Abscisic Acid Induces Adventitious Rooting in Cucumber (Cucumis sativus L.) by Enhancing Sugar Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Explant Treatments
2.3. Determination of Glucose Content
2.4. Determination of Sucrose Content
2.5. Determination of Starch Content
2.6. Determination of Total Sugar Content
2.7. G6P, F6P and G1P Content Measurements
2.8. SS, SPS, HK and PK Enzyme Activity Measurement
2.9. Quantitative Real-Time PCR (qRT-PCR)
2.10. Data Statistics and Analysis
3. Results
3.1. Effect of Exogenous ABA on Adventitious Root Development
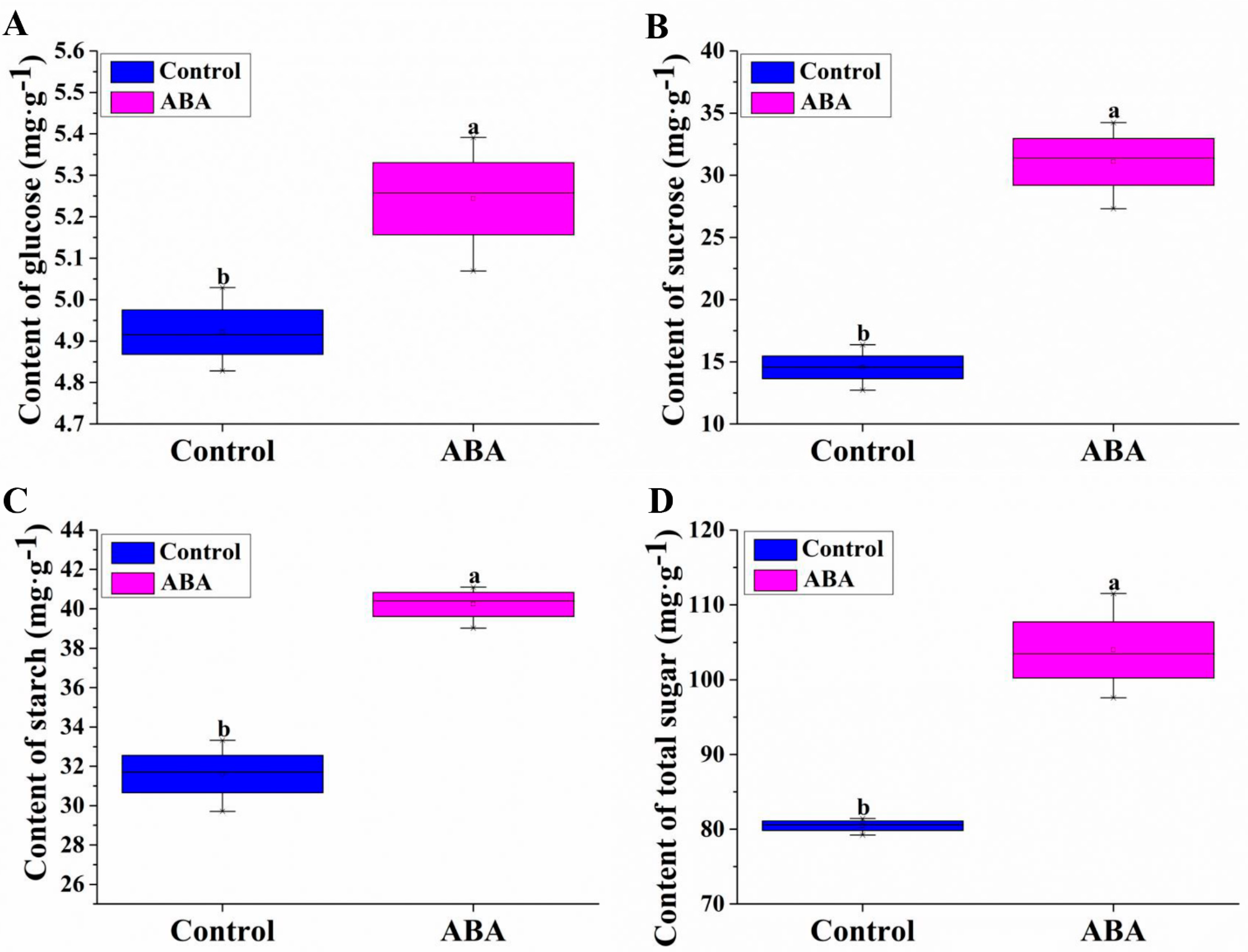
3.2. Effect of Exogenous ABA on Glucose, Sucrose, Starch and Total Sugar Contents during Adventitious Rooting
3.3. Effect of Exogenous ABA on G6P, F6P and G1P Contents during Adventitious Root Development
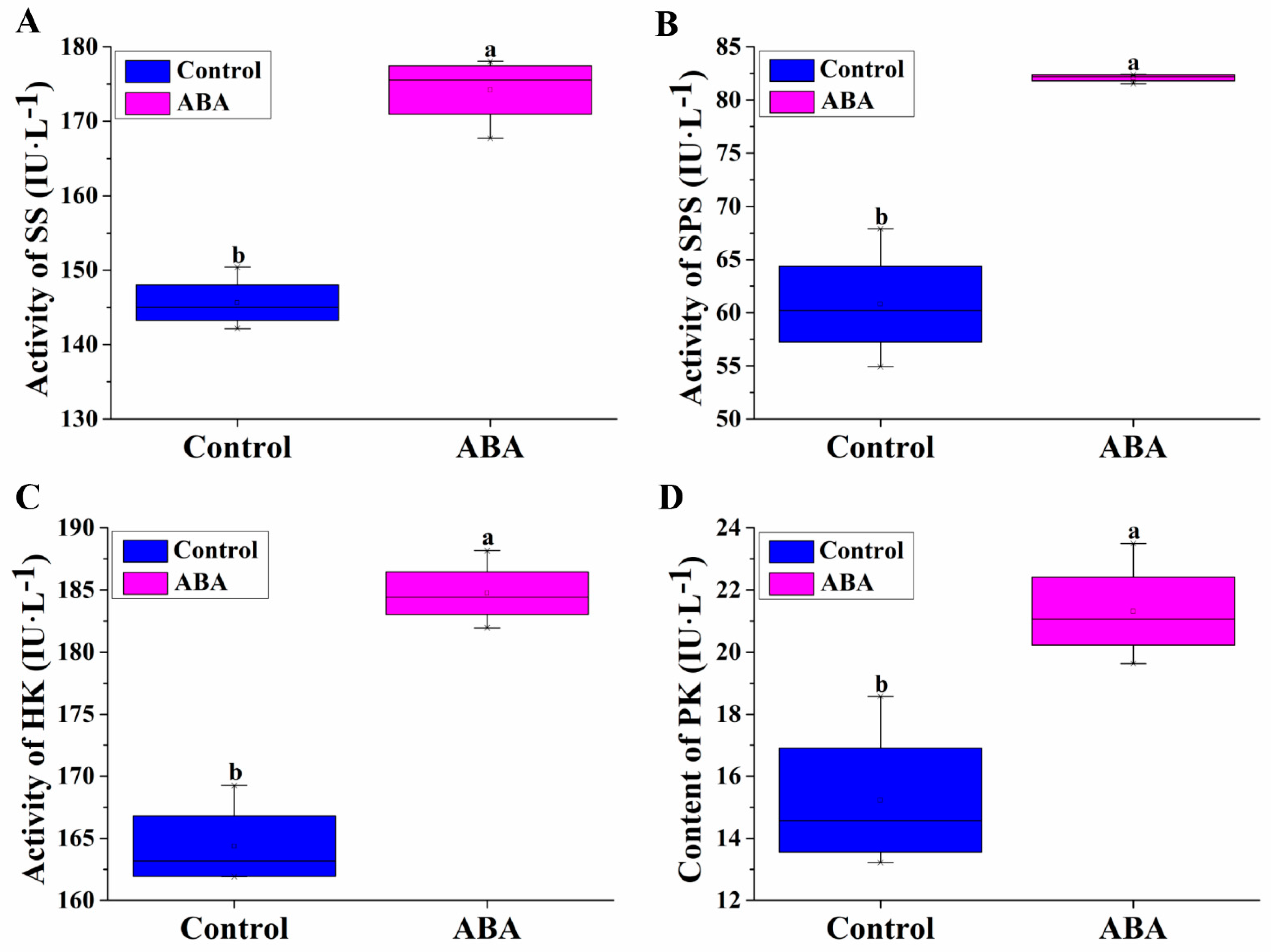
3.4. Effect of Exogenous ABA on SS, SPS, HK and PK Activities during Adventitious Rooting
3.5. Effect of Exogenous ABA on CsSuSy1, CsSuSy6, CsHK1 and CsHK3 Expression Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reyes, J.L.; Chua, N.H. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef]
- Qu, Y.; Song, P.; Hu, Y.; Jin, X.; Jia, Q.; Zhang, X.; Chen, L.; Zhang, Q. Regulation of stomatal movement by cortical microtubule organization in response to darkness and ABA signaling in Arabidopsis. Plant Growth Regul. 2018, 84, 467–479. [Google Scholar] [CrossRef]
- Endo, T.; Shimada, T.; Nakata, Y.; Fujii, H.; Matsumoto, H.; Nakajima, N.; Ikoma, Y.; Omura, M. Abscisic acid affects expression of citrus FT homologs upon floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.). Tree Physiol. 2017, 38, 755–771. [Google Scholar] [CrossRef]
- Hong, J.H.; Seah, S.W.; Xu, J. The root of ABA action in environmental stress response. Plant Cell Rep. 2013, 32, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.Y.; Meyer, M.M.; Beevers, A.L. Abscisic-acid-stimulated rooting of stem cuttings. Planta 1969, 88, 192–196. [Google Scholar] [CrossRef]
- Tartoura, K.A.H. Effect of abscisic acid on endogenous IAA, auxin protector levels and peroxidase activity during adventitious root initiation in Vigna radiata cuttings. Acta Physiol. Plant. 2001, 23, 149–156. [Google Scholar] [CrossRef]
- Guo, D.; Liang, J.; Li, L. Abscisic acid (ABA) inhibition of lateral root formation involves endogenous ABA biosynthesis in Arachis hypogaea L. Plant Growth Regul. 2009, 58, 173–179. [Google Scholar] [CrossRef]
- Guo, D.; Liang, J.; Qiao, Y.; Yan, Y.; Li, L.; Dai, Y. Involvement of G1-to-S transition and AhAUX-dependent auxin transport in abscisic acid-induced inhibition of lateral root primordia initiation in Arachis hypogaea L. J. Plant Physiol. 2012, 169, 1102–1111. [Google Scholar] [CrossRef]
- Steffens, B.; Wang, J.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.A.M.; Brodribb, T.J.; Ross, J.J. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ. 2016, 39, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-L.; Chen, F.; Zou, X.-L.; Shen, S.-S.; Wang, X.-G.; Yao, G.-X.; Xu, B.-B. Graphene oxide and ABA cotreatment regulates root growth of Brassica napus L. by regulating IAA/ABA. J. Plant Physiol. 2019, 240, 153007. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Yin, X.; Wang, H.; Lu, P.; Liu, X.; Gong, C.; Wu, Y. ABA-INDUCED expression 1 is involved in ABA-inhibited primary root elongation via modulating ROS homeostasis in Arabidopsis. Plant Sci. 2021, 304, 110821. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Wirtz, W.; Heldt, H.W. Regulation of sucrose synthesis by cytoplasmic fructosebisphosphatase and sucrose phosphate synthase during photosynthesis in varying light and carbon dioxide. Plant Physiol. 1983, 72, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Strand, A.; Zrenner, R.; Trevanion, S.; Stitt, M.; Gustafsson, P.; Gardestrom, P. Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1,6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant J. 2000, 23, 759–770. [Google Scholar] [CrossRef]
- Sturm, A. Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999, 121, 1–8. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Xiao, W.; Sheen, J.; Jang, J.C. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol. Biol. 2000, 44, 451–461. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Hu, M.Y.; Li, H.; Zhang, Y.J.; Liu, Q. Photosynthesis and related physiological characteristics affected by exogenous glucose in wheat seedlings under water stress. Acta Agron. Sin. 2009, 35, 724–732. [Google Scholar] [CrossRef]
- Hu, M.Y.; Shi, Z.G.; Zhang, Z.B.; Zhang, Y.J.; Li, H. Effects of exogenous glucose on seed germination and antioxidant capacity in wheat seedlings under salt stress. Plant Growth Regul. 2012, 68, 177–188. [Google Scholar] [CrossRef]
- Jiang, W.; Ding, M.; Duan, Q.; Zhou, Q.; Huang, D. Exogenous glucose preserves the quality of watermelon (Citrullus lanatus) plug seedlings for low temperature storage. Sci. Hortic. 2012, 148, 23–29. [Google Scholar] [CrossRef]
- MacGregor, D.R.; Deak, K.I.; Ingram, P.A.; Malamy, J.E. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell 2008, 20, 2643–2660. [Google Scholar] [CrossRef]
- Takahashi, F.; Sato-Nara, K.; Kobayashi, K.; Suzuki, M.; Suzuki, H. Sugar-induced adventitious roots in Arabidopsis seedling. J. Plant Res. 2003, 116, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef] [PubMed]
- Booker, K.S.; Schwarz, J.; Garrett, M.B.; Jones, A.M. Glucose attenuation of auxin-mediated bimodality in lateral root formation is partly coupled by the heterotrimeric G protein complex. PLoS ONE 2010, 5, e12833. [Google Scholar] [CrossRef]
- Yuan, T.T.; Xu, H.H.; Zhang, K.X.; Guo, T.T.; Lu, Y.T. Glucose inhibits root meristem growth via ABA INSENSITIVE 5, which represses PIN1 accumulation and auxin activity in Arabidopsis. Plant Cell Environ. 2014, 37, 1338–1350. [Google Scholar] [CrossRef]
- Calamar, A.; de Klerk, G.J. Effect of sucrose on adventitious root regeneration in apple. Plant Cell Tissue Organ Cult. 2002, 70, 207–212. [Google Scholar] [CrossRef]
- Tossi, V.; Lamattina, L.; Cassia, R. An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol. 2009, 181, 871–879. [Google Scholar] [CrossRef]
- Tari, I.; Nagy, M. Abscisic acid and Ethrel abolish the inhibition of adventitious root formation of paclobutrazol-treated bean primary leaf cuttings. Biol. Plant. 1996, 38, 369. [Google Scholar] [CrossRef]
- Li, X.P.; Xu, Q.Q.; Liao, W.B.; Ma, Z.J.; Xu, X.T.; Wang, M.; Ren, P.J.; Niu, L.J.; Jin, X.; Zhu, Y.C. Hydrogen peroxide is involved in abscisic acid-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J. Plant Biol. 2016, 59, 536–548. [Google Scholar]
- Leach, K.A.; Braun, D.M. Soluble sugar and starch extraction and quantification from maize (Zea mays) leaves. Curr. Protoc. Plant Biol. 2016, 1, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.T.; Li, W.T.; Liu, Z.Y.; Liu, H.W.; Gao, R.; Luo, Y.Y.; Liao, W.B. Trehalose promotes the formation of Lanzhou lily bulblets by increasing carbohydrate content. J. Hortic. Sci. Biotechnol. 2022, 97, 503–513. [Google Scholar] [CrossRef]
- Hou, X.; Qi, N.; Wang, C.; Li, C.; Huang, D.; Li, Y.; Wang, N.; Liao, W. Hydrogen-rich water promotes the formation of bulblets in Lilium davidii var. unicolor through regulating sucrose and starch metabolism. Planta 2021, 254, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Li, C.X.; Liu, H.W.; Yang, J.J.; Huang, P.P.; Liao, W.B. The involvement of glucose in hydrogen gas-medicated adventitious rooting in cucumber. Plants 2021, 10, 1937. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.J.; Wang, C.L.; Liao, W.B. Hydrogen sulfide improves the vase life and quality of cut roses and chrysanthemums. J. Plant Growth Regul. 2021, 40, 2532–2547. [Google Scholar] [CrossRef]
- Shi, S.Y.; Wang, W.; Liu, L.Q.; Shu, B.; Wei, Y.Z.; Jue, D.W.; Fu, J.X.; Xie, J.H.; Liu, C.M. Physico-chemical properties of longan fruit during development and ripening. Sci. Hortic. 2016, 207, 160–167. [Google Scholar] [CrossRef]
- Huang, D.J.; Li, W.T.; Dawuda, M.M.; Huo, J.Q.; Li, C.X.; Wang, C.L.; Liao, W.B. Hydrogen sulfide reduced colour change in Lanzhou lily-bulb scales. Postharvest Biol. Technol. 2021, 176, 111520. [Google Scholar] [CrossRef]
- Wan, H.J.; Zhao, Z.G.; Qian, C.T.; Sui, Y.H.; Malik, A.A.; Chen, J.F. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal. Biochem. 2010, 399, 257–261. [Google Scholar] [CrossRef]
- Liu, H.Y.; Ren, X.Q.; Zhu, J.Z.; Wu, X.; Liang, C.J. Effect of exogenous abscisic acid on morphology, growth and nutrient uptake of rice (Oryza sativa) roots under simulated acid rain stress. Planta 2018, 248, 647–659. [Google Scholar] [CrossRef]
- Li, S.W.; Leng, Y.; Feng, L.; Zeng, X.Y. Involvement of abscisic acid in regulating antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean [Vigna radiata (L.) Wilczek] seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2014, 21, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wu, R.; Wee, C.W.; Xie, F.; Wei, X.; Chan, P.M.Y.; Tham, C.; Duan, L.; Dinneny, J.R. A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell 2013, 25, 2132–2154. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Kitano, H.; Komatsu, S. Identification of rice root proteins regulated by gibberellin using proteome analysis. Plant Cell Environ. 2005, 28, 328–339. [Google Scholar] [CrossRef]
- McSteen, P. Auxin and monocot development. CSH Perspect. Biol. 2010, 2, a001479. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, C.W.; Sun, C.L.; Wang, J.H.; Ye, Y.Q.; Zhou, W.W.; Lu, L.L.; Lin, X.Y. Inhibition of ethylene production by putrescine alleviates aluminum-induced root inhibition in wheat plants. Sci. Rep. 2016, 6, 18888. [Google Scholar] [CrossRef]
- Dekkers, B.J.; Schuurmans, J.A.; Smeekens, S.C. Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol. Biol. 2008, 67, 151–167. [Google Scholar] [CrossRef]
- Wang, X.H.; Yin, W.; Wu, J.X.; Chai, L.J.; Yi, H.L. Effects of exogenous abscisic acid on the expression of citrus fruit ripening-related genes and fruit ripening. Sci. Hortic. 2016, 201, 175–183. [Google Scholar] [CrossRef]
- Tan, S.; Xie, J.; Wang, W.; Shi, S.W. Effects of exogenous plant hormones on sugar accumulation and related enzyme activities during the development of longan (Dimocarpus Longan Lour.) fruits. J. Hortic. Sci. Biotechnol. 2019, 94, 790–797. [Google Scholar] [CrossRef]
- Pan, Q.H.; Li, M.J.; Peng, C.C.; Zhang, N.; Zou, X.; Zou, K.Q.; Wang, X.L.; Yu, X.C.; Wang, X.F.; Zhang, D.P. Abscisic acid activates acid invertases in developing grape berry. Physiol. Plant. 2005, 125, 157–170. [Google Scholar] [CrossRef]
- Chen, J.; Huang, B.Q.; Li, Y.P.; Du, H.; Gu, Y.; Liu, H.M.; Zhang, J.J.; Huang, Y.B. Synergistic influence of sucrose and abscisic acid on the genes involved in starch synthesis in maize endosperm. Carbohydr. Res. 2011, 346, 1684–1691. [Google Scholar] [CrossRef]
- Akihiro, T.; Mizuno, K.; Fujimura, T. Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol. 2005, 46, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Rook, F.; Corke, F.; Card, R.; Munz, G.; Smith, C.; Bevan, M.W. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J. 2001, 26, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Xie, S.D.; Xiao, Q.L.; Wei, B.; Zheng, L.J.; Wang, Y.B.; Cao, Y.; Zhang, X.G.; Long, T.D.; Li, Y.P.; et al. Sucrose and ABA regulate starch biosynthesis in maize through a novel transcription factor, ZmEREB156. Sci. Rep. 2016, 6, 27590. [Google Scholar] [CrossRef]
- Tang, T.; Xie, H.; Wang, Y.X.; Lü, B.; Liang, J.S. The effect of sucrose and abscisic acid interaction on sucrose synthase and its relationship to grainfilling of rice (Oryza sativa L.). J. Exp. Bot. 2009, 60, 2641–2652. [Google Scholar] [CrossRef]
- Dominguez, P.G.; Frankel, N.; Mazuch, J.; Balbo, I.; Iusem, N.; Fernie, A.R.; Carrari, F. ASR1 mediates glucose-hormone cross talk by affecting sugar trafficking in tobacco plants. Plant Physiol. 2013, 161, 1486–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; He, J.X. Sugar-induced plant growth is dependent on brassinosteroids. Plant Signal. Behav. 2015, 10, e1082700. [Google Scholar] [CrossRef]
- Loreti, E.; Povero, G.; Novi, G.; Solfanelli, C.; Alpi, A.; Perata, P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008, 179, 1004–1016. [Google Scholar] [CrossRef]
- Jia, H.F.; Jiu, S.T.; Zhang, C.; Wang, C.; Tariq, P.; Liu, Z.J.; Wang, B.J.; Cui, L.W.; Fang, J.G. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol. J. 2016, 14, 2045–2065. [Google Scholar] [CrossRef] [Green Version]


| Gene Symbol | Accession Number | Primer Sequence (5′-3′) |
|---|---|---|
| CsSuSy1-F CsSuSy1-R CsSuSy6-F CsSuSy6-R CsHK3-F CsHK3-R CsHK1-F CsHK1-R | LOC101213767 LOC101216865 LOC101218300 LOC101215511 | CGTGTGCTAAGGAAGGCGGAAG CAGTGTCACCCCACCCTCTCTC TCCAACCGCCACAACTTCATCAC CCATTCCCACTCTGCCCAAGC CACGGTCCTAGTCAGTCGGAGAG GCCATAGCATCAACCACCTGTCTC CGCCATGACCGTCGAGATGC TTTGTACCGCCGAGATCCAATGC |
| CsTUA-F CsTUA-R | AJ715498 | ACGCTGTTGGTGGTGGTAC GAGAGGGGTAAACAGTGAATC |
| ABA/μM | Root Number | Root Length (mm) | Fresh Weight (g) |
|---|---|---|---|
| 0.00 | 3.21 ± 032 d | 6.16 ± 0.23 d | 0.1708 ± 0.0036 c |
| 0.01 | 4.75 ± 0.52 c | 8.04 ± 0.25 c | 0.2025 ± 0.0054 b |
| 0.02 | 5.29 ± 0.40 b | 8.39 ± 0.42 b | 0.2104 ± 0.0064 b |
| 0.05 | 7.62 ± 0.37 a | 10.55 ± 0.04 a | 0.2460 ± 0.0017 a |
| 0.10 | 4.71 ± 0.25 b | 7.69 ± 0.35 c | 0.2225 ± 0.0034 b |
| 1.00 | 1.41 ± 0.22 e | 4.79 ± 0.31 e | 0.1610 ± 0.0013 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhang, M.; Qi, N.; Liu, H.; Zhao, Z.; Huang, P.; Liao, W. Abscisic Acid Induces Adventitious Rooting in Cucumber (Cucumis sativus L.) by Enhancing Sugar Synthesis. Plants 2022, 11, 2354. https://doi.org/10.3390/plants11182354
Li C, Zhang M, Qi N, Liu H, Zhao Z, Huang P, Liao W. Abscisic Acid Induces Adventitious Rooting in Cucumber (Cucumis sativus L.) by Enhancing Sugar Synthesis. Plants. 2022; 11(18):2354. https://doi.org/10.3390/plants11182354
Chicago/Turabian StyleLi, Changxia, Meiling Zhang, Nana Qi, Huwei Liu, Zongxi Zhao, Panpan Huang, and Weibiao Liao. 2022. "Abscisic Acid Induces Adventitious Rooting in Cucumber (Cucumis sativus L.) by Enhancing Sugar Synthesis" Plants 11, no. 18: 2354. https://doi.org/10.3390/plants11182354
APA StyleLi, C., Zhang, M., Qi, N., Liu, H., Zhao, Z., Huang, P., & Liao, W. (2022). Abscisic Acid Induces Adventitious Rooting in Cucumber (Cucumis sativus L.) by Enhancing Sugar Synthesis. Plants, 11(18), 2354. https://doi.org/10.3390/plants11182354






