2 | 82.7 ± 3.3 | 83.1 ± 2.3 | 165.9 ± 4.2 | ||
| Einkorn | T1 | 66.0 ± 5.5 | 59.2 ± 2.3 | 125.2 ± 7.7 | RAC 875 | T1 | 49.0 ± 5.8 | 62.2 ± 4.3 | 111.2 ± 10.0 |
| T2 | 80.5 ± 3.0 | 73.5 ± 4.9 | 154.0 ± 7.6 | T2 | 55.4 ± 1.2 | 66.9 ± 2.3 | 122.2 ± 2.0 | ||
| Ciano 67 | T1 | 51.3 ± 2.0 | 53.3 ± 2.2 | 104.7 ± 4.1 | Espada | T1 | 47.9 ± 2.9 | 53.5 ± 2.4 | 101.4 ± 4.1 |
| T2 | 54.8 ± 3.2 | 56.5 ± 2.7 | 111.3 ± 5.9 | T2 | 57.5 ± 5.1 | 62.0 ± 6.1 | 119.5 ± 11.2 | ||
| Yecora 70 | T1 | 52.3 ± 4.1 | 70.2 ± 0.4 | 122.5 ± 4.1 | Mace | T1 | 50.0 ± 0.6 | 52.0 ± 0.3 | 102.0 ± 0.7 |
| T2 | 60.0 ± 2.5 | 63.2 ± 1.2 | 123.2 ± 3.7 | T2 | 57.9 ± 5.6 | 66.0 ± 1.8 | 123.9 ± 6.5 | ||
| Magenta | T1 | 46.5 ± 3.2 | 60.0 ± 3.1 | 106.5 ± 2.5 | Gladius | T1 | 53.2 ± 1.9 | 65.9 ± 2.2 | 119.1 ± 4.1 |
| T2 | 53.1 ± 4.9 | 72.6 ± 3.7 | 125.7 ± 8.1 | T2 | 64.0 ± 4.2 | 74.2 ± 4.1 | 138.2 ± 5.6 | ||
| Janz | T1 | 49.2 ± 2.1 | 56.2 ± 1.7 | 105.5 ± 2.4 | Kukri | T1 | 54.5 ± 4.3 | 62.2 ± 1.6 | 116.7 ± 5.4 |
| T2 | 51.1 ± 3.8 | 67.0 ± 2.1 | 118.1 ± 5.6 | T2 | 54.5 ± 3.3 | 62.8 ± 2.1 | 117.3 ± 5.2 | ||
| Wyalkatchem | T1 | 65.4 ± 1.4 | 55.6 ± 0.9 | 121.0 ± 2.0 | Emu Rock | T1 | 61.9 ± 1.9 | 63.5 ± 1.5 | 125.4 ± 1.5 |
| T2 | 53.4 ± 1.2 | 65.0 ± 2.0 | 118.4 ± 2.5 | T2 | 63.4 ± 3.4 | 65.9 ± 3.7 | 129.2 ± 5.3 | ||
| Wyalkatchem | T1 | 59.4 ± 1.8 | 63.4 ± 4.5 | 122.7 ± 5.8 | |||||
| (Reference) | T2 | 60.5 ± 1.9 | 68.6 ± 3.6 | 129.1 ± 5.1 | |||||
| p value: G | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| T | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| G × T | 0.003 | 0.056 | 0.037 | 0.502 | 0.309 | 0.392 | |||
| LSD value: G | 6.010 | 6.508 | 10.194 | 5.960 | 5.669 | 9.861 | |||
| T | 2.655 | 2.875 | 4.503 | 2.539 | 2.415 | 4.200 | |||
| G × T | 8.540 | NS | 14.486 | NS | NS | NS | |||
| Batch 01 | Batch 02 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype (G) | Treatment (T) | Green Leaf Area (m2 plant−1) | Shoot Dry Weight (g plant−1) | Specific Leaf Weight (g m−2) | Genotype (G) | Treatment (T) | Green Leaf Area (m2 plant−1) | Shoot Dry Weight (g plant−1) | Specific Leaf Weight (g m−2) |
| Excalibur | T1 | 0.14 ± 0.03 | 13.9 ± 1.9 | 42.7 ± 1.3 | Drysdale | T1 | 0.08 ± 0.00 | 19.3 ± 0.6 | 52.5 ± 1.0 |
| T2 | 0.07 ± 0.01 | 11.7 ± 0.4 | 49.6 ± 0.8 | T2 | 0.03 ± 0.00 | 15.0 ± 0.5 | 51.4 ± 2.2 | ||
| Glennson 81 | T1 | 0.17 ± 0.01 | 15.3 ± 1.6 | 46.8 ± 1.8 | LongReach-Envoy | T1 | 0.14 ± 0.01 | 12.5 ± 1.2 | 45.9 ± 2.2 |
| T2 | 0.07 ± 0.01 | 10.6 ± 0.6 | 51.9 ± 1.4 | T2 | 0.05 ± 0.00 | 12.0 ± 1.3 | 57.9 ± 2.7 | ||
| Sonora 64 | T1 | 0.09 ± 0.00 | 14.6 ± 0.7 | 49.3 ± 1.9 | Hartog | T1 | 0.07 ± 0.01 | 14.3 ± 1.6 | 50.0 ± 1.5 |
| T2 | 0.06 ± 0.00 | 12.7 ± 0.6 | 47.9 ± 0.8 | T2 | 0.02 ± 0.00 | 9.5 ± 1.7 | 51.6 ± 1.1 | ||
| Downey | T1 | 0.24 ± 0.02 | 12.7 ± 1.0 | 34.7 ± 3.1 | Glossy-Huguenot | T1 | 0.11 ± 0.00 | 19.5 ± 1.3 | 56.7 ± 2.6 |
| T2 | 0.23 ± 0.01 | 10.8 ± 0.5 | 39.2 ± 2.8 | T2 | 0.06 ± 0.00 | 14.0 ± 0.7 | 57.0 ± 1.1 | ||
| Einkorn | T1 | 0.20 ± 0.00 | 8.1 ± 0.5 | 31.6 ± 2.2 | RAC 875 | T1 | 0.10 ± 0.00 | 20.6 ± 1.2 | 54.6 ± 1.7 |
| T2 | 0.12 ± 0.00 | 7.2 ± 0.2 | 40.8 ± 1.7 | T2 | 0.05 ± 0.00 | 15.4 ± 0.3 | 57.3 ± 1.8 | ||
| Ciano 67 | T1 | 0.09 ± 0.00 | 16.9 ± 0.7 | 46.2 ± 0.6 | Espada | T1 | 0.10 ± 0.00 | 19.3 ± 2.3 | 55.1 ± 2.1 |
| T2 | 0.04 ± 0.00 | 12.0 ± 0.3 | 45.8 ± 0.9 | T2 | 0.05 ± 0.00 | 17.0 ± 0.7 | 55.8 ± 1.6 | ||
| Yecora 70 | T1 | 0.12 ± 0.01 | 15.9 ± 0.4 | 45.4 ± 2.7 | Mace | T1 | 0.08 ± 0.01 | 16.4 ± 2.1 | 51.1 ± 0.7 |
| T2 | 0.05 ± 0.00 | 11.7 ± 0.3 | 43.9 ± 1.4 | T2 | 0.04 ± 0.00 | 12.3 ± 1.2 | 56.3 ± 1.2 | ||
| Magenta | T1 | 0.18 ± 0.00 | 16.9 ± 1.2 | 45.1 ± 2.5 | Gladius | T1 | 0.08 ± 0.01 | 12.8 ± 1.5 | 51.5 ± 1.2 |
| T2 | 0.07 ± 0.01 | 12.5 ± 0.4 | 44.6 ± 1.4 | T2 | 0.04 ± 0.00 | 13.0 ± 2.2 | 50.2 ± 4.1 | ||
| Janz | T1 | 0.16 ± 0.01 | 16.2 ± 1.2 | 47.6 ± 1.7 | Kukri | T1 | 0.08 ± 0.01 | 16.1 ± 2.7 | 49.3 ± 1.8 |
| T2 | 0.08 ± 0.01 | 13.1 ± 0.6 | 44.2 ± 2.1 | T2 | 0.03 ± 0.00 | 8.9 ± 1.2 | 52.4 ± 0.5 | ||
| Wyalkatchem | T1 | 0.15 ± 0.02 | 13.2 ± 0.5 | 40.4 ± 0.6 | Emu Rock | T1 | 0.05 ± 0.01 | 11.9 ± 2.2 | 49.7 ± 1.2 |
| T2 | 0.08 ± 0.01 | 11.5 ± 0.3 | 44.3 ± 4.8 | T2 | 0.03 ± 0.00 | 11.6 ± 2.0 | 51.9 ± 0.5 | ||
| Wyalkatchem | T1 | 0.07 ± 0.01 | 11.2 ± 1.3 | 45.9 ± 2.1 | |||||
| (reference) | T2 | 0.04 ± 0.01 | 10.9 ± 1.9 | 56.0 ± 2.9 | |||||
| p value: G | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.004 | |||
| T | <0.001 | <0.001 | 0.019 | <0.001 | <0.001 | 0.000 | |||
| G × T | 0.003 | 0.139 | 0.048 | 0.006 | 0.241 | 0.012 | |||
| LSD value: G | 0.023 | 1.662 | 4.154 | 0.013 | 3.152 | 3.835 | |||
| T | 0.010 | 0.743 | 1.858 | 0.005 | 1.344 | 1.635 | |||
| G × T | 0.032 | NS | 5.874 | 0.018 | NS | 5.424 | |||
| Genotype (G) | Treatment (T) | Root Dry Weight (g plant−1) | Shoot:Root Ratio |
|---|---|---|---|
| Drysdale | T1 | 2.2 ± 0.3 | 8.9 ± 1.0 |
| T2 | 0.9 ± 0.0 | 15.9 ± 0.5 | |
| LongReach-Envoy | T1 | 2.0 ± 0.2 | 6.2 ± 0.5 |
| T2 | 1.1 ± 0.1 | 10.6 ± 0.2 | |
| Hartog | T1 | 1.7 ± 0.1 | 8.6 ± 0.8 |
| T2 | 0.5 ± 0.1 | 18.2 ± 0.7 | |
| Glossy-Huguenot | T1 | 3.1 ± 0.2 | 6.4 ± 0.5 |
| T2 | 2.1 ± 0.2 | 6.8 ± 0.5 | |
| RAC 875 | T1 | 2.1 ± 0.2 | 10.0 ± 0.8 |
| T2 | 1.3 ± 0.1 | 12.4 ± 0.7 | |
| Espada | T1 | 2.3 ± 0.4 | 8.5 ± 0.5 |
| T2 | 1.5 ± 0.2 | 11.4 ± 1.1 | |
| Mace | T1 | 1.5 ± 0.2 | 10.8 ± 1.1 |
| T2 | 0.9 ± 0.1 | 13.8 ± 0.4 | |
| Gladius | T1 | 1.7 ± 0.3 | 7.8 ± 0.8 |
| T2 | 1.3 ± 0.2 | 10.2 ± 0.9 | |
| Kukri | T1 | 1.6 ± 0.3 | 10.5 ± 0.5 |
| T2 | 0.6 ± 0.1 | 15.4 ± 1.6 | |
| Emu Rock | T1 | 1.1 ± 0.3 | 12.3 ± 1.7 |
| T2 | 0.9 ± 0.1 | 13.3 ± 1.5 | |
| Wyalkatchem | T1 | 1.6 ± 0.5 | 8.2 ± 1.4 |
| T2 | 0.9 ± 0.2 | 12.5 ± 1.5 | |
| p value: G | <0.001 | <0.001 | |
| T | <0.001 | <0.001 | |
| G × T | 0.540 | <0.001 | |
| LSD value: G | 0.462 | 1.946 | |
| T | 0.197 | 0.830 | |
| G × T | NS | 2.752 |
| Degrees of Freedom (df) | Sum of Squares | Wald Statistic | Pr (Chisq) | |
|---|---|---|---|---|
| (Intercept) | 1 | 24197 | 91.640 | <0.0001 |
| (Tair–Tleaf) | 1 | 16 | 0.061 | 0.806 |
| Total water use per plant | 1 | 11524 | 43.644 | <0.0001 |
| (Tair–Tleaf) × genotype | 19 | 12273 | 46.482 | 0.0004 |
| Total water use × genotype | 19 | 13467 | 51.003 | <0.0001 |
| Residual (Mean Square) | <0.0001 | |||
| Genotype | Slope (°C °C−1) | Tleaf–Intercept (°C) | R2 | p |
|---|---|---|---|---|
| Einkorn | 0.49 ± 0.15 | 13.73 ± 4.55 | 0.45 | 0.008 |
| RAC 875 | 0.57 ± 0.12 | 12.44 ± 3.24 | 0.60 | 0.0002 |
| Excalibur | 0.57 ± 0.12 | 10.58 ± 3.36 | 0.63 | 0.0003 |
| Espada | 0.58 ± 0.14 | 12.57 ± 3.97 | 0.53 | 0.001 |
| LongReach-Envoy | 0.62 ± 0.14 | 12.57 ± 3.84 | 0.59 | 0.001 |
| Emu Rock | 0.64 ± 0.17 | 11.35 ± 4.84 | 0.49 | 0.002 |
| Kukri | 0.65 ± 0.16 | 11.15 ± 4.48 | 0.53 | 0.001 |
| Wyalkatchem (Batch 02) | 0.67 ± 0.13 | 10.01 ± 3.72 | 0.65 | 0.0001 |
| Mace | 0.69 ± 0.16 | 9.50 ± 4.40 | 0.56 | 0.001 |
| Glossy-Huguenot | 0.68 ± 0.12 | 9.90 ± 3.51 | 0.65 | <0.0001 |
| Gladius | 0.74 ± 0.13 | 8.48 ± 3.68 | 0.69 | <0.0001 |
| Hartog | 0.78 ± 0.13 | 7.51 ± 3.54 | 0.72 | <0.0001 |
| Drysdale | 0.80 ± 0.14 | 6.68 ± 4.01 | 0.68 | <0.0001 |
| Downey | 0.81 ± 0.13 | 4.96 ± 3.72 | 0.74 | <0.0001 |
| Wyalkatchem (Batch 01) | 0.81 ± 0.13 | 3.53 ± 3.85 | 0.79 | 0.0001 |
| Yecora 70 | 0.87 ± 0.19 | 1.64 ± 5.60 | 0.61 | 0.001 |
| Glennson 81 | 0.89 ± 0.08 | 0.81 ± 2.26 | 0.92 | <0.0001 |
| Ciano 67 | 0.91 ± 0.19 | 1.26 ± 5.42 | 0.64 | 0.0004 |
| Magenta | 0.92 ± 0.12 | 0.34 ± 3.53 | 0.81 | <0.0001 |
| Sonora 64 | 0.93 ± 0.18 | 0.75 ± 5.26 | 0.71 | 0.0003 |
| Janz | 1.02 ± 0.14 | −2.01 ± 4.14 | 0.80 | <0.0001 |
| Batch No | Character of Interest | Status | Genotype | Reference |
|---|---|---|---|---|
| 1 | Canopy Temperature Depression | High | Glennson 81 | [54] |
| Low | Sonora 64 | [54] | ||
| Transpiration rate or stomatal conductance (gs) | High | Excalibur | [88] | |
| Pubescence | Pubescent | Downy | [89] | |
| Glabrous | Einkorn * | [90] | ||
| Leaf angle | Erectophile | Ciano 67 | [91] | |
| Planophile | Yecora 70 | [91] | ||
| Early vigour | High | Magenta | [92] | |
| Low | Janz | [93,94,95] | ||
| Adaptability to Western Australian conditions | High | Wyalkatchem | [96] Dr. D. Mullan, InterGrain, pers. Comm. | |
| 2 | Carbon Isotope Discrimination (CID) or Transpiration Efficiency (TE) | Low CID or High TE | Drysdale LongReach-Envoy | [97]; Dr. D. Mullan, InterGrain, pers. Comm.; Dr. G. Rebetzke, CSIRO, pers. Comm. Dr. B. Jacobs, LongReach Plant Breeders, pers. Comm. |
| High CID or Low TE | Hartog | [97] | ||
| Glaucousness (waxiness) | Non–glaucous | Glossy–Huguenot ** | [98] | |
| Glaucous (high) | RAC 875, Espada | [85,99] | ||
| Grain size | High | Emu Rock | [100] | |
| Drought adaptability | Drought-tolerant | Mace Gladius | [101] [102]; Dr. D. Mullan, InterGrain, pers. Comm. | |
| Drought-susceptible | Kukri | [88,103] | ||
| Adaptability to Western Australian conditions | High | Wyalkatchem (reference for comparisons) | [96]; Dr. D. Mullan, InterGrain, pers. Comm. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bramley, H.; Ranawana, S.R.W.M.C.J.K.; Palta, J.A.; Stefanova, K.; Siddique, K.H.M. Transpirational Leaf Cooling Effect Did Not Contribute Equally to Biomass Retention in Wheat Genotypes under High Temperature. Plants 2022, 11, 2174. https://doi.org/10.3390/plants11162174
Bramley H, Ranawana SRWMCJK, Palta JA, Stefanova K, Siddique KHM. Transpirational Leaf Cooling Effect Did Not Contribute Equally to Biomass Retention in Wheat Genotypes under High Temperature. Plants. 2022; 11(16):2174. https://doi.org/10.3390/plants11162174
Chicago/Turabian StyleBramley, Helen, S. R. W. M. Chandima J. K. Ranawana, Jairo A. Palta, Katia Stefanova, and Kadambot H. M. Siddique. 2022. "Transpirational Leaf Cooling Effect Did Not Contribute Equally to Biomass Retention in Wheat Genotypes under High Temperature" Plants 11, no. 16: 2174. https://doi.org/10.3390/plants11162174
APA StyleBramley, H., Ranawana, S. R. W. M. C. J. K., Palta, J. A., Stefanova, K., & Siddique, K. H. M. (2022). Transpirational Leaf Cooling Effect Did Not Contribute Equally to Biomass Retention in Wheat Genotypes under High Temperature. Plants, 11(16), 2174. https://doi.org/10.3390/plants11162174







