Plant Development and Crop Yield: The Role of Gibberellins
Abstract
:1. Introduction
2. History of Gibberellins Research
3. Vegetative Development
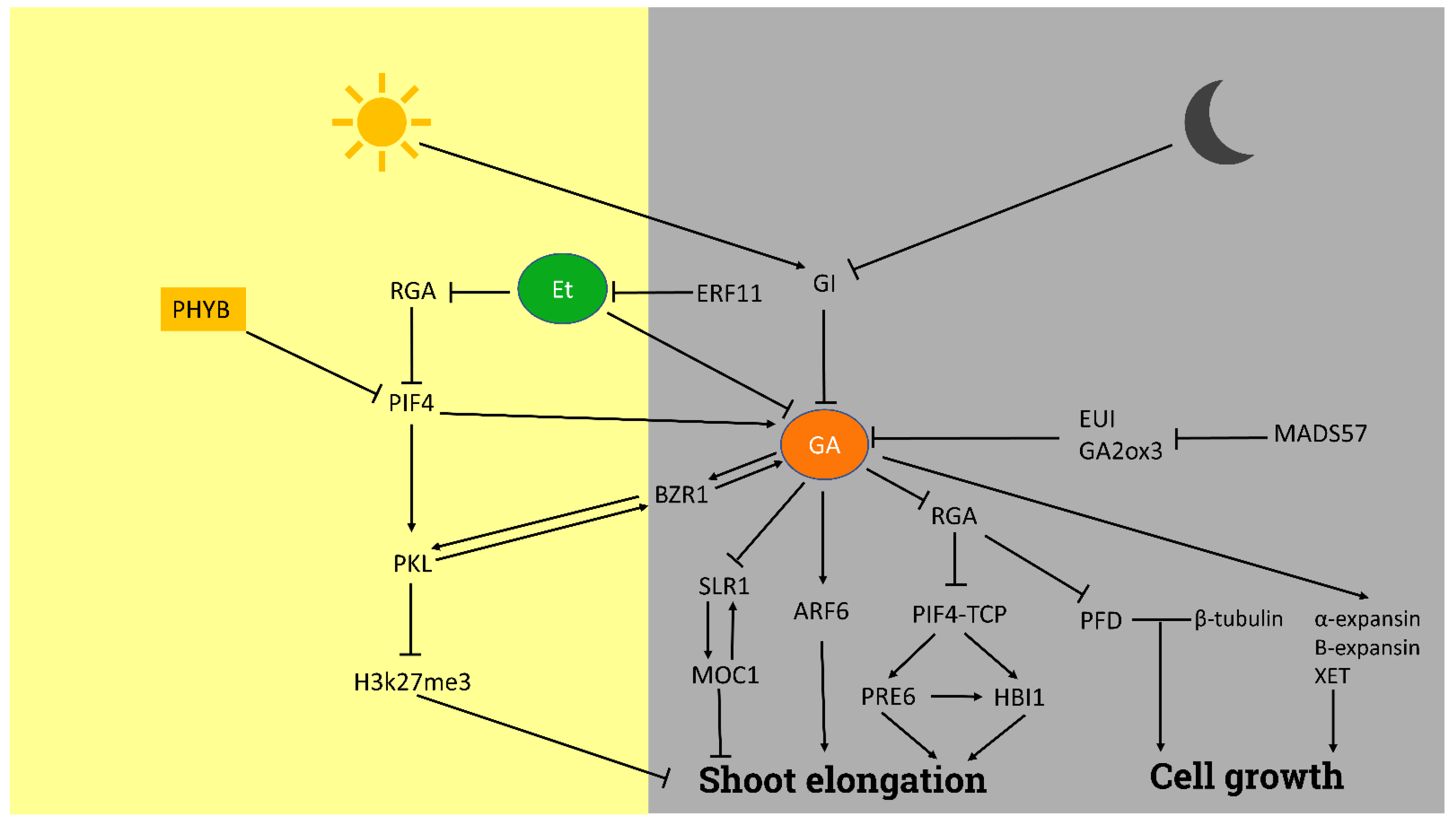
3.1. Shoot Elongation
3.2. Xylogenesis and Cellulose Production
3.3. Root Development
3.4. Other Vegetative Processes
4. Reproductive Development
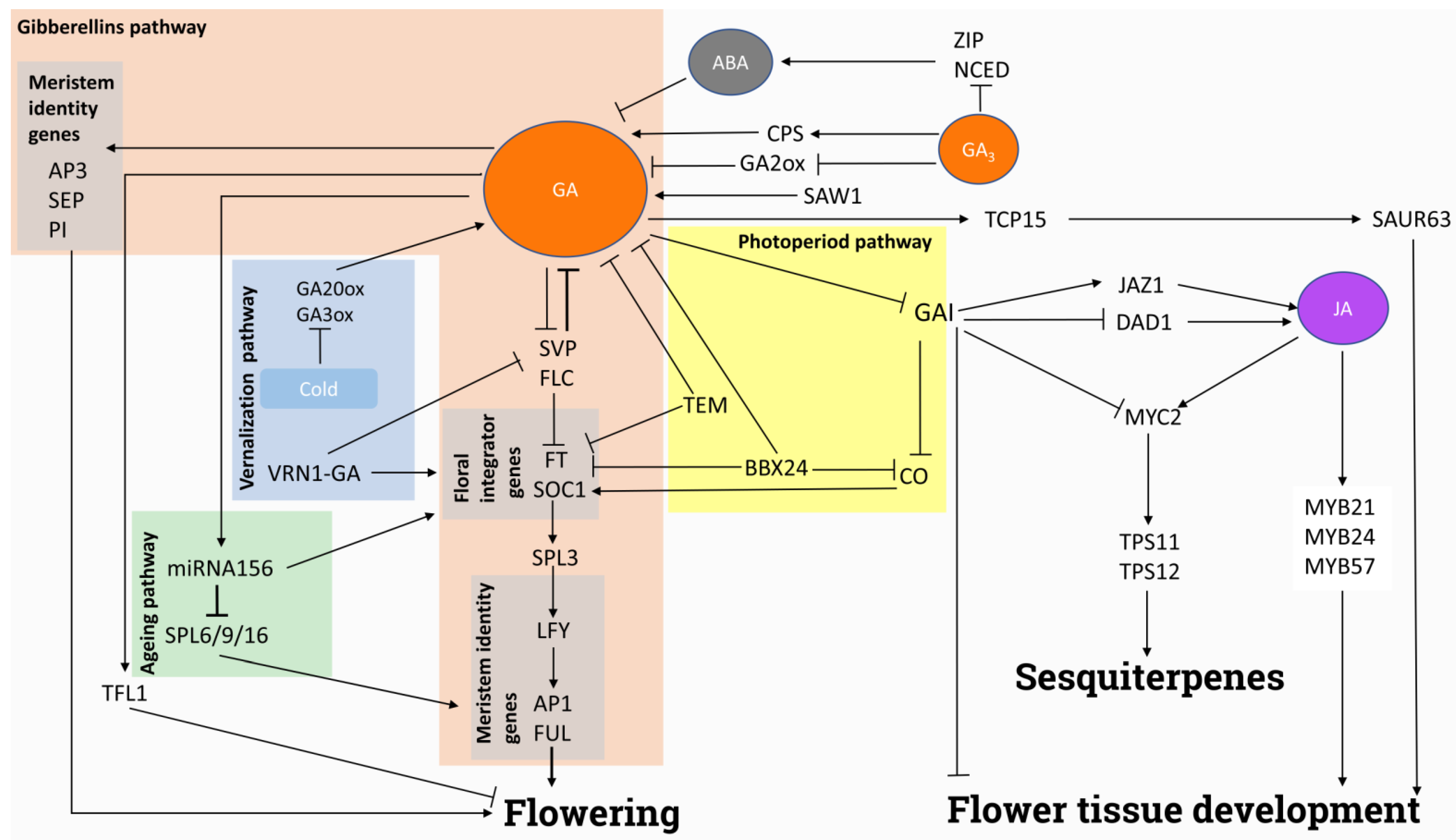
4.1. Flowering
4.2. Flower Formation and Fertilization
5. Fruit Development
6. Seed Germination
7. Current and Potential Applications
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kende, H.; Zeevaart, J. The Five “Classical” Plant Hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, J.; Lu, W.; Deng, D. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Groot, S.P.C.; Karssen, C.M. Gibberellins regulate seed germination in tomato by endosperm weakening: A study with gibberellin-deficient mutants. Planta 1987, 171, 525–531. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Xu, H.; Gao, X.; Fu, X. New insights into gibberellin signaling in regulating plant growth–metabolic coordination. Curr. Opin. Plant Biol. 2021, 63, 102074. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhang, Y.; Xiao, G. Neo-gibberellin Signaling: Guiding the Next Generation of the Green Revolution. Trends Plant Sci. 2020, 25, 520–522. [Google Scholar] [CrossRef]
- Stowe, B.B.; Yamaki, T. The History and Physiological Action of the Gibberellins. Annu. Rev. Plant Physiol. 1957, 8, 181–216. [Google Scholar] [CrossRef]
- Piombo, E.; Bosio, P.; Acquadro, A.; Abbruscato, P.; Spadaro, D. Different Phenotypes, Similar Genomes: Three Newly Sequenced Fusarium fujikuroi Strains Induce Different Symptoms in Rice Depending on Temperature. Phytopathology 2020, 110, 656–665. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Kitamura, H.; Kawarada, A.; Seta, Y.; Takai, M.; Tamura, S.; Sumiki, Y. Biochemical Studies on “Bakanae” Fungus. Bull. Agric. Chem. Soc. Japan 1955, 19, 267–281. [Google Scholar] [CrossRef]
- Takahashi, N.; Seta, Y.; Kitamura, H.; Sumiki, Y.; Kawarada, A. Biochemical Studies on “Bakanae” Fungus. Part 48–50. Bull. Agric. Chem. Soc. Jpn. 1959, 23, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Phinney, B.O. Growth response of single-gene dwarf mutants in maize to gibberellic acid. Proc. Natl. Acad. Sci. USA 1956, 42, 185–189. [Google Scholar] [CrossRef] [Green Version]
- MacMillan, J. Occurrence of Gibberellins in Vascular Plants, Fungi, and Bacteria. J. Plant Growth Regul. 2002, 20, 387–442. [Google Scholar] [CrossRef]
- Rademacher, W. Chemical Regulators of Gibberellin Status and Their Application in Plant Production. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Chichester, UK, 2016; Volume 49, pp. 359–403. ISBN 9781119312994. [Google Scholar]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Eshed, Y.; Lippman, Z.B. Revolutions in agriculture chart a course for targeted breeding of old and new crops. Science 2019, 366, eaax0025. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J. Interactions of Gibberellins with Phytohormones and Their Role in Stress Responses. Horticulturae 2022, 8, 241. [Google Scholar] [CrossRef]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Krishnan, S.; Merewitz, E.; Xu, J.; Huang, B. Gibberellin-Regulation and Genetic Variations in Leaf Elongation for Tall Fescue in Association with Differential Gene Expression Controlling Cell Expansion. Sci. Rep. 2016, 6, 30258. [Google Scholar] [CrossRef]
- Locascio, A.; Blázquez, M.A.; Alabadí, D. Dynamic regulation of cortical microtubule organization through prefoldin-DELLA interaction. Curr. Biol. 2013, 23, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Touriñán, N.; Esteve-Bruna, D.; Serrano-Mislata, A.; Esquinas-Ariza, R.M.; Resentini, F.; Forment, J.; Carrasco-López, C.; Novella-Rausell, C.; Palacios-Abella, A.; Carrasco, P.; et al. A genetic approach reveals different modes of action of prefoldins. Plant Physiol. 2021, 187, 1534–1550. [Google Scholar] [CrossRef]
- Liao, Z.; Yu, H.; Duan, J.; Yuan, K.; Yu, C.; Meng, X.; Kou, L.; Chen, M.; Jing, Y.; Liu, G.; et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019, 10, 2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.; Hong, J.; Chen, X.; Zhang, C.; Chen, M.; Luo, Z.; Chang, S.; Bai, S.; Liang, W.; Liu, Q.; et al. Gibberellins orchestrate panicle architecture mediated by DELLA–KNOX signalling in rice. Plant Biotechnol. J. 2021, 19, 2304–2318. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, N.; Wu, Q.; Yu, B.; Li, X.; Chen, R.; Huang, J. Rice transcription factor OsMADS57 regulates plant height by modulating gibberellin catabolism. Rice 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Liu, X.; Wang, M.; Xie, L.; Wu, Z.; Yu, J.; Wang, Y.; Zhang, Z.; Jia, Y.; Liu, Q. The miR528-D3 Module Regulates Plant Height in Rice by Modulating the Gibberellin and Abscisic Acid Metabolisms. Rice 2022, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shu, J.; Mohamed, A.M.A.; Deng, X.; Zhi, X.; Bai, J.; Cui, Y.; Lu, X.; Du, Y.; Wang, X.; et al. Identification and characterization of EI (elongated internode) gene in tomato (solanum lycopersicum). Int. J. Mol. Sci. 2019, 20, 2204. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 2015, 15, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizza, A.; Walia, A.; Lanquar, V.; Frommer, W.B.; Jones, A.M. In vivo gibberellin gradients visualized in rapidly elongating tissues. Nat. Plants 2017, 3, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Gil, K.-E.; Ha, J.-H.; Park, C.-M. Abscisic acid-mediated phytochrome B signaling promotes primary root growth in Arabidopsis. Plant Signal. Behav. 2018, 13, e1473684. [Google Scholar] [CrossRef] [Green Version]
- De Lucas, M.; Davière, J.M.; Rodríguez-Falcón, M.; Pontin, M.; Iglesias-Pedraz, J.M.; Lorrain, S.; Fankhauser, C.; Blázquez, M.A.; Titarenko, E.; Prat, S. A molecular framework for light and gibberellin control of cell elongation. Nature 2008, 451, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Arana, M.V.; Marín-De La Rosa, N.; Maloof, J.N.; Blázqueza, M.A.; Alabadí, D. Circadian oscillation of gibberellin signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 9292–9297. [Google Scholar] [CrossRef]
- Nohales, M.A.; Kay, S.A. GIGANTEA gates gibberellin signaling through stabilization of the DELLA proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 21893–21899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Gao, Z.; Zhu, Z. DELLA–PIF Modules: Old Dogs Learn New Tricks. Trends Plant Sci. 2016, 21, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Bartolome, J.; Minguet, E.G.; Grau-Enguix, F.; Abbas, M.; Locascio, A.; Thomas, S.G.; Alabadi, D.; Blazquez, M.A. Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 13446–13451. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Jing, Y.; Jiang, Z.; Lin, R. The chromatin-remodeling factor PICKLE integrates brassinosteroid and gibberellin signaling during skotomorphogenic growth in Arabidopsis. Plant Cell 2014, 26, 2472–2485. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.; Zhu, J.Y.; Bai, M.Y.; Arenhart, R.A.; Sun, Y.; Wang, Z.Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife 2014, 2014, e03031. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.Y.; Wang, Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef] [Green Version]
- Stavang, J.A.; Gallego-Bartolomé, J.; Gómez, M.D.; Yoshida, S.; Asami, T.; Olsen, J.E.; García-Martínez, J.L.; Alabadí, D.; Blázquez, M.A. Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J. 2009, 60, 589–601. [Google Scholar] [CrossRef]
- Camut, L.; Regnault, T.; Sirlin-Josserand, M.; Sakvarelidze-Achard, L.; Carrera, E.; Zumsteg, J.; Heintz, D.; Leonhardt, N.; Lange, M.J.P.; Lange, T.; et al. Root-derived GA12 contributes to temperature-induced shoot growth in Arabidopsis. Nat. Plants 2019, 5, 1216–1221. [Google Scholar] [CrossRef]
- Ohtaka, K.; Yoshida, A.; Kakei, Y.; Fukui, K.; Kojima, M.; Takebayashi, Y.; Yano, K.; Imanishi, S.; Sakakibara, H. Difference between Day and Night Temperatures Affects Stem Elongation in Tomato (Solanum lycopersicum) Seedlings via Regulation of Gibberellin and Auxin Synthesis. Front. Plant Sci. 2020, 11, 577235. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, G.; Wang, L.; Zhou, Q.; Huang, X. Effects of elevated ultraviolet-B radiation on root growth and chemical signaling molecules in plants. Ecotoxicol. Environ. Saf. 2019, 171, 683–690. [Google Scholar] [CrossRef]
- Miao, T.; Li, D.; Huang, Z.; Huang, Y.; Li, S.; Wang, Y. Gibberellin regulates UV-B-induced hypocotyl growth inhibition in Arabidopsis thaliana. Plant Signal. Behav. 2021, 16, 1966587. [Google Scholar] [CrossRef]
- Tavridou, E.; Pireyre, M.; Ulm, R. Degradation of the transcription factors PIF4 and PIF5 under UV-B promotes UVR8-mediated inhibition of hypocotyl growth in Arabidopsis. Plant J. 2020, 101, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Cheng, Q.; Qin, C.; Li, Y.; Xu, X.; Ji, R.; Mu, R.; Li, H.; Zhao, T.; Liu, J.; et al. GmCRY1s modulate gibberellin metabolism to regulate soybean shade avoidance in response to reduced blue light. Mol. Plant 2021, 14, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Zeng, B.; Tang, D.; Yang, J.; Qu, L.; Yan, J.; Wang, X.; Li, X.; Liu, X.; Zhao, X. The blue light receptor CRY1 interacts with GID1 and DELLA proteins to repress GA signaling during photomorphogenesis in Arabidopsis. Mol. Plant 2021, 14, 1328–1342. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, N.; Shen, Z.; Zhu, C.; Liu, H.; Xu, Z.; Li, R.; Shen, Q.; Salles, J.F. Soil microbiome manipulation triggers direct and possible indirect suppression against Ralstonia solanacearum and Fusarium oxysporum. NPJ Biofilms Microbiomes 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, C.; Li, L. Shade-induced stem elongation in rice seedlings: Implication of tissue-specific phytohormone regulation. J. Integr. Plant Biol. 2016, 58, 614–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Katin-Grazzini, L.; Gu, X.; Wang, X.; El-Tanbouly, R.; Yer, H.; Thammina, C.; Inguagiato, J.; Guillard, K.; McAvoy, R.J.; et al. Transcriptome analysis reveals differential gene expression and a possible role of gibberellins in a shade-tolerant mutant of perennial ryegrass. Front. Plant Sci. 2017, 8, 868. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, Z.L.; Park, J.; Tyler, L.; Yusuke, J.; Qiu, K.; Nam, E.A.; Lumba, S.; Desveaux, D.; McCourt, P.; et al. The ERF11 transcription factor promotes internode elongation by activating gibberellin biosynthesis and signaling. Plant Physiol. 2016, 171, 2760–2770. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-M.; Shieh, Y.-J.; Chou, C.-H. Abscisic acid inhibits shoot elongation of Scirpus mucronatus. Physiol. Plant. 1996, 97, 1–4. [Google Scholar] [CrossRef]
- Saab, I.N.; Sharp, R.E.; Pritchard, J.; Voetberg, G.S. Increased Endogenous Abscisic Acid Maintains Primary Root Growth and Inhibits Shoot Growth of Maize Seedlings at Low Water Potentials. Plant Physiol. 1990, 93, 1329–1336. [Google Scholar] [CrossRef]
- Zou, X.; Wang, Q.; Chen, P.; Yin, C.; Lin, Y. Strigolactones regulate shoot elongation by mediating gibberellin metabolism and signaling in rice (Oryza sativa L.). J. Plant Physiol. 2019, 237, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shui, Z.; Xu, L.; Yang, Y.; Li, Y.; Yuan, X.; Shang, J.; Asghar, M.A.; Wu, X.; Yu, L.; et al. Gibberellins modulate shade-induced soybean hypocotyl elongation downstream of the mutual promotion of auxin and brassinosteroids. Plant Physiol. Biochem. 2020, 150, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Lachaud, S. Participation of auxin and abscisic acid in the regulation of seasonal variations in cambial activity and xylogenesis. Trees 1989, 3, 125–137. [Google Scholar] [CrossRef]
- Sorce, C.; Giovannelli, A.; Sebastiani, L.; Anfodillo, T. Hormonal signals involved in the regulation of cambial activity, xylogenesis and vessel patterning in trees. Plant Cell Rep. 2013, 32, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Su, H.; Cao, H.; Wei, H.; Fu, X.; Jiang, X.; Song, Q.; He, X.; Xu, C.; Luo, K. AUXIN RESPONSE FACTOR7 integrates gibberellin and auxin signaling via interactions between DELLA and AUX/IAA proteins to regulate cambial activity in poplar. Plant Cell 2022, 34, 2688–2707. [Google Scholar] [CrossRef]
- Mauriat, M.; Moritz, T. Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J. 2009, 58, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Guo, G.S.; Qiu, Z.F.; Li, X.D.; Zeng, B.S.; Fan, C.J. Exogenous GA3 application altered morphology, anatomic and transcriptional regulatory networks of hormones in Eucalyptus grandis. Protoplasma 2018, 255, 1107–1119. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Liu, H.; Hu, P.; Jia, Y.; Zhang, C.; Wang, Y.; Gu, S.; Yang, C.; Wang, C. Exogenous GA3 application enhances xylem development and induces the expression of secondary wall biosynthesis related genes in Betula platyphylla. Int. J. Mol. Sci. 2015, 16, 22960–22975. [Google Scholar] [CrossRef] [Green Version]
- Busov, V.B. Manipulation of growth and architectural characteristics in trees for increased woody biomass production. Front. Plant Sci. 2018, 871, 1505. [Google Scholar] [CrossRef]
- Cho, J.S.; Jeon, H.W.; Kim, M.H.; Vo, T.K.; Kim, J.; Park, E.J.; Choi, Y.I.; Lee, H.; Han, K.H.; Ko, J.H. Wood forming tissue-specific bicistronic expression of PdGA20ox1 and PtrMYB221 improves both the quality and quantity of woody biomass production in a hybrid poplar. Plant Biotechnol. J. 2019, 17, 1048–1057. [Google Scholar] [CrossRef]
- Eriksson, M.E.; Israelsson, M.; Olsson, O.; Moritz, T. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 2000, 18, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Song, Y.; Du, Q.; Yang, X.; Ci, D.; Chen, J.; Xie, J.; Li, B.; Zhang, D. Population genomic analysis of gibberellin-responsive long non-coding RNAs in Populus. J. Exp. Bot. 2016, 67, 2467–2482. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yu, W.; Ran, L.; Chen, Z.; Wang, C.; Dou, Y.; Qin, Y.; Suo, Q.; Li, Y.; Zeng, J.; et al. DELLA-NAC Interactions Mediate GA Signaling to Promote Secondary Cell Wall Formation in Cotton Stem. Front. Plant Sci. 2021, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Duan, A.Q.; Feng, K.; Liu, J.X.; Que, F.; Xu, Z.S.; Xiong, A.S. Elevated gibberellin altered morphology, anatomical structure, and transcriptional regulatory networks of hormones in celery leaves. Protoplasma 2019, 256, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Illouz-Eliaz, N.; Nissan, I.; Nir, I.; Ramon, U.; Shohat, H.; Weiss, D. Mutations in the tomato gibberellin receptors suppress xylem proliferation and reduce water loss under water-deficit conditions. J. Exp. Bot. 2020, 71, 3603–3612. [Google Scholar] [CrossRef]
- Shtin, M.; Dello Ioio, R.; Del Bianco, M. It’s Time for a Change: The Role of Gibberellin in Root Meristem Development. Front. Plant Sci. 2022, 13, 882517. [Google Scholar] [CrossRef]
- Chu, X.; Su, H.; Hayashi, S.; Gresshoff, P.M.; Ferguson, B.J. Spatiotemporal changes in gibberellin content are required for soybean nodulation. New Phytol. 2022, 234, 479–493. [Google Scholar] [CrossRef]
- Nett, R.S.; Bender, K.S.; Peters, R.J. Production of the plant hormone gibberellin by rhizobia increases host legume nodule size. ISME J. 2022, 16, 1809–1817. [Google Scholar] [CrossRef]
- Rizza, A.; Tang, B.; Stanley, C.E.; Grossmann, G.; Owen, M.R.; Band, L.R.; Jones, A.M. Differential biosynthesis and cellular permeability explain longitudinal gibberellin gradients in growing roots. Proc. Natl. Acad. Sci. USA 2021, 118, e1921960118. [Google Scholar] [CrossRef]
- Moubayidin, L.; Perilli, S.; Dello Ioio, R.; Di Mambro, R.; Costantino, P.; Sabatini, S. The Rate of Cell Differentiation Controls the Arabidopsis Root Meristem Growth Phase. Curr. Biol. 2010, 20, 1138–1143. [Google Scholar] [CrossRef]
- Marín-de la Rosa, N.; Pfeiffer, A.; Hill, K.; Locascio, A.; Bhalerao, R.P.; Miskolczi, P.; Grønlund, A.L.; Wanchoo-Kohli, A.; Thomas, S.G.; Bennett, M.J.; et al. Genome Wide Binding Site Analysis Reveals Transcriptional Coactivation of Cytokinin-Responsive Genes by DELLA Proteins. PLoS Genet. 2015, 11, e1005337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achard, P.; Gusti, A.; Cheminant, S.; Alioua, M.; Dhondt, S.; Coppens, F.; Beemster, G.T.S.; Genschik, P. Gibberellin Signaling Controls Cell Proliferation Rate in Arabidopsis. Curr. Biol. 2009, 19, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Pandey, B.K.; Li, Y.; Huang, G.; Wang, J.; Quan, R.; Zhou, J.; Zhou, Y.; Miao, Y.; Zhang, D.; et al. Orchestration of ethylene and gibberellin signals determines primary root elongation in rice. Plant Cell 2022, 34, 1273–1288. [Google Scholar] [CrossRef]
- Bidadi, H.; Yamaguchi, S.; Asahina, M.; Satoh, S. Effects of shoot-applied gibberellin/gibberellin-biosynthesis inhibitors on root growth and expression of gibberellin biosynthesis genes in arabidopsis thaliana. Plant Root 2010, 4, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhou, T.; Zhang, C.; Zheng, W.; Li, J.; Jiang, W.; Xiao, C.; Wei, D.; Yang, C.; Xu, R.; et al. Gibberellin disturbs the balance of endogenesis hormones and inhibits adventitious root development of Pseudostellaria heterophylla through regulating gene expression related to hormone synthesis. Saudi J. Biol. Sci. 2021, 28, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sergeeva, L.; Ligterink, W.; Aloni, R.; Zemach, H.; Doron-Faigenboim, A.; Yang, J.; Zhang, P.; Shabtai, S.; Firon, N. Gibberellin Promotes Sweetpotato Root Vascular Lignification and Reduces Storage-Root Formation. Front. Plant Sci. 2019, 10, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Seng, S.; Li, D.; Zhang, F.; Liu, Y.; Yao, T.; Liang, J.; Yi, M.; Wu, J. Antagonism between abscisic acid and gibberellin regulates starch synthesis and corm development in Gladiolus hybridus. Hortic. Res. 2021, 8, 155. [Google Scholar] [CrossRef]
- Hong, C.P.; Kim, J.; Lee, J.; Yoo, S.I.; Bae, W.; Geem, K.R.; Yu, J.; Jang, I.; Jo, I.H.; Cho, H.; et al. Gibberellin signaling promotes the secondary growth of storage roots in panax ginseng. Int. J. Mol. Sci. 2021, 22, 8694. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Gong, M.; Qin, F.; Xiao, D.; Zhan, J.; Wang, A.; He, L. Regulatory mechanism of GA3 on tuber growth by DELLA-dependent pathway in yam (Dioscorea opposita). Plant Mol. Biol. 2021, 106, 433–448. [Google Scholar] [CrossRef]
- Chen, P.; Yang, R.; Bartels, D.; Dong, T.; Duan, H. Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development. Int. J. Mol. Sci. 2022, 23, 4955. [Google Scholar] [CrossRef]
- Tan, H.; Man, C.; Xie, Y.; Yan, J.; Chu, J.; Huang, J. A Crucial Role of GA-Regulated Flavonol Biosynthesis in Root Growth of Arabidopsis. Mol. Plant 2019, 12, 521–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramon, U.; Weiss, D.; Illouz-Eliaz, N. Underground gibberellin activity: Differential gibberellin response in tomato shoots and roots. New Phytol. 2021, 229, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Fonouni-Farde, C.; Miassod, A.; Laffont, C.; Morin, H.; Bendahmane, A.; Diet, A.; Frugier, F. Gibberellins negatively regulate the development of Medicago truncatula root system. Sci. Rep. 2019, 9, 2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshed, Y.; Riov, J.; Atzmon, N. Rooting Oak Cuttings from Gibberellin-treated Stock Plants. HortScience 1996, 31, 872–873. [Google Scholar] [CrossRef] [Green Version]
- Ford, Y.-Y.; Taylor, J.M.; Blake, P.S.; Marks, T.R. Gibberellin A 3 stimulates adventitious rooting of cuttings from cherry (Prunus avium). Plant Growth Regul. 2002, 37, 127–133. [Google Scholar] [CrossRef]
- Niu, S.; Li, Z.; Yuan, H.; Fang, P.; Chen, X.; Li, W. Proper gibberellin localization in vascular tissue is required to regulate adventitious root development in tobacco. J. Exp. Bot. 2013, 64, 3411–3424. [Google Scholar] [CrossRef] [Green Version]
- Busov, V.; Meilan, R.; Pearce, D.W.; Rood, S.B.; Ma, C.; Tschaplinski, T.J.; Strauss, S.H. Transgenic modification of gai or rgl1 causes dwarfing and alters gibberellins, root growth, and metabolite profiles in Populus. Planta 2006, 224, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, A.; Díaz-Sala, C. Effect of polar auxin transport and gibberellins on xylem formation in pine cuttings under adventitious rooting conditions. Isr. J. Plant Sci. 2020, 67, 27–39. [Google Scholar] [CrossRef]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Liang, X.; Lv, S.; Guan, T.; Jiang, T.; Cheng, Y. Histone deacetylase gene PtHDT902 modifies adventitious root formation and negatively regulates salt stress tolerance in poplar. Plant Sci. 2020, 290, 110301. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.F.; Yang, S.Y.; Chen, K.T.; Hsing, Y.I.; Zeevaart, J.A.D.; Chen, L.J.; Yu, S.M. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef] [Green Version]
- Salari, H.; Baninasab, B.; Akbari, M.; Rohani, M.A. Effect of Paclobutrazol on Adventitious Root Formation of IBA-Treated Cuttings of “Zard” and “Dakal” Olive (Olea europaea L.). Cultivars. Asian J. Appl. Sci. 2017, 5, 692–699. [Google Scholar]
- Qadri, R.; Akram, M.T.; Khan, I.; Azam, M.; Nisar, N.; Ghani, M.A.; Tanveer, M.; Khan, M. Response of guava (Psidium guajava L.) softwood cuttings to paclobutrazol application in different rooting media. Bangladesh J. Bot. 2018, 47, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Lombardi-Crestana, S.; Da Silva Azevedo, M.; E Silva, G.F.F.; Pino, L.E.; Appezzato-Da-Glória, B.; Figueira, A.; Nogueira, F.T.S.; Peres, L.E.P. The Tomato (Solanum Lycopersicum cv. Micro-Tom) Natural Genetic Variation Rg1 and the della Mutant Procera Control the Competence Necessary to Form Adventitious Roots and Shoots. J. Exp. Bot. 2012, 63, 5689–5703. [Google Scholar] [CrossRef] [Green Version]
- Mignolli, F.; Vidoz, M.L.; Picciarelli, P.; Mariotti, L. Gibberellins modulate auxin responses during tomato (Solanum lycopersicum L.) fruit development. Physiol. Plant 2019, 165, 768–779. [Google Scholar] [CrossRef] [Green Version]
- Li, S.W.; Shi, R.F.; Leng, Y.; Zhou, Y. Transcriptomic analysis reveals the gene expression profile that specifically responds to IBA during adventitious rooting in mung bean seedlings. BMC Genom. 2016, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Lei, C.; Fan, S.; Li, K.; Meng, Y.; Mao, J.; Han, M.; Zhao, C.; Bao, L.; Zhang, D. iTRAQ-based proteomic analysis reveals potential regulation networks of iba-induced adventitious root formation in apple. Int. J. Mol. Sci. 2018, 19, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Liang, Y.; Xing, L.; Mao, J.; Liu, Z.; Dong, F.; Meng, Y.; Han, M.; Zhao, C.; Bao, L.; et al. Transcriptome analysis reveals multiple hormones, wounding and sugar signaling pathways mediate adventitious root formation in apple rootstock. Int. J. Mol. Sci. 2018, 19, 2201. [Google Scholar] [CrossRef] [Green Version]
- Gou, J.; Strauss, S.H.; Tsai, C.J.; Fang, K.; Chen, Y.; Jiang, X.; Busov, V.B. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 2010, 22, 623–639. [Google Scholar] [CrossRef] [Green Version]
- Lange, M.J.P.; Lange, T. Touch-induced changes in Arabidopsis morphology dependent on gibberellin breakdown. Nat. Plants 2015, 1, 14025. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, S.; Piazza, P.; Craft, J.; Hay, A.; Woolley, L.; Rieu, I.; Phillips, A.; Hedden, P.; Tsiantis, M. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. 2005, 15, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wen, Y.; Cui, M.; Qi, X.; Deng, R.; Gao, J.; Cheng, Z. Histological, physiological and transcriptomic analysis reveal gibberellin-induced axillary meristem formation in garlic (Allium sativum). Plants 2020, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Kwame Acheampong, A.; Shi, Z.; Halaly, T.; Kamiya, Y.; Ophir, R.; Galbraith, D.W.; Or, E. Distinct gibberellin functions during and after grapevine bud dormancy release. J. Exp. Bot. 2018, 69, 1635–1648. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Soto, D.; Ramos-Sánchez, J.M.; Alique, D.; Conde, D.; Triozzi, P.M.; Perales, M.; Allona, I. Overexpression of a SOC1-Related Gene Promotes Bud Break in Ecodormant Poplars. Front. Plant Sci. 2021, 12, 670497. [Google Scholar] [CrossRef]
- Perazza, D.; Vachon, G.; Herzog, M. Gibberellins Promote Trichome Formation by Up-Regulating GLABROUS1 in Arabidopsis1. Plant Physiol. 1998, 117, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Su, D.; Li, J.; Ying, S.; Deng, H.; He, X.; Zhu, Y.; Li, Y.; Chen, Y.; Pirrello, J.; et al. Overexpression of bHLH95, a basic helix–loop–helix transcription factor family member, impacts trichome formation via regulating gibberellin biosynthesis in tomato. J. Exp. Bot. 2020, 71, 3450–3462. [Google Scholar] [CrossRef]
- Han, J.; Tan, J.; Tu, L.; Zhang, X. A peptide hormone gene, GhPSK promotes fibre elongation and contributes to longer and finer cotton fibre. Plant Biotechnol. J. 2014, 12, 861–871. [Google Scholar] [CrossRef]
- An, L.; Zhou, Z.; Su, S.; Yan, A.; Gan, Y. GLABROUS INFLORESCENCE STEMS (GIS) is Required for Trichome Branching Through Gibberellic Acid Signaling in Arabidopsis. Plant Cell Physiol. 2012, 53, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Matías-Hernández, L.; Aguilar-Jaramillo, A.E.; Osnato, M.; Weinstain, R.; Shani, E.; Suárez-López, P.; Pelaz, S. TEMPRANILLO reveals the mesophyll as crucial for epidermal trichome formation. Plant Physiol. 2016, 170, 1624–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Han, Q.; Li, Y.; Yang, F.; Li, J.; Li, P.; Xu, X.; Lin, H.; Zhang, D. A HAT1-DELLA signaling module regulates trichome initiation and leaf growth by achieving gibberellin homeostasis. New Phytol. 2021, 231, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Ran, L.; Hu, J.; Ye, X.; Xu, D.; Li, J.; Su, H.; Wang, X.; Ren, S.; Luo, K. miR319a/TCP module and DELLA protein regulate trichome initiation synergistically and improve insect defenses in Populus tomentosa. New Phytol. 2020, 227, 867–883. [Google Scholar] [CrossRef]
- Lei, W.; Li, Y.; Yao, X.; Qiao, K.; Wei, L.; Liu, B.; Zhang, D.; Lin, H. NAP is involved in GA-mediated chlorophyll degradation and leaf senescence by interacting with DELLAs in Arabidopsis. Plant Cell Rep. 2020, 39, 75–87. [Google Scholar] [CrossRef]
- Kappers, I.F.; Jordi, W.; Maas, F.M.; Stoopen, G.M.; Van Der Plas, L.H.W. Gibberellin and phytochrome control senescence in alstroemeria leaves independently. Physiol. Plant. 1998, 103, 91–98. [Google Scholar] [CrossRef]
- Xiao, X.M.; Xu, Y.M.; Zeng, Z.X.; Tan, X.L.; Liu, Z.L.; Chen, J.W.; Su, X.G.; Chen, J.Y. Activation of the transcription of BrGA20ox3 by a BrTCP21 transcription factor is associated with gibberellin-delayed leaf senescence in chinese flowering cabbage during storage. Int. J. Mol. Sci. 2019, 20, 3860. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.Q.; Tan, X.L.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Characterization of a Transcriptional Regulator, BrWRKY6, Associated with Gibberellin-Suppressed Leaf Senescence of Chinese Flowering Cabbage. J. Agric. Food Chem. 2018, 66, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhang, H.; Song, X.; Jiang, Y.; Liang, R.; Li, G. Functional characterization of the maize phytochrome-interacting factors PIF4 and PIF5. Front. Plant Sci. 2018, 8, 2273. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Jeong, J.; Kang, M.Y.; Kim, J.; Paek, N.C.; Choi, G. Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nat. Commun. 2014, 5, 4636. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xiang, S.; Chen, Y.; Li, D.; Yu, D. Arabidopsis WRKY45 Interacts with the DELLA Protein RGL1 to Positively Regulate Age-Triggered Leaf Senescence. Mol. Plant 2017, 10, 1174–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhang, L.; Wu, S.; Chen, Y.; Yu, D.; Chen, L. AtWRKY75 positively regulates age-triggered leaf senescence through gibberellin pathway. Plant Divers. 2021, 43, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.; Shen, L.; Wu, Y.; Chen, H.; Robertson, M.; Helliwell, C.A.; Ito, T.; Meyerowitz, E.; Yu, H. A Repressor Complex Governs the Integration of Flowering Signals in Arabidopsis. Dev. Cell 2008, 15, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Torti, S.; Coupland, G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 2009, 60, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Ohashi, Y.; Takahashi, R.; Nakai, K.; Takahashi, Y. Della degradation by gibberellin promotes flowering via gaf1-tpr-dependent repression of floral repressors in arabidopsis. Plant Cell 2021, 33, 2258–2272. [Google Scholar] [CrossRef] [PubMed]
- Andrés, F.; Porri, A.; Torti, S.; Mateos, J.; Romera-Branchat, M.; García-Martínez, J.L.; Fornara, F.; Gregis, V.; Kater, M.M.; Coupland, G. SHORT VEGETATIVE PHASE reduces gibberellin biosynthesis at the Arabidopsis shoot apex to regulate the floral transition. Proc. Natl. Acad. Sci. USA 2014, 111, E2760–E2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susila, H.; Nasim, Z.; Gawarecka, K.; Jung, J.Y.; Jin, S.; Youn, G.; Ahn, J.H. PHOSPHORYLETHANOLAMINE CYTIDYLYLTRANSFERASE 1 modulates flowering in a florigen-independent manner by regulating SVP. Development 2021, 148, dev193870. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Wu, M.F.; Yang, L.; Wu, G.; Poethig, R.S.; Wagner, D. The MicroRNA-Regulated SBP-Box Transcription Factor SPL3 Is a Direct Upstream Activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 2009, 17, 268–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Ju, Y.; Seo, P.J.; Lee, J.H.; Park, C.M. The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J. 2012, 69, 577–588. [Google Scholar] [CrossRef]
- Hyun, Y.; Richter, R.; Vincent, C.; Martinez-Gallegos, R.; Porri, A.; Coupland, G. Multi-layered Regulation of SPL15 and Cooperation with SOC1 Integrate Endogenous Flowering Pathways at the Arabidopsis Shoot Meristem. Dev. Cell 2016, 37, 254–266. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Liu, Y.; Zhang, Q.; Gao, Y.; Fang, K.; Cao, Q.; Qin, L.; Xing, Y. Roles of the ga-mediated spl gene family and mir156 in the floral development of chinese chestnut (Castanea mollissima). Int. J. Mol. Sci. 2019, 20, 1577. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Li, T.; Xu, P.B.; Li, L.; Du, S.S.; Lian, H.L.; Yang, H.Q. DELLA proteins physically interact with CONSTANS to regulate flowering under long days in Arabidopsis. FEBS Lett. 2016, 590, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.Q.; Chen, Q.; Lin, R.N.; Li, Y.Q.; Li, L.; Chen, S.; He, X.J. The HDA19 histone deacetylase complex is involved in the regulation of flowering time in a photoperiod-dependent manner. Plant J. 2019, 98, 448–464. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, C.; Xu, Y.; Wei, Q.; Imtiaz, M.; Lan, H.; Gao, S.; Cheng, L.; Wang, M.; Fei, Z.; et al. A zinc finger protein regulates flowering time and abiotic stress tolerance in chrysanthemum by modulating gibberellin biosynthesis. Plant Cell 2014, 26, 2038–2054. [Google Scholar] [CrossRef]
- Osnato, M.; Castillejo, C.; Matías-Hernández, L.; Pelaz, S. TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis. Nat. Commun. 2012, 3, 808. [Google Scholar] [CrossRef] [Green Version]
- Hui, W.K.; Wang, Y.; Chen, X.Y.; Zayed, M.Z.; Wu, G.J. Analysis of transcriptional responses of the inflorescence meristems in Jatropha curcas following gibberellin treatment. Int. J. Mol. Sci. 2018, 19, 432. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.; Deng, Y.; Wang, H.; Gao, R.; Stephen, G.K.; Chen, S.; Jiang, J.; Chen, F. Gibberellic acid signaling is required to induce flowering of chrysanthemums grown under both short and long days. Int. J. Mol. Sci. 2017, 18, 1259. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Li, T.; Wang, S.; Xue, Y.; Hu, F.; Zhang, X. Elucidation of the mechanism of reflowering in tree peony (Paeonia suffruticosa) ‘Zi Luo Lan’ by defoliation and gibberellic acid application. Plant Physiol. Biochem. 2018, 132, 571–578. [Google Scholar] [CrossRef]
- Yuxi, Z.; Yanchao, Y.; Zejun, L.; Tao, Z.; Feng, L.; Chunying, L.; Shupeng, G. GA3 is superior to GA4 in promoting bud endodormancy release in tree peony (Paeonia suffruticosa) and their potential working mechanism. BMC Plant Biol. 2021, 21, 323. [Google Scholar] [CrossRef]
- Tilmes, V.; Mateos, J.L.; Madrid, E.; Vincent, C.; Severing, E.; Carrera, E.; López-Díaz, I.; Coupland, G. Gibberellins act downstream of Arabis PERPETUAL FLOWERING1 to accelerate floral induction during vernalization. Plant Physiol. 2019, 180, 1549–1563. [Google Scholar] [CrossRef] [Green Version]
- Zanewich, K.P.; Rood, S.B. Vernalization and Gibberellin Physiology of Winter Canola (Endogenous Gibberellin (GA) Content and Metabolism of [3H]GA1 and [3H]GA20. Plant Physiol. 1995, 108, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Shang, M.; Wang, X.; Zhang, J.; Qi, X.; Ping, A.; Hou, L.; Xing, G.; Li, G.; Li, M. Genetic regulation of GA metabolism during vernalization, floral bud initiation and development in Pak Choi (Brassica rapa ssp. Chinensis Makino). Front. Plant Sci. 2017, 8, 1533. [Google Scholar] [CrossRef]
- Deng, W.; Casao, M.C.; Wang, P.; Sato, K.; Hayes, P.M.; Finnegan, E.J.; Trevaskis, B. Direct links between the vernalization response and other key traits of cereal crops. Nat. Commun. 2015, 6, 5882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, S.; Vanzetti, L.S.; Dubcovsky, J. Exogenous gibberellins induce wheat spike development under short days only in the presence of VERNALIZATION1. Plant Physiol. 2013, 163, 1433–1445. [Google Scholar] [CrossRef]
- King, R.W.; Evans, L.T. Gibberellins and Flowering of Grasses and Cereals: Prizing Open the Lid of the “Florigen” Black Box. Annu. Rev. Plant Biol. 2003, 54, 307–328. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Fambuena, N.; Mesejo, C.; González-Mas, M.C.; Iglesias, D.J.; Primo-Millo, E.; Agustí, M. Gibberellic Acid Reduces Flowering Intensity in Sweet Orange [Citrus sinensis (L.) Osbeck] by Repressing CiFT Gene Expression. J. Plant Growth Regul. 2012, 31, 529–536. [Google Scholar] [CrossRef]
- Garmendia, A.; Beltrán, R.; Zornoza, C.; García-Breijo, F.J.; Reig, J.; Merle, H. Gibberellic acid in Citrus spp. Flowering and fruiting: A systematic review. PLoS ONE 2019, 14, e0223147. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Gottschalk, C.; Van Nocker, S. Genetic mechanisms in the repression of flowering by gibberellins in apple (Malus x domestica Borkh.). BMC Genom. 2019, 20, 747. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, X.; Yan, J.; Fan, L.; Rong, C.; Mo, C.; Zhang, M. Effect of exogenous spermidine on floral induction, endogenous polyamine and hormone production, and expression of related genes in ‘Fuji’ apple (Malus domestica Borkh.). Sci. Rep. 2019, 9, 12777. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Qin, L.; Lee, S.; Fu, X.; Richards, D.E.; Cao, D.; Luo, D.; Harberd, N.P.; Peng, J. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 2004, 131, 1055–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; De Storme, N.; Geelen, D. Gibberellin Induces Diploid Pollen Formation by Interfering with Meiotic Cytokinesis. Plant Physiol. 2017, 173, 338–353. [Google Scholar] [CrossRef] [Green Version]
- Gastaldi, V.; Lucero, L.E.; Ferrero, L.V.; Ariel, F.D.; Gonzalez, D.H. Class-I TCP transcription factors activate the SAUR63 gene subfamily in gibberellin-dependent stamen filament elongation. Plant Physiol. 2020, 182, 2096–2110. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Song, S.; Xiao, L.; Soo, H.M.; Cheng, Z.; Xie, D.; Peng, J. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in arabidopsis. PLoS Genet. 2009, 5, e1000440. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gong, Y.; Liu, B.; Wu, D.; Zhang, M.; Xie, D.; Song, S. The della proteins interact with MYB21 and MYB24 to regulate filament elongation in Arabidopsis. BMC Plant Biol. 2020, 20, 64. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Fang, R.; Chen, F.; Han, J.; Liu, Y.G.; Chen, L.; Zhu, Q. A novel CCCH-type zinc finger protein SAW1 activates OsGA20ox3 to regulate gibberellin homeostasis and anther development in rice. J. Integr. Plant Biol. 2020, 62, 1594–1606. [Google Scholar] [CrossRef]
- Marciniak, K.; Przedniczek, K. Anther dehiscence is regulated by gibberellic acid in yellow lupine (Lupinus luteus L.). BMC Plant Biol. 2021, 21, 314. [Google Scholar] [CrossRef]
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef] [Green Version]
- Sakata, T.; Oda, S.; Tsunaga, Y.; Shomura, H.; Kawagishi-Kobayashi, M.; Aya, K.; Saeki, K.; Endo, T.; Nagano, K.; Kojima, M.; et al. Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiol. 2014, 164, 2011–2019. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Tian, J.; Guo, C.; Luo, S.; Li, J. Interaction of gibberellin and other hormones in almond anthers: Phenotypic and physiological changes and transcriptomic reprogramming. Hortic. Res. 2021, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Hao, Y.J.; Lu, J.X.; Lu, G.; Zhang, T. Transcriptomic analysis reveals the mechanism of thermosensitive genic male sterility (TGMS) of Brassica napus under the high temperature inducement. BMC Genom. 2019, 20, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barro-Trastoy, D.; Carrera, E.; Baños, J.; Palau-Rodríguez, J.; Ruiz-Rivero, O.; Tornero, P.; Alonso, J.M.; López-Díaz, I.; Gómez, M.D.; Pérez-Amador, M.A. Regulation of ovule initiation by gibberellins and brassinosteroids in tomato and Arabidopsis: Two plant species, two molecular mechanisms. Plant J. 2020, 102, 1026–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.; Wani, S.H.; Razzaq, A.; Skalicky, M.; Samantara, K.; Gupta, S.; Pandita, D.; Goel, S.; Grewal, S.; Hejnak, V.; et al. Abscisic Acid: Role in Fruit Development and Ripening. Front. Plant Sci. 2022, 13, 817500. [Google Scholar] [CrossRef]
- Jia, H.-F.; Chai, Y.-M.; Li, C.-L.; Lu, D.; Luo, J.-J.; Qin, L.; Shen, Y.-Y. Abscisic Acid Plays an Important Role in the Regulation of Strawberry Fruit Ripening. Plant Physiol. 2011, 157, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, N.; Girin, T.; Sorefan, K.; Fuentes, S.; Wood, T.A.; Lawrenson, T.; Sablowski, R.; Østergaard, L. Gibberellins control fruit patterning in Arabidopsis thaliana. Genes Dev. 2010, 24, 2127–2132. [Google Scholar] [CrossRef] [Green Version]
- Ariizumi, T.; Shinozaki, Y.; Ezura, H. Genes that influence yield in tomato. Breed. Sci. 2013, 63, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindo-García, V.; Muñoz, P.; Larrigaudière, C.; Munné-Bosch, S.; Giné-Bordonaba, J. Interplay between hormones and assimilates during pear development and ripening and its relationship with the fruit postharvest behaviour. Plant Sci. 2020, 291, 110339. [Google Scholar] [CrossRef]
- Gu, T.; Jia, S.; Huang, X.; Wang, L.; Fu, W.; Huo, G.; Gan, L.; Ding, J.; Li, Y. Transcriptome and hormone analyses provide insights into hormonal regulation in strawberry ripening. Planta 2019, 250, 145–162. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.-J.; Tan, G.-F.; Zhou, W.-Q.; Wang, G.-L. Gibberellin and the plant growth retardant Paclobutrazol altered fruit shape and ripening in tomato. Protoplasma 2020, 257, 853–861. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Qi, Q.; Li, H.; Li, Z.; Chen, S.; Ding, Q.; Wang, Q.; Yan, Z.; Gai, Y.; et al. Gibberellins Play a Role in Regulating Tomato Fruit Ripening. Plant Cell Physiol. 2019, 60, 1619–1629. [Google Scholar] [CrossRef]
- Li, Y.H.; Wu, Y.J.; Wu, B.; Zou, M.H.; Zhang, Z.; Sun, G.M. Exogenous gibberellic acid increases the fruit weight of “Comte de Paris” pineapple by enlarging flesh cells without negative effects on fruit quality. Acta Physiol. Plant. 2011, 33, 1715–1722. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, P.; Jiang, F.; Wang, S.; Liu, Z.; Song, G.; Li, W.; Lv, T.; Li, J.; Wang, D.; et al. Exogenous gibberellin treatment improves fruit quality in self-pollinated apple. Plant Physiol. Biochem. 2022, 174, 11–21. [Google Scholar] [CrossRef]
- Keawmanee, N.; Ma, G.; Zhang, L.; Yahata, M.; Murakami, K.; Yamamoto, M.; Kojima, N.; Kato, M. Exogenous gibberellin induced regreening through the regulation of chlorophyll and carotenoid metabolism in Valencia oranges. Plant Physiol. Biochem. 2022, 173, 14–24. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, F.; Deng, Y.; Zhong, F.; Tian, P.; Lin, D.; Deng, J.; Zhang, Y.; Huang, T. Sly-miR159 regulates fruit morphology by modulating GA biosynthesis in tomato. Plant Biotechnol. J. 2022, 20, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, S.; Wang, H.; Wang, K.; Yu, H.; Zhou, Z.; Xin, P.; Chu, J.; Zhao, T.; Wang, H.; et al. FIS1 encodes a GA2-oxidase that regulates fruit firmness in tomato. Nat. Commun. 2020, 11, 5844. [Google Scholar] [CrossRef]
- Fuentes, S.; Ljung, K.; Sorefan, K.; Alvey, E.; Harberd, N.P.; Østergaard, L. Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell 2012, 24, 3982–3996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, R.C.; Nietsche, S.; Pereira, M.C.T.; Ribeiro, L.M.; Mercadante-Simões, M.O.; Carneiro dos Santos, B.H. Atemoya fruit development and cytological aspects of GA3-induced growth and parthenocarpy. Protoplasma 2019, 256, 1345–1360. [Google Scholar] [CrossRef]
- Cong, L.; Yue, R.; Wang, H.; Liu, J.; Zhai, R.; Yang, J.; Wu, M.; Si, M.; Zhang, H.; Yang, C.; et al. 2,4-D-induced parthenocarpy in pear is mediated by enhancement of GA 4 biosynthesis. Physiol. Plant. 2019, 166, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, T.; Liu, J.; Cong, L.; Zhu, Y.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. PbGA20ox2 Regulates Fruit Set and Induces Parthenocarpy by Enhancing GA4 Content. Front. Plant Sci. 2020, 11, 113. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Tuan, P.A.; Ayele, B.T. Jasmonate regulates seed dormancy in wheat via modulating the balance between gibberellin and abscisic acid. J. Exp. Bot. 2022, 73, 2434–2453. [Google Scholar] [CrossRef]
- Gong, D.; He, F.; Liu, J.; Zhang, C.; Wang, Y.; Tian, S.; Sun, C.; Zhang, X. Understanding of Hormonal Regulation in Rice Seed Germination. Life 2022, 12, 1021. [Google Scholar] [CrossRef]
- Li, Q.-F.; Zhou, Y.; Xiong, M.; Ren, X.-Y.; Han, L.; Wang, J.-D.; Zhang, C.-Q.; Fan, X.-L.; Liu, Q.-Q. Gibberellin recovers seed germination in rice with impaired brassinosteroid signalling. Plant Sci. 2020, 293, 110435. [Google Scholar] [CrossRef]
- Ribeiro, L.M.; Garcia, Q.S.; Müller, M.; Munné-Bosch, S. Tissue-specific hormonal profiling during dormancy release in macaw palm seeds. Physiol. Plant. 2015, 153, 627–642. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Kuo, S.-R.; Chien, C.-T. Roles of gibberellins and abscisic acid in dormancy and germination of red bayberry (Myrica rubra) seeds. Tree Physiol. 2008, 28, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cheng, H.; King, K.E.; Wang, W.; He, Y.; Hussain, A.; Lo, J.; Harberd, N.P.; Peng, J. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 2002, 16, 646–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombolá-Caldentey, B.; Rueda-Romero, P.; Iglesias-Fernández, R.; Carbonero, P.; Oñate-Sánchez, L. Arabidopsis DELLA and two HD-ZIP transcription factors regulate GA signaling in the epidermis through the L1 box cis-element. Plant Cell 2014, 26, 2905–2919. [Google Scholar] [CrossRef] [Green Version]
- Palmer, G.H. Relationship between levels of gibberellic acid and the production and action of carbohydrases of barley. J. Inst. Brew. 1973, 79, 513–518. [Google Scholar] [CrossRef]
- Atzorn, R.; Weiler, E.W. The role of endogenous gibberellins in the formation of ?-amylase by aleurone layers of germinating barley caryopses. Planta 1983, 159, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M. The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol. 2002, 128, 1264–1270. [Google Scholar] [CrossRef] [Green Version]
- Nakabayashi, K.; Bartsch, M.; Xiang, Y.; Miatton, E.; Pellengahr, S.; Yano, R.; Seo, M.; Soppe, W.J.J. The time required for dormancy release in arabidopsis is determined by DELAY OF GERMINATION1 protein levels in freshly harvested seeds. Plant Cell 2012, 24, 2826–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbrecher, T.; Leubner-Metzger, G. Tissue and cellular mechanics of seeds. Curr. Opin. Genet. Dev. 2018, 51, 1–10. [Google Scholar] [CrossRef]
- Graeber, K.; Linkies, A.; Steinbrecher, T.; Mummenhoff, K.; Tarkowská, D.; Turečková, V.; Ignatz, M.; Sperber, K.; Voegele, A.; De Jong, H.; et al. Delay of germination 1 mediates a conserved coat-dormancy mechanism for the temperature- and gibberellin-dependent control of seed germination. Proc. Natl. Acad. Sci. USA 2014, 111, E3571–E3580. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, U.; Zhao, X.; Luo, X.; Wei, S.; Shu, K. Endosperm weakening: The gateway to a seed’s new life. Plant Physiol. Biochem. 2022, 178, 31–39. [Google Scholar] [CrossRef]
- Shu, K.; Zhang, H.; Wang, S.; Chen, M.; Wu, Y.; Tang, S.; Liu, C.; Feng, Y.; Cao, X.; Xie, Q. ABI4 Regulates Primary Seed Dormancy by Regulating the Biogenesis of Abscisic Acid and Gibberellins in Arabidopsis. PLoS Genet. 2013, 9, e1003577. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Chen, Q.; Wu, Y.; Liu, R.; Zhang, H.; Wang, P.; Li, Y.; Wang, S.; Tang, S.; Liu, C.; et al. ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels. Plant J. 2016, 85, 348–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, G.; Liu, X.; Sun, F.; Cao, J.; Huo, N.; Wuda, B.; Xin, M.; Hu, Z.; Du, J.; Xia, R.; et al. Wheat miR9678 Affects Seed Germination by Generating Phased siRNAs and Modulating Abscisic Acid/Gibberellin Signaling. Plant Cell 2018, 30, 796–814. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, L.; Liu, Y.; Lv, Q.; Zhang, H.; Zhu, J.; Li, X. Influence of TaGW2-6A on seed development in wheat by negatively regulating gibberellin synthesis. Plant Sci. 2017, 263, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, B.; Tian, L.; Wang, S.; Zhang, J.; Guo, S.; Zhang, H.; Xu, L.; Chen, Y. Comprehensive dynamic transcriptome analysis at two seed germination stages in maize (Zea mays L.). Physiol. Plant. 2020, 168, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Park, J.; Lee, N.; Jeong, J.; Toh, S.; Watanabe, A.; Kim, J.; Kang, H.; Kim, D.H.; Kawakami, N.; et al. ABA-insensitive3, ABA-insensitive5, and DELLAs interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis. Plant Cell 2013, 25, 4863–4878. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhang, Q.; He, D.; Zhou, Y.; Ni, H.; Tian, D.; Chang, G.; Jing, Y.; Lin, R.; Huang, J.; et al. AGAMOUS-LIKE67 Cooperates with the Histone Mark Reader EBS to Modulate Seed Germination under High Temperature. Plant Physiol. 2020, 184, 529–545. [Google Scholar] [CrossRef]
- Chiu, R.S.; Nahal, H.; Provart, N.J.; Gazzarrini, S. The role of the Arabidopsis FUSCA3 transcription factor during inhibition of seed germination at high temperature. BMC Plant Biol. 2012, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Chen, Z.; Huang, Y.; Chang, G.; Li, P.; Wei, J.; Yuan, X.; Huang, J.; Hu, X. Powerdress as the novel regulator enhances Arabidopsis seeds germination tolerance to high temperature stress by histone modification of SOM locus. Plant Sci. 2019, 284, 91–98. [Google Scholar] [CrossRef]
- Penfield, S.; Josse, E.M.; Kannangara, R.; Gilday, A.D.; Halliday, K.J.; Graham, I.A. Cold and light control seed germination through the bHLH transcription factor SPATULA. Curr. Biol. 2005, 15, 1998–2006. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Yamaguchi, S.; Lim, S.; Oh, E.; Park, J.; Hanada, A.; Kamiya, Y.; Choi, G. SOMNUS, a CCCH-Type Zinc Finger Protein in Arabidopsis, Negatively Regulates Light-Dependent Seed Germination Downstream of PIL5. Plant Cell 2008, 20, 1260–1277. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, S.; Rizza, A.; Martone, J.; Circelli, P.; Costantino, P.; Vittorioso, P. The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene AtGA3ox1. Plant J. 2010, 61, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.; Santopolo, S.; Capauto, D.; Lorrai, R.; Minutello, E.; Serino, G.; Costantino, P.; Vittorioso, P. The DOF protein DAG1 and the della protein GAI cooperate in negatively regulating the AtGA3ox1 gene. Mol. Plant 2014, 7, 1486–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubler, F.; Hughes, T.; Waterhouse, P.; Jacobsen, J. Regulation of dormancy in barley by blue light and after-ripening: Effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008, 147, 886–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, H.H.; Sechet, J.; Bailly, C.; Leymarie, J.; Corbineau, F. Inhibition of germination of dormant barley (Hordeum vulgare L.) grains by blue light as related to oxygen and hormonal regulation. Plant Cell Environ. 2014, 37, 1393–1403. [Google Scholar] [CrossRef]
- Oh, E.; Yamaguchi, S.; Kamiya, Y.; Bae, G.; Chung, W.I.; Choi, G. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 2006, 47, 124–139. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Z.; Liu, S.; Lin, R. Interplay between REVEILLE1 and RGA-LIKE2 regulates seed dormancy and germination in Arabidopsis. New Phytol. 2020, 225, 1593–1605. [Google Scholar] [CrossRef]
- Liu, S.; Yang, L.; Li, J.; Tang, W.; Li, J.; Lin, R. FHY3 interacts with phytochrome B and regulates seed dormancy and germination. Plant Physiol. 2021, 187, 289–302. [Google Scholar] [CrossRef]
- Luo, D.; Qu, L.; Zhong, M.; Li, X.; Wang, H.; Miao, J.; Liu, X.; Zhao, X. Vascular plant one-zinc finger 1 (VOZ1) and VOZ2 negatively regulate phytochrome B-mediated seed germination in Arabidopsis. Biosci. Biotechnol. Biochem. 2020, 84, 1384–1393. [Google Scholar] [CrossRef]
- Stawska, M.; Oracz, K. Phyb and hy5 are involved in the blue light-mediated alleviation of dormancy of arabidopsis seeds possibly via the modulation of expression of genes related to light, ga, and aba. Int. J. Mol. Sci. 2019, 20, 5882. [Google Scholar] [CrossRef] [Green Version]
- De Souza, B.L.; Ribeiro-Oliveira, J.P.; Bravo, J.P.; Dias, G.F.; da Silva, E.A.A. What happens when the rain is back? A hypothetical model on how germination and post-germination occur in a species from transient seed banks. PLoS ONE 2020, 15, e0229215. [Google Scholar] [CrossRef] [PubMed]
- Postma, F.M.; Ågren, J. Maternal environment affects the genetic basis of seed dormancy in Arabidopsis thaliana. Mol. Ecol. 2015, 24, 785–797. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, W.; Yin, H.; Luo, X.; Chen, W.; Liu, X.; Wang, X.; Meng, Y.; Feng, L.; Qin, Y.; et al. Shading of the mother plant during seed development promotes subsequent seed germination in soybean. J. Exp. Bot. 2020, 71, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef]
- Huarte, H.R.; Puglia, G.D.; Prjibelski, A.D.; Raccuia, S.A. Seed Transcriptome Annotation Reveals Enhanced Expression of Genes Related to ROS Homeostasis and Ethylene Metabolism at Alternating Temperatures in Wild Cardoon. Plants 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ruan, J.; Chu, P.; Fu, W.; Liang, Z.; Li, Y.; Tong, J.; Xiao, L.; Liu, J.; Li, C.; et al. AtPER1 enhances primary seed dormancy and reduces seed germination by suppressing the ABA catabolism and GA biosynthesis in Arabidopsis seeds. Plant J. 2020, 101, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, L.; Huang, M.; He, X.; Yang, Y.; Liu, X.; Li, Y.; Hou, X. Gibberellins play an essential role in late embryogenesis of Arabidopsis. Nat. Plants 2018, 4, 289–298. [Google Scholar] [CrossRef]
- Kępczyńska, E.; Orłowska, A. Profiles of endogenous ABA, bioactive GAs, IAA and their metabolites in Medicago truncatula Gaertn. non-embryogenic and embryogenic tissues during induction phase in relation to somatic embryo formation. Planta 2021, 253, 67. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, N.; Wang, H.; Stromberg, A.J.; Perry, S.E. Global identification of targets of the arabidopsis MADS domain protein AGAMOUS-like15. Plant Cell 2009, 21, 2563–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plant Growth Regulators Market Size, Share & Trends Analysis Report by Product (Cytokinins, Auxins), by Region (North America, Europe, Asia Pacific, RoW), Vendor Landscape, and Segment Forecasts, 2012–2020. Available online: https://www.grandviewresearch.com/industry-analysis/plant-growth-regulators-market (accessed on 2 September 2022).
- Plant Growth Regulators Market Size, Share & COVID-19 Impact Analysis by Type (Cytokinins, Auxins, Gibberellins, Ethylene, and Others) Crop Type (Cereals, Oilseeds & Pulses, Fruits & Vegetables, Turf, Ornamentals & Others), and Regional Forecast, 2021–2. Available online: https://www.fortunebusinessinsights.com/plant-growth-regulators-market-103064 (accessed on 2 September 2022).
- IndustryArc. Cytokinins Market—Forecast (2022–2027). Furion analytics Research & Consulting LLP™, India, 2022. Available online: https://www.industryarc.com/Report/15910/cytokinins-market.html (accessed on 2 September 2022).
- IndustryArc. Auxins Market Report—Forecast (2022–2027). Furion analytics Research & Consulting LLP™, India, 2022. Available online: https://www.industryarc.com/Research/Global-Auxins-Industry-Market-Research-511818 (accessed on 2 September 2022).
- Ethylene Market Share, Size, Trends, Industry Analysis Report by Feedstock (Naphtha, Ethane, Propane, Butane); by Application (Polyethylene, Ethylene Oxide, Ethyl Benzene, Ethylene Dichloride); by End-Use; By Region, Segment Forecast, 2021–2029. Available online: https://www.polarismarketresearch.com/industry-analysis/ethylene-market (accessed on 2 September 2022).
- Global Abscisic Acid (ABA) Market—Industry Trends and Forecast to 2028. Available online: https://www.databridgemarketresearch.com/reports/global-abscisic-acid-aba-market (accessed on 2 September 2022).
- Gibberellins Market Size, Share and Analysis by Method (Methylerythritol Phosphate (MEP) Pathway, Trans-Geranylgeranyl Diphosphate (GGDP) Pathway), by Type (19-Carbon Gibberellins, 20-Carbon Gibberellins), by Distribution Channel (Online, Offline), by App. Available online: https://www.reportsanddata.com/report-detail/gibberellins-market (accessed on 13 July 2021).
- Zhang, L.; Luo, Z.; Cui, S.; Xie, L.; Yu, J.; Tang, D.; Ma, X.; Mou, Y. Residue of Paclobutrazol and Its Regulatory Effects on the Secondary Metabolites of Ophiopogon japonicas. Molecules 2019, 24, 3504. [Google Scholar] [CrossRef] [PubMed]
- Global Paclobutrazol Market—Industry Trends and Forecast to 2028. Available online: https://www.databridgemarketresearch.com/reports/global-paclobutrazol-market# (accessed on 2 September 2022).
- Soumya, P.R.; Kumar, P.; Pal, M. Paclobutrazol: A novel plant growth regulator and multi-stress ameliorant. Indian J. Plant Physiol. 2017, 22, 267–278. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Xu, Y.; Wang, Y.; Zhu, L.; Yu, X.; Kong, F.; Zhou, C.; Han, L. Systematic Analysis of Gibberellin Pathway Components in Medicago truncatula Reveals the Potential Application of Gibberellin in Biomass Improvement. Int. J. Mol. Sci. 2020, 21, 7180. [Google Scholar] [CrossRef]
- Li, W.; Liu, S.W.; Ma, J.J.; Liu, H.M.; Han, F.X.; Li, Y.; Niu, S.H. Gibberellin signaling is required for far-red light-induced shoot elongation in pinus tabuliformis seedlings1. Plant Physiol. 2020, 182, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Kim, M.H.; Bae, E.K.; Choi, Y.I.; Jeon, H.W.; Han, K.H.; Ko, J.H. Field evaluation of transgenic hybrid poplars with desirable wood properties and enhanced growth for biofuel production by bicistronic expression of PdGA20ox1 and PtrMYB3 in wood-forming tissue. Biotechnol. Biofuels 2021, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zheng, B. Molecular responses during plant grafting and its regulation by auxins, cytokinins, and gibberellins. Biomolecules 2019, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, K.T.; Liu, S.H.; Wang, I.W.; Chen, L.J. Phalaenopsis orchid miniaturization by overexpression of OsGA2ox6, a rice GA2-oxidase gene. Bot. Stud. 2020, 61, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, C.; Wang, Z.; Zhang, L.; Yao, J.; Hua, K.; Liu, X.; Shi, H.; Zhu, J.K. The grain yield modulator miR156 regulates seed dormancy through the gibberellin pathway in rice. Nat. Commun. 2019, 10, 3822. [Google Scholar] [CrossRef] [Green Version]
- Balaguera-López, H.E.; Cárdenas-Hernández, J.F.; Álvarez-Herrera, J.G. Effect of gibberellic acid (GA3) on seed germination and growth of tomato (Solanum lycopersicum L.). Acta Hortic. 2009, 821, 141–148. [Google Scholar] [CrossRef]
- Wu, K.; Wang, S.; Song, W.; Zhang, J.; Wang, Y.; Liu, Q.; Yu, J.; Ye, Y.; Li, S.; Chen, J.; et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 2020, 367, eaaz2046. [Google Scholar] [CrossRef] [PubMed]
- Groszmann, M.; Chandler, P.M.; Ross, J.J.; Swain, S.M. Manipulating Gibberellin Control Over Growth and Fertility as a Possible Target for Managing Wild Radish Weed Populations in Cropping Systems. Front. Plant Sci. 2020, 11, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.P.; Bicalho, E.M.; da Silva, F.V.; Souza, A.M.; Silva, B.M.R.; de Almeida Gonçalves, C.; Silva dos Santos, T.R.; Garcia, Q.S. Does integrative effects of glyphosate, gibberellin and hydrogen peroxide ameliorate the deleterious effects of the herbicide on sorghum seed through its germination? Chemosphere 2019, 233, 905–912. [Google Scholar] [CrossRef]
- Koryzniene, D.; Jurkoniene, S.; Žalnierius, T.; Gaveliene, V.; Jankovska-Bortkevič, E.; Bareikiene, N.; Būda, V. Heracleum sosnowskyi seed development under the effect of exogenous application of GA3. PeerJ 2019, 2019, e6906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiaens, A.; Dhooghe, E.; Pinxteren, D.; Van Labeke, M.C. Flower development and effects of a cold treatment and a supplemental gibberellic acid application on flowering of Helleborus niger and Helleborus x ericsmithii. Sci. Hortic. 2012, 136, 145–151. [Google Scholar] [CrossRef]
- Zang, Y.X.; Chun, I.J.; Zhang, L.L.; Hong, S.B.; Zheng, W.W.; Xu, K. Effect of gibberellic acid application on plant growth attributes, return bloom, and fruit quality of rabbiteye blueberry. Sci. Hortic. 2016, 200, 13–18. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Koshioka, M.; Kubota, S.; King, R.W. Effect of Gibberellin A4 and GA Biosynthesis Inhibitors on Growth and Flowering of Stock (Matthiola incana (L.) R. Br.). Engei Gakkai zasshi 1998, 67, 537–543. [Google Scholar] [CrossRef]
- Casanova, L.; Casanova, R.; Moret, A.; Agustí, M. The application of gibberellic acid increases berry size of “Emperatriz” seedless grape. Spanish J. Agric. Res. 2009, 7, 919. [Google Scholar] [CrossRef] [Green Version]
- Weaver, R.J. Effect of Gibberellic Acid on Fruit Set and Berry Enlargement in Seedless Grapes of Vitis vinifera. Nature 1958, 181, 851–852. [Google Scholar] [CrossRef]
- Dokoozlian, N.K.; Peacock, W.L. Gibberellic Acid Applied at Bloom Reduces Fruit Set and Improves Size of “Crimson Seedless” Table Grapes. HortScience 2001, 36, 706–709. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Liu, Y.; Chen, H.; Yang, B.; Ge, M.; Zhang, Z. Effects of gibberellin applications before flowering on the phenotype, ripening, and flavonoid compounds of Syrah grape berries. J. Sci. Food Agric. 2022, 102, 6100–6111. [Google Scholar] [CrossRef] [PubMed]
- Hed, B.; Centinari, M. Gibberellin Application Improved Bunch Rot Control of Vignoles Grape, but Response to Mechanical Defoliation Varied Between Training Systems. Plant Dis. 2021, 105, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.S.; Barreto, C.F.; Kirinus, M.B.M.; Schiavon, A.V.; Malgarim, M.B.; Mello-Farias, P. Effects of Gibberellic acid (GA3) on reduction of rot disease and physico-chemical quality of “Pinot Noir” grape. Aust. J. Crop Sci. 2018, 12, 1363–1369. [Google Scholar] [CrossRef]
- Weaver, R.J.; Kasimatis, A.N.; Mccunei, S.B. Studies with gibberellin on wine grapes to decrease bunch rot. Am. J. Enol. Vitic. 1962, 13, 78–82. [Google Scholar]
- Molitor, D.; Behr, M.; Hoffmann, L.; Evers, D. Research Note: Benefits and Drawbacks of Pre-bloom Applications of Gibberellic Acid (GA3) for Stem Elongation in Sauvignon blanc. S. Afr. J. Enol. Vitic. 2012, 33, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Meneses, M.; García-Rojas, M.; Muñoz-Espinoza, C.; Carrasco-Valenzuela, T.; Defilippi, B.; González-Agüero, M.; Meneses, C.; Infante, R.; Hinrichsen, P. Transcriptomic study of pedicels from GA3-treated table grape genotypes with different susceptibility to berry drop reveals responses elicited in cell wall yield, primary growth and phenylpropanoids synthesis. BMC Plant Biol. 2020, 20, 66. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.R.; Singh, R. Gibberellic acid influences the production of malformed and button berries, and fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2009, 119, 430–433. [Google Scholar] [CrossRef]
- Yim, K.-O.; Kwon, Y.W.; Bayer, D.E. Growth Responses and Allocation of Assimilates of Rice Seedlings by Paclobutrazol and Gibberellin Treatment. J. Plant Growth Regul. 1997, 16, 35–41. [Google Scholar] [CrossRef]
- Gavino, R.B.; Pi, Y.; Abon, C.C.J. Application of gibberellic acid (GA3) in dosage for three hybridrice seed production in the Philippines. J. Agric. Technol. 2008, 4, 183–192. [Google Scholar]
- Chen, Z.; Liu, Y.; Yin, Y.; Liu, Q.; Li, N.; Liu, X.; Li, X.; Guo, C.; Hao, D. Development of dwarfish and yield-effective GM maize through passivation of bioactive gibberellin. Transgenic Res. 2019, 28, 589–599. [Google Scholar] [CrossRef]
- Han, X.; Wang, D.; Song, G. Expression of a maize SOC1 gene enhances soybean yield potential through modulating plant growth and flowering. Sci. Rep. 2021, 11, 12758. [Google Scholar] [CrossRef]
- Hu, D.; Li, X.; Yang, Z.; Liu, S.; Hao, D.; Chao, M.; Zhang, J.; Yang, H.; Su, X.; Jiang, M.; et al. Downregulation of a gibberellin 3β-hydroxylase enhances photosynthesis and increases seed yield in soybean. New Phytol. 2022, 235, 502–517. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.W.; Wong, F.L.; Luk, C.Y.; Chung, C.Y.L.; Yung, W.S.; Wang, Z.; Xie, M.; Song, S.; Chung, G.; et al. Increased copy number of gibberellin 2-oxidase 8 genes reduced trailing growth and shoot length during soybean domestication. Plant J. 2021, 107, 1739–1755. [Google Scholar] [CrossRef] [PubMed]
- Mabvongwe, O.; Manenji, B.T.; Gwazane, M.; Chandiposha, M. The Effect of Paclobutrazol Application Time and Variety on Growth, Yield, and Quality of Potato (Solanum tuberosum L.). Adv. Agric. 2016, 2016, 1585463. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, R.; Moraes, J.C.; Auad, A.M.; Coelho, M.; Nascimento, A.M. Induction of resistance of corn plants to Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) by application of silicon and gibberellic acid. Bull. Entomol. Res. 2017, 107, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.C.; Hendrich, C.; Huang, Y.; et al. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Asghar, H.N.; Khan, M.Y.; Zahir, Z.A. Gibberellic acid in combination with pressmud enhances the growth of sunflower and stabilizes chromium(VI)-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 10610–10617. [Google Scholar] [CrossRef]
- Chen, L.; Long, C.; Wang, D.; Yang, J. Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere 2019, 242, 125112. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Fahad, S.; Adnan, M.; Ali, M.; Rana, M.S.; Kamran, M.; Ali, Q.; Hashem, I.A.; Bhantana, P.; Ali, M.; et al. Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ. Sci. Pollut. Res. 2020, 27, 37121–37133. [Google Scholar] [CrossRef]
- Hwang, O.J.; Back, K. Exogenous Gibberellin Treatment Enhances Melatonin Synthesis for Melatonin-Enriched Rice Production. Biomolecules 2022, 12, 198. [Google Scholar] [CrossRef]
- Takei, M.; Nin, C.; Iizuka, T.; Pawlikowski, M.; Selva, M.-A.; Chantran, Y.; Nakajima, Y.; Zheng, J.; Aizawa, T.; Ebisawa, M.; et al. Capsicum Allergy: Involvement of Cap a 7, a New Clinically Relevant Gibberellin-Regulated Protein Cross-Reactive With Cry j7, the Gibberellin-Regulated Protein From Japanese Cedar Pollen. Allergy Asthma Immunol. Res. 2022, 14, 328. [Google Scholar] [CrossRef]
- Inuo, C.; Okazaki, F.; Shiraki, R.; Tanaka, Y.; Momma, K.; Kondo, Y. Generalized allergic reaction in response to exercise due to strawberry gibberellin-regulated protein: A case report. Allergy Asthma Clin. Immunol. 2022, 18, 4–8. [Google Scholar] [CrossRef]
- Nawaz, G.; Usman, B.; Zhao, N.; Han, Y.; Li, Z.; Wang, X.; Liu, Y.; Li, R. CRISPR/Cas9 Directed Mutagenesis of OsGA20ox2 in High Yielding Basmati Rice (Oryza sativa L.) Line and Comparative Proteome Profiling of Unveiled Changes Triggered by Mutations. Int. J. Mol. Sci. 2022, 21, 6170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Chen, R.; Yang, L.; Fan, K.; Liu, Y.; Wang, G.; Ren, Z.; Liu, Y. Generation of Transgene-Free Semidwarf Maize Plants by Gene Editing of Gibberellin-Oxidase20-3 Using CRISPR/Cas9. Front. Plant Sci. 2020, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Paciorek, T.; Chiapelli, B.J.; Wang, J.Y.; Paciorek, M.; Yang, H.; Sant, A.; Val, D.L.; Boddu, J.; Liu, K.; Gu, C.; et al. Targeted suppression of gibberellin biosynthetic genes ZmGA20ox3 and ZmGA20ox5 produces a short stature maize ideotype. Plant Biotechnol. J. 2022, 20, 1140–1153. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Chebotar, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, S.P.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Rajput, V.D.; Minkina, T.M.; Ortiz, A.; Sansinenea, E. Biosynthesis and beneficial effects of microbial gibberellins on crops for sustainable agriculture. J. Appl. Microbiol. 2021, 132, 1597–1615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, J.; Liang, H.; Huang, J.; Chen, Z.; Nie, Y.; Wang, C.; Wang, Y. Manipulation of the rhizosphere microbial community through application of a new bio-organic fertilizer improves watermelon quality and health. PLoS ONE 2018, 13, e0192967. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant Development and Crop Yield: The Role of Gibberellins. Plants 2022, 11, 2650. https://doi.org/10.3390/plants11192650
Castro-Camba R, Sánchez C, Vidal N, Vielba JM. Plant Development and Crop Yield: The Role of Gibberellins. Plants. 2022; 11(19):2650. https://doi.org/10.3390/plants11192650
Chicago/Turabian StyleCastro-Camba, Ricardo, Conchi Sánchez, Nieves Vidal, and Jesús Mª Vielba. 2022. "Plant Development and Crop Yield: The Role of Gibberellins" Plants 11, no. 19: 2650. https://doi.org/10.3390/plants11192650
APA StyleCastro-Camba, R., Sánchez, C., Vidal, N., & Vielba, J. M. (2022). Plant Development and Crop Yield: The Role of Gibberellins. Plants, 11(19), 2650. https://doi.org/10.3390/plants11192650








