Marrubium alysson L. Ameliorated Methotrexate-Induced Testicular Damage in Mice through Regulation of Apoptosis and miRNA-29a Expression: LC-MS/MS Metabolic Profiling
Abstract
1. Introduction
2. Results
2.1. In Vivo Investigation
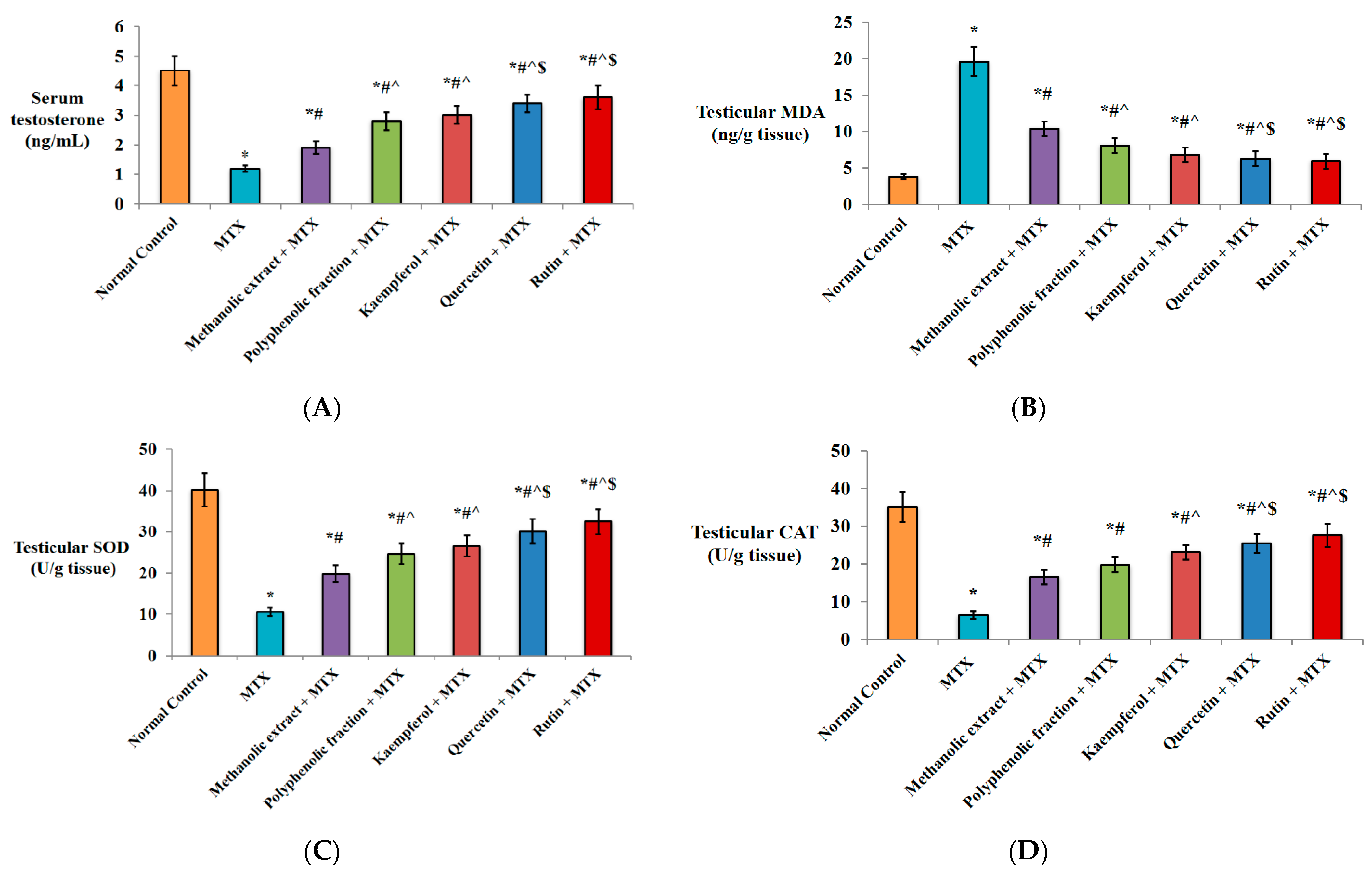
2.1.1. Effect on Serum Testosterone Level
2.1.2. Antioxidant Activity
2.1.3. Anti-Inflammatory Activity
2.1.4. Effect on Apoptotic Markers
2.1.5. Effect on miRNA-29a Expression
2.1.6. Histopathological Findings
2.2. LC-ESI-TOF-MS/MS Analysis of Marrubium Alysson L.
2.3. Total Phenolic and Total Flavonoid Content Determination
2.4. Quantitative Estimation of Kaempferol, Quercetin and Rutin Using HPLC
2.4.1. Analytical Solution Stability
2.4.2. Linearity
2.4.3. Precision of the System
2.4.4. Method Precision
2.4.5. Limits of Detection and Quantification
2.4.6. Sample Analysis
3. Discussion
4. Materials and Procedures
4.1. Plant Material
4.2. Preparation of Plant Crude Extract
4.3. Preparation of Phenolic Extract
4.4. Chemicals
4.5. Determination of Total Phenolic and Total Flavonoid Contents:
4.6. HPLC-DAD Quantitative Analysis
4.6.1. Instrumentation
4.6.2. Operating Conditions
4.6.3. Standard and Sample Preparation
4.7. Metabolomic Profiling by LC/MS/MS
4.8. In Vivo Biological Study
4.8.1. Animals
4.8.2. Study Design
4.8.3. Biochemical Assays
Serum Level of Testosterone
Evaluation of Testicular Oxidative Stress Level
Evaluation of Inflammation and Apoptosis Biomarkers
Quantitative Real-Time Polymerase Chain Reaction for Expression of NF-κB, TNF-α, p53, and miRNA-29a
4.8.4. Histopathological Examination
4.8.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [PubMed]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef] [PubMed]
- Famurewa, A.C.; Aja, P.M.; Nwankwo, O.E.; Awoke, J.N.; Maduagwuna, E.K.; Aloke, C. Moringa oleifera seed oil or virgin coconut oil supplementation abrogates cerebral neurotoxicity induced by antineoplastic agent methotrexate by suppression of oxidative stress and neuro-inflammation in rats. J. Food Biochem. 2019, 43, e12748. [Google Scholar] [PubMed]
- Howard, S.C.; McCormick, J.; Pui, C.H.; Buddington, R.K.; Harvey, R.D. Preventing and Managing Toxicities of High-Dose Methotrexate. Oncologist 2016, 21, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Sukhotnik, I.; Nativ, O.; Roitburt, A.; Bejar, D.; Coran, A.G.; Mogilner, J.G.; Nativ, O. Methotrexate induces germ cell apoptosis and impairs spermatogenesis in a rat. Pediatr. Surg. Int. 2013, 29, 179–184. [Google Scholar] [CrossRef]
- Soliman, A.M.; Desouky, S.; Marzouk, M.; Sayed, A.A. Origanum majorana attenuates nephrotoxicity of cisplatin anticancer drug through ameliorating oxidative stress. Nutrients 2016, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Park, J.H.; Lee, S.H.; Lee, D.E.; Kim, I.S.; Lee, K.Y.; Lee, B.M.; et al. Protective effects of Dendropanax morbifera against cisplatin-induced nephrotoxicity without altering chemotherapeutic efficacy. Antioxidants 2019, 8, 256. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Elhady, S.S.; Nafie, M.S.; Ahmed, H.A.; Abo-Elmatty, D.M.; Ahmed, S.A.; Badr, J.B.; Abdel-Hamed, A.R. The Antioxidant Carrichtera annua DC. ethanolic extract counteracts cisplatin triggered hepatic and renal toxicities. Antioxidants 2021, 10, 825. [Google Scholar] [CrossRef]
- Al-Abkal, F.; Abdel-Wahab, B.A.; Abd El-Kareem, H.F.; Moustafa, Y.M.; Khodeer, D.M. Protective effect of pycnogenol against methotrexate-induced hepatic, renal, and cardiac toxicity: An in vivo study. Pharmaceuticals 2022, 15, 674. [Google Scholar] [CrossRef] [PubMed]
- Popoola, O.K.; Elbagory, A.M.; Ameer, F.; Hussein, A.A. Marrubiin. Molecules 2013, 18, 9049–9060. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.H. Anticancer activity of Marrubium alysson L. and its phenolic constituents. Drug Plants 2010, 27, 185–193. [Google Scholar]
- Pourreza, N. Phenolic Compounds as Potential Antioxidant. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Essawy, S.S.; Abo-elmatty, D.M.; Ghazy, N.M.; Badr, J.M.; Sterner, O. Antioxidant and anti-inflammatory effects of Marrubium alysson extracts in high cholesterol-fed rabbits. Saudi Pharm. J. 2014, 22, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, D.; Bartosińska, E.; Jacyna, J.; Patejko, M.; Siluk, D.; Kaliszan, R. Quantitative determination of trigonelline in mouse serum by means of hydrophilic interaction liquid chromatography–MS/MS analysis: Application to a pharmacokinetic study. Biomed. Chromatogr. 2018, 32, 4054–4062. [Google Scholar] [CrossRef]
- Chang, C.; Wu, R. Quantification of (+)-catechin and (−)-epicatechin in coconut water by LC–MS. Food Chem. 2011, 126, 710–717. [Google Scholar] [CrossRef]
- Seraglio, S.; Valese, A.; Daguer, H.; Bergamo, G.; Azevedo, M.; Gonzaga, L.; Fett, R.; Costa, A. Development and validation of a LC-ESI-MS/MS method for the determination of phenolic compounds in honeydew honeys with the diluted-and-shoot approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef]
- Flieger, M.; Bandouchova, H.; Cerny, J.; Chudíčková, M.; Kolarik, M.; Kovacova, V.; Martínková, N.; Novák, P.; Šebesta, O.; Stodůlková, E.; et al. Vitamin B2 as a virulence factor in Pseudogymnoascus destructans skin infection. Sci. Rep. 2016, 6, 33200. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.; Xu, W.; Huang, M.; Xu, W.; Li, H.; Ye, M.; Zhang, Z.; Chu, K. Qualitative and quantitative analysis of phenolic acids, flavonoids and iridoid glycosides in Yinhua Kanggan tablet by uplc-qqq-ms/ms. Molecules 2015, 20, 12209–12228. [Google Scholar] [CrossRef]
- Tine, Y.; Yang, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. LC-MS/MS Analysis of flavonoid compounds from Zanthoxylum zanthoxyloides extracts and their antioxidant activities. Nat. Prod. Commun. 2017, 12, 1865–1868. [Google Scholar] [CrossRef]
- AbdelHamed, A.R.; Mehanna, E.T.; Hazem, R.M.; Badr, J.M.; Abo-Elmatty, D.M.; Abdel-Kader, M.S.; Goda, M.S. Plicosepalus acacia extract and its major constituents, methyl gallate and quercetin, potentiate therapeutic angiogenesis in diabetic hind limb ischemia: HPTLC quantification and LC-MS/MS metabolic profiling. Antioxidants 2021, 10, 1701. [Google Scholar] [CrossRef]
- Ma, Y.; Li, P.; Chen, D.; Fang, T.; Li, H.; Su, W. LC/MS/MS quantitation assay for pharmacokinetics of naringenin and double peaks phenomenon in rats plasma. Int. J. Pharm. 2006, 307, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and Quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in pollen typhae for transformation rule exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, V.C.; Krishna, B.G.; Viswanatha, G.L. Simultaneous determination of quercetin, rutin and kaempferol in the leaf extracts of Moringa oleifera Lam. and Raphinus sativus Linn. by liquid chromatography-tandem mass spectrometry. Zhong Xi Yi Jie He Xue Bao 2011, 9, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.F.; Elhady, S.S.; Sirwi, A.; Samir, H.; Ibrahim, E.A.; Thomford, A.K.; ElGindy, A.; Hadad, G.M.; Badr, J.M.; Nafie, M.S. Thonningia sanguinea extract: Antioxidant and cytotoxic activities supported by chemical composition and molecular docking simulations. Plants 2021, 10, 2156. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS Profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Ferreira, R.O.; De Carvalho Junior, A.R.; Da Silva, T.M.G.; Castro, R.N.; Da Silva, T.M.S.; De Carvalho, M.G. Distribution of metabolites in galled and non-galled leaves of Clusia lanceolata and its antioxidant activity. Rev. Bras. Farmacogn. 2014, 24, 617–625. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Song, H.P.; Zhang, H.; Fu, Y.; Mo, H.Y.; Zhang, M.; Chen, J.; Li, P. Screening for selective inhibitors of xanthine oxidase from Flos Chrysanthemum using ultrafiltration LC–MS combined with enzyme channel blocking. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 961, 56–61. [Google Scholar] [CrossRef]
- Yuan, Y.; Hou, W.; Tang, M.; Luo, H.; Chen, L.; Guan, Y.; Sutherland, I.A. Separation of flavonoids from the leaves of Oroxylum indicum by HSCCC. Chromatographia. 2008, 68, 885–892. [Google Scholar] [CrossRef]
- Švehlíková, V.; Bennett, R.N.; Mellon, F.A.; Needs, P.W.; Piacente, S.; Kroon, P.A.; Bao, Y. Isolation, Identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert). Phytochemistry 2004, 65, 2323–2332. [Google Scholar] [CrossRef]
- Pereira, O.R.; Peres, A.M.S.; Silva, A.M.; Domingues, M.R.M.; Cardoso, S.M. Simultaneous characterization and quantification of phenolic compounds in Thymus x citriodorus using a validated HPLC-UV and ESI- MS combined method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Zhang, B.; Weston, P.A.; Gu, L.; Zhang, B.; Li, M.; Wang, F.; Tu, W.; Wang, J.; Weston, L.A.; Zhang, Z. Identification of phytotoxic metabolites released from Rehmannia glutinosa suggest their importance in the formation of its replant problem. Plant. Soil 2019, 441, 439–454. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Montoro, P.; Piacente, S.; Pizza, C.; Dönmez, A.; Caliş, I. Identification by HPLC-PAD-MS and quantification by HPLC-PAD of phenylethanoid glycosides of five Phlomis species. Phytochem. Anal. 2005, 16, 1–6. [Google Scholar] [CrossRef]
- Yun, E.; Park, S.; Kim, B.; Chae, Y.; Cho, S.; Yi, H.; Cho, H.; Shin, H. Determination of the esculetin contents of medicinal plants by liquid chromatography–tandem mass spectrometry. Biomed. Chromatogr. 2012, 26, 1247–1251. [Google Scholar] [CrossRef]
- Tine, Y.; Yang, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. A method for LC-MS/MS profiling of coumarins in Zanthoxylum zanthoxyloides (Lam.) B. zepernich and timler extracts and essential Oils. Molecules 2017, 22, 174. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Manakov, Y.; Ercisli, S.; Derbush, I.; Kislin, E.; Seryodkin, I.; Sabitov, A.; Kalenik, T.; et al. LC-MS/MS screening of phenolic compounds in wild and Cultivated Grapes Vitis amurensis Rupr. Molecules 2021, 26, 3650. [Google Scholar] [CrossRef] [PubMed]
- Geenen, S.; Guallar-Hoyas, C.; Michopoulos, F.; Kenna, J.G.; Kolaja, K.L.; Westerhoff, H.V.; Thomas, P.; Wilson, I.D. HPLC–MS/MS methods for the quantitative analysis of 5-oxoproline (pyroglutamate) in rat plasma and hepatic cell line culture medium. J. Pharm. Biomed. Anal. 2011, 56, 655–663. [Google Scholar] [CrossRef]
- Rashed, M.S.; Al-Ahaidib, L.Y.; Aboul-Enein, H.Y.; Al-Amoudi, M.; Jacob, M. Determination of l-Pipecolic acid in plasma using chiral liquid chromatography-electrospray tandem mass spectrometry. Clin. Chem. 2001, 47, 2124–2130. [Google Scholar] [CrossRef]
- MacKinnon, S.L.; Craft, C. Analysis of betaines from marine algae using LC-MS-MS. Nat. Prod. Mar. Algae 2015, 1308, 267–275. [Google Scholar]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacumtissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem. Cent. J. 2014, 8, 66–75. [Google Scholar] [CrossRef]
- Hu, L.; Li, X.; Hu, J.; Ni, X.; Lu, H.; Wang, J.; Huang, X.; Lin, C.; Shang, D.; Wen, Y. A simple HPLC–MS/MS method for determination of tryptophan, kynurenine and kynurenic acid in human serum and its potential for monitoring antidepressant therapy. J. Anal. Toxicol. 2017, 41, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Al Kadhi, O.; Melchini, A.; Mithen, R.; Saha, S. Development of a LC-MS/MS method for the simultaneous detection of tricarboxylic acid cycle intermediates in a range of biological matrices. J. Anal. Methods Chem. 2017, 2017, 5391832. [Google Scholar] [CrossRef] [PubMed]
- Penner, N.; Ramanathan, R.; Zgoda-Pols, J.; Chowdhury, S. Quantitative determination of hippuric and benzoic acids in urine by LC–MS/MS using surrogate standards. J. Pharm. Biomed. Anal. 2010, 52, 534–543. [Google Scholar] [CrossRef]
- Du Bois de Maquillé, L.; Wund, P.; Renaudin, L.; Gautier, C.; Jardy, A.; Vial, J.; Thiébaut, D.; Fichet, P.; Goutelard, F. Determination of gluconate in nuclear waste by high-performance liquid chromatography: Comparison of pulsed amperometric detection and electrospray mass spectrometry detection. J. Radioanal. Nucl. Chem. 2015, 306, 213–220. [Google Scholar] [CrossRef]
- Cocuron, J.; Ross, Z.; Alonso, A.P. Liquid chromatography tandem mass spectrometry quantification of 13C-labeling in sugars. Metabolites 2020, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, G.R.; Shah, I.; Gariballa, S.; Yasin, J.; Barker, J.; Ashraf, S.S. Significantly elevated levels of plasma nicotinamide, pyridoxal, and pyridoxamine phosphate levels in obese emirati population: A cross-sectional study. Molecules 2020, 25, 3932. [Google Scholar] [CrossRef] [PubMed]
- Doyle, R.M. LC-MS/MS Quantitative Analysis of Polyunsaturated Omega 3, 6, 7 and 9 Fatty Acids in Serum for Research Use; Thermo Scientific, Inc.: Somerset, NJ, USA, 2017. [Google Scholar]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Felemban, S.; Aldubyan, M.; Alhowail, A.; Almami, I. Vitamin B17 ameliorates methotrexate-induced reproductive toxicity, oxidative stress, and testicular injury in male rats. Oxid. Med. Cell. Longev. 2020, 2020, 4372719. [Google Scholar] [CrossRef]
- Kavram Sarihan, K.; Yardimoğlu Yilmaz, M.; Eraldemir, F.C.; Yazir, Y.; Acar, E. Protective effects of apocynin on damaged testes of rats exposed to methotrexate. Turk. J. Med. Sci. 2020, 50, 1409–1420. [Google Scholar] [CrossRef]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Gutierrez, J.C.; Hwang, K. The toxicity of methotrexate in male fertility and paternal teratogenicity. Expert Opin. Drug Metab. Toxicol. 2017, 13, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Yuluğ, E.; Türedi, S.; Alver, A.; Türedi, S.; Kahraman, C. Effects of resveratrol on methotrexate-induced testicular damage in rats. Sci. World J. 2013, 2013, 489659. [Google Scholar] [CrossRef] [PubMed]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Tatsimo, S.J.; Tamokou, J.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Aziz, A.M.; Abdel-Hafez, S.M.N.; Venugopala, K.N.; Nair, A.B.; Abdel-Gaber, S.A. The possible contribution of p-glycoprotein in the protective effect of paeonol against methotrexate-induced testicular injury in rats. Pharmaceuticals 2020, 13, 223. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Al-Taher, A.Y.; Morsy, M.A.; Rifaai, R.A.; Zenhom, N.M.; Abdel-Gaber, S.A. Paeonol attenuates methotrexate-induced cardiac toxicity in rats by inhibiting oxidative stress and suppressing TLR4-induced NF-κB inflammatory pathway. Mediat. Inflamm. 2020, 2020, 8641026. [Google Scholar] [CrossRef]
- Owumi, S.; Ochaoga, S.; Odunola, O.; Farombi, O. Protocatechuic acid inhibits testicular and epididymal toxicity associated with methotrexate in rats. Andrologia 2019, 51, e13350. [Google Scholar] [CrossRef] [PubMed]
- Edziri, H.; Marzouk, B.; Mabrouk, H.; Garreb, M.; Douki, W.; Mahjoub, A.; Verschaeve, L.; Najjar, F.; Mastouri, M. Phytochemical screening, butyrylcholinesterase inhibitory activity and anti-inflammatory effect of some Tunisian medicinal plants. S. Afr. J. Bot. 2018, 114, 84–88. [Google Scholar] [CrossRef]
- Thouri, A.; Chahdoura, H.; Amira, E.M.; Hassin, R.; Hammami, M. Effect of solvents extraction on phytochemical components and biological activities of Tunisian date seeds (var. Korkobbi and Arechti). BMC Complement. Altern. Med. 2017, 17, 248. [Google Scholar] [CrossRef]
- Lu, C.L.; Zhu, W.; Wang, M.; Xu, X.J.; Lu, C.J. Antioxidant and Anti-Inflammatory activities of phenolic-enriched extracts of Smilax glabra. Evid. Based Complement. Alternat. Med. 2014, 2014, 910438. [Google Scholar] [CrossRef]
- Zhang, R.; Ai, X.; Duan, Y.; Xue, M.; He, W.; Wang, C.; Xu, T.; Xu, M.; Liu, B.; Li, C.; et al. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomed. Pharmacother. 2017, 89, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N. The berry constituent’s quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary anti-inflammatory agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Yang, M.; Liu, M. Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB signalling pathway. J. Pharm. Pharmacol. 2021, 73, 110–117. [Google Scholar] [CrossRef]
- Selloum, L.; Bouriche, H.; Tigrine, C.; Boudoukha, C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp. Toxicol. Pathol. 2003, 54, 313–318. [Google Scholar] [CrossRef]
- Omar, N.; El-Kot, A.A.; Khalifa, E.I. Protective role of thyme leave extract on methotrexate-induced histological and immunohistochemical changes in testes of rats. Egypt. J. Hosp. Med. 2021, 83, 1230–1238. [Google Scholar] [CrossRef]
- Popgeorgiev, N.; Jabbour, L.; Gillet, G. Subcellular localization and dynamics of the Bcl-2 family of proteins. Front. Cell Dev. Biol. 2018, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, M.F.; Çilenk, K.T.; Karabulut, D.; Ünalmış, S.; Deligönül, E.; Öztürk, İ.; Kaymak, E. Protective effects of propolis on methotrexate-induced testis injury in rat. Biomed. Pharmacother. 2016, 79, 44–51. [Google Scholar] [CrossRef]
- Sheikhbahaei, F.; Khazaei, M.; Rabzia, A.; Mansouri, K.; Ghanbari, A. Protective effects of thymoquinone against methotrexate-induced germ cell apoptosis in male mice. Int. J. Fertil. Steril. 2016, 9, 541–547. [Google Scholar] [PubMed]
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol protects against cerebral ischemia reperfusion injury through intervening oxidative and inflammatory stress induced apoptosis. Front. Pharmacol. 2020, 11, 424. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Med. (Baltim.) 2020, 99, e22241. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Li, J.; Yao, Z. Rutin alleviates cardiomyocyte injury induced by high glucose through inhibiting apoptosis and endoplasmic reticulum stress. Exp. Ther. Med. 2021, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Liu, Z.; Wang, S.; Liu, F.; Yang, X.; Hou, J.; Hou, Z.; Chen, B. MiR-29a promotes intestinal epithelial apoptosis in ulcerative colitis by down-regulating Mcl-1. Int. J. Clin. Exp. Pathol. 2014, 7, 8542–8552. [Google Scholar]
- Ding, S.; Liu, D.; Wang, L.; Wang, G.; Zhu, Y. Inhibiting MicroRNA-29a protects myocardial ischemia-reperfusion injury by targeting sirt1 and suppressing oxidative stress and NLRP3-mediated pyroptosis pathway. J. Pharmacol. Exp. Ther. 2020, 372, 128–135. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, L.; Yu, J.; Fu, H.; Zhou, D.; Liu, H. Inhibition of microRNA-29a alleviates hyperoxia-induced bronchopulmonary dysplasia in neonatal mice via upregulation of GAB1. Mol. Med. 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Meunier, L.; Siddeek, B.; Vega, A.; Lakhdari, N.; Inoubli, L.; Bellon, R.P.; Lemaire, G.; Mauduit, C.; Benahmed, M. Perinatal programming of adult rat germ cell death after exposure to xenoestrogens: Role of microRNA miR-29 family in the down-regulation of DNA methyltransferases and Mcl-1. Endocrinology 2012, 153, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Akinjo, O.O.; Gant, T.W.; Marczylo, E.L. Perturbation of epigenetic processes by doxorubicin in the mouse testis. Toxicol. Res. 2016, 5, 1229–1243. [Google Scholar] [CrossRef]
- Tang, B.; Li, X.; Ren, Y.; Wang, J.; Xu, D.; Hang, Y.; Zhou, T.; Li, F.; Wang, L. MicroRNA-29a regulates lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages through the Akt1/ NF-κB pathway. Exp. Cell Res. 2017, 360, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Ma, L.; Liu, Z.; Yang, Z.; Wang, J.; Liu, J.; Jiang, G. Targeting microRNAs: A new action mechanism of natural compounds. Oncotarget 2017, 8, 15961–15970. [Google Scholar] [CrossRef] [PubMed]
- Biersack, B. Current state of phenolic and terpenoidal dietary factors and natural products as non-coding RNA/microRNA modulators for improved cancer therapy and prevention. Noncoding RNA Res. 2016, 1, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.R.; Sarg, T.M.; Metwally, A.M.; Rakha, A.A. Chemical constituents from Marrubium alysson. Planta Med. 1981, 41, 202–203. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Elhady, S.S.; Ahmed, H.A.; Badr, J.M.; Noor, A.O.; Ahmed, S.A.; Nafie, M.S. Chemical profiling, antioxidant, cytotoxic activitiesand molecular docking simulation of Carrichtera annua DC. (Cruciferae). Antioxidants 2020, 9, 1286. [Google Scholar] [CrossRef]
- Kirnanmai, M.; Kumar, C.; Ibrahim, M. Comparison of total flavonoid content of Azadirachta indica root bark extracts prepared by different methods of extraction. Res. Pharm. Biol. Chem. Sci. 2011, 2, 254–261. [Google Scholar]
- Brenton, A.G.; Godfrey, A.R. Accurate mass measurement: Terminology and treatment of data. J. Am. Soc. Mass. Spectrom. 2010, 21, 1821–1835. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Najafi, G.; Atashfaraz, E.; Farokhi, F. Attenuation of Methotrexate-induced embryotoxicity and oxidative stress by ethyl pyruvate. Int. J. Fertil. Steril. 2016, 10, 232–238. [Google Scholar] [PubMed]
- Alaa, R.; Abd-Alhaseeb, M.; Habib, E.; Ibrahim, A.; Ahmed, S. Screening of Marrubium alysson L. extract for pharmacological activity. J. Chem. Pharm. Res. 2016, 8, 283–289. [Google Scholar]
- Yang, Q.S.; He, L.P.; Zhou, X.L.; Zhao, Y.; Shen, J.; Xu, P.; Ni, S.Z. Kaempferol pretreatment modulates systemic inflammation and oxidative stress following hemorrhagic shock in mice. Chin. Med. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Chan, S.T.; Lin, Y.C.; Chuang, C.H.; Shiau, R.J.; Liao, J.W.; Yeh, S.L. Oral and intraperitoneal administration of quercetin decreased lymphocyte DNA damage and plasma lipid peroxidation induced by TSA in vivo. Biomed. Res. Int. 2014, 2014, 580626. [Google Scholar] [CrossRef]
- Domitrović, R.; Jakovac, H.; Vasiljev Marchesi, V.; Vladimir-Knežević, S.; Cvijanović, O.; Tadić, Z.; Romić, Z.; Rahelić, D. Differential hepatoprotective mechanisms of rutin and quercetin in CCl(4)-intoxicated BALB/cN mice. Acta pharmacol. Sin. 2012, 33, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.E.; Mosalam, E.M.; Mehanna, E.T.; Awad, B.M.; Mosaad, S.M.; Abdel-Kader, M.S.; Ibrahim, A.K.; Badr, J.M.; Goda, M.S. Potential Gonado-Protective Effect of Cichorium endivia and Its Major Phenolic Acids against Methotrexate-Induced Testicular Injury in Mice. Biomedicines 2022, 10, 1986. [Google Scholar] [CrossRef] [PubMed]






| No. | Polarity Mode | Retention Time (min) | Precursor Type | Measured m/z | Expected or Calculated m/z | Name | Molecular Formula | Fragments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | |||||||||
| 1 | Positive | 1.33 | [M + H]+ | 138.0540 | 138.0550 | Trigonelline | C7H7NO2 | 94, 92 | [14] |
| Catechins | |||||||||
| 2 | Positive | 5.51 | [M + H]+ | 291.0857 | 291.0869 | Catechin | C15H14O6 | 123, 139, 147 | [15] |
| 3 | Positive | 4.67 | [M + H]+ | 291.0880 | 291.0869 | (-)-Epicatechin | C15H14O6 | 139, 123 | [15,16] |
| Flavines | |||||||||
| 4 | Positive | 5.45 | [M + H]+ | 377.1434 | 377.1461 | (-)-Riboflavin | C17H20N4O6 | 377, 243 | [17] |
| Flavonoids and their glycosides | |||||||||
| 5 | Positive | 6.62 | [M + H]+ | 611.1652 | 611.1612 | Rutin | C27H30O16 | 609, 300 | [18] |
| 6 | Positive | 14.02 | [M + H]+ | 303.0503 | 303.0505 | Quercetin | C15H10O7 | 301, 151 | [19,20] |
| 7 | Positive | 7.83 | [M + H]+ | 273.0744 | 273.0763 | Naringenin | C15H12O5 | 273,153, 147 | [21] |
| 8 | Negative | 9.16 | [M − H]− | 315.0542 | 315.0505 | Isorhamnetin | C16H12O7 | 301, 272 | [22] |
| 9 | Positive | 9.84 | [M + H]+ | 287.0566 | 287.0556 | Kaempferol | C15H10O6 | 287, 153 | [23] |
| 10 | Negative | 10.26 | [M − H]− | 299.0583 | 299.0556 | 3 5 7-trihydroxy-4′-methoxyflavone (Diosmetin) | C16H12O6 | 299, 284 | [24] |
| 11 | Positive | 11.02 | [M + H]+ | 271.0618 | 271.0606 | Apigenin | C15H10O5 | 268, 269 179 225, 201, 151 | [25] |
| 12 | Positive | 6.20 | [M + H]+ | 449.1082 | 449.1084 | Luteolin-6-C-glucoside (Isoorientin) | C21H20O11 | 449 | [26] |
| 13 | Negative | 6.67 | [M − H]− | 623.1609 | 623.1612 | Isorhamnetin-3-O-rutinoside | C28H32O16 | 315, 300 | [22] |
| 14 | Positive | 6.76 | [M + H]+ | 433.1110 | 433.1135 | Apigenin 8-C-glucoside (Vitexin) | C21H20O10 | 431, 433, 311 | [25,27] |
| 15 | Negative | 6.86 | [M − H]− | 447.0906 | 447.0928 | Luteolin-7-O-glucoside | C21H20O11 | 447, 285 | [28] |
| 16 | Negative | 6.95 | [M − H]− | 477.1028 | 477.1033 | Isorhamnetin-3-O-glucoside | C22H22O12 | 477 | [22] |
| 17 | Positive | 7.69 | [M + H]+ | 447.0930 | 447.0927 | Baicalein-7-O-glucuronide | C21H18O11 | 245 | [29] |
| 18 | Positive | 7.79 | [M + H]+ | 433.1108 | 433.1135 | Apigenin-7-O-glucoside (Cosmosiin) | C21H20O10 | 433,271 | [30] |
| 19 | Negative | 7.96 | [M − H]− | 433.1144 | 433.1135 | Naringenin-7-O-glucoside (Prunin) | C21H22O10 | 433, 271 | [31] |
| 20 | Positive | 10.09 | [M + H]+ | 579.1768 | 579.1714 | Apigenin 7-O-neohesperidoside (Rhoifolin) | C27H30O14 | 577, 579, 269, 225. | [25] |
| Phenylethanoid glycosides | |||||||||
| 21 | Positive | 7.71 | [M + H]+ | 639.2277 | 639.2289 | Leucosceptoside A | C30H38O15 | 177 | [32] |
| 22 | Positive | 8.80 | [M + H]+ | 653.2425 | 653.2446 | Martynoside | C31H40O15 | 485, 339, 177 | [32] |
| 23 | Negative | 7.10 | [M − H]− | 769.2575 | 769.2555 | Alyssonoside | C35H46O19 | 769, 575 | [33] |
| Coumarins and their glycosides | |||||||||
| 24 | Positive | 5.38 | [M + H]+ | 179.0334 | 179.0344 | 6,7-dihydroxycoumarin-Aesculetin | C9H6O4 | 77, 133 | [34] |
| 25 | Positive | 7.01 | [M + H]+ | 177.0547 | 177.0552 | 7-hydroxy-4-methylcoumarin Hymecromone 4-Methylumbelliferon | C10H8O3 | 177, 77 | [35] |
| 26 | Negative | 2.32 | [M − H]− | 339.0697 | 339.0716 | Esculin | C15H16O9 | 2. 399, 177 | [36] |
| Amino acids | |||||||||
| 27 | Negative | 1.22 | [M − H]− | 128.0341 | 128.0348 | L-5-Oxoproline (pyroglutamate)amino a | C5H7NO3 | 130,84 | [37] |
| 28 | Positive | 1.43 | [M + H]+ | 130.0867 | 130.0868 | Pipecolate | C6H11NO2 | 130, 84 | [38] |
| 29 | Positive | 26.93 | [M + H]+ | 118.0867 | 118.0868 | Glycine-Betaine-Trimethylglycine | C5H11NO2 | 118, 58 | [39] |
| Miscellaneous | |||||||||
| 30 | Negative | 1.38 | [M − H]− | 353.0866 | 353.0873 | Chlorogenic Acid | C16H18O9 | 191, 179 | [40] |
| 31 | Positive | 4.36 | [M + H]+ | 190.0495 | 190.0504 | Kynurenic acid | C10H7NO3 | 190, 144 | [41] |
| 32 | Negative | 1.18 | [M − H]− | 117.0187 | 117.0188 | Succinic acid | C4H6O4 | 117,73,99 | [42] |
| 33 | Positive | 17.58 | [M + H]+ | 123.0450 | 123.0446 | Benzoic acid | C7H6O2 | 77 | [43] |
| 34 | Negative | 1.87 | [M − H]− | 163.0386 | 163.0395 | 3-(4-hydroxyphenyl) prop-2-enoic acid | C9H8O3 | 165,119 | [36] |
| 35 | Negative | 1.21 | [M − H]− | 133.0126 | 133.0137 | D-(+)-Malic acid | C4H6O5 | 115,71 | [42] |
| 36 | Negative | 1.17 | [M − H]− | 195.0491 | 195.0505 | Gluconate | C6H12O7 | 195 | [44] |
| 37 | Negative | 1.36 | [M − H]− | 179.0556 | 179.0556 | D-(-)-Tagatose-Monosaccharide | C6H12O6 | 179, 89 | [45] |
| 38 | Negative | 4.91 | [M − H]− | 166.0488 | 166.0504 | Pyridoxal | C8H9NO3 | 94,168 | [46] |
| 39 | Negative | 19.84 | [M − H]− | 277.2186 | 277.2168 | gamma-Linolenic acid | C18H30O2 | 275, 259, 233 | [47] |
| 40 | Positive | 4.68 | [M + H]+ | 183.0655 | 183.0657 | Syringaldehyde | C9H10O4 | 123, 77 | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhameed, R.F.A.; Ali, A.I.; Elhady, S.S.; Abo Mansour, H.E.; Mehanna, E.T.; Mosaad, S.M.; Ibrahim, S.A.; Hareeri, R.H.; Badr, J.M.; Eltahawy, N.A. Marrubium alysson L. Ameliorated Methotrexate-Induced Testicular Damage in Mice through Regulation of Apoptosis and miRNA-29a Expression: LC-MS/MS Metabolic Profiling. Plants 2022, 11, 2309. https://doi.org/10.3390/plants11172309
Abdelhameed RFA, Ali AI, Elhady SS, Abo Mansour HE, Mehanna ET, Mosaad SM, Ibrahim SA, Hareeri RH, Badr JM, Eltahawy NA. Marrubium alysson L. Ameliorated Methotrexate-Induced Testicular Damage in Mice through Regulation of Apoptosis and miRNA-29a Expression: LC-MS/MS Metabolic Profiling. Plants. 2022; 11(17):2309. https://doi.org/10.3390/plants11172309
Chicago/Turabian StyleAbdelhameed, Reda F. A., Asmaa I. Ali, Sameh S. Elhady, Hend E. Abo Mansour, Eman T. Mehanna, Sarah M. Mosaad, Salma A. Ibrahim, Rawan H. Hareeri, Jihan M. Badr, and Nermeen A. Eltahawy. 2022. "Marrubium alysson L. Ameliorated Methotrexate-Induced Testicular Damage in Mice through Regulation of Apoptosis and miRNA-29a Expression: LC-MS/MS Metabolic Profiling" Plants 11, no. 17: 2309. https://doi.org/10.3390/plants11172309
APA StyleAbdelhameed, R. F. A., Ali, A. I., Elhady, S. S., Abo Mansour, H. E., Mehanna, E. T., Mosaad, S. M., Ibrahim, S. A., Hareeri, R. H., Badr, J. M., & Eltahawy, N. A. (2022). Marrubium alysson L. Ameliorated Methotrexate-Induced Testicular Damage in Mice through Regulation of Apoptosis and miRNA-29a Expression: LC-MS/MS Metabolic Profiling. Plants, 11(17), 2309. https://doi.org/10.3390/plants11172309








