UV-B Irradiation Effect on Microalgae Performance in the Remediation of Effluent Derived from the Cigarette Butt Cleaning Process
Abstract
:1. Introduction
2. Results
2.1. Photosynthetic Pigments in Microalgal Strains
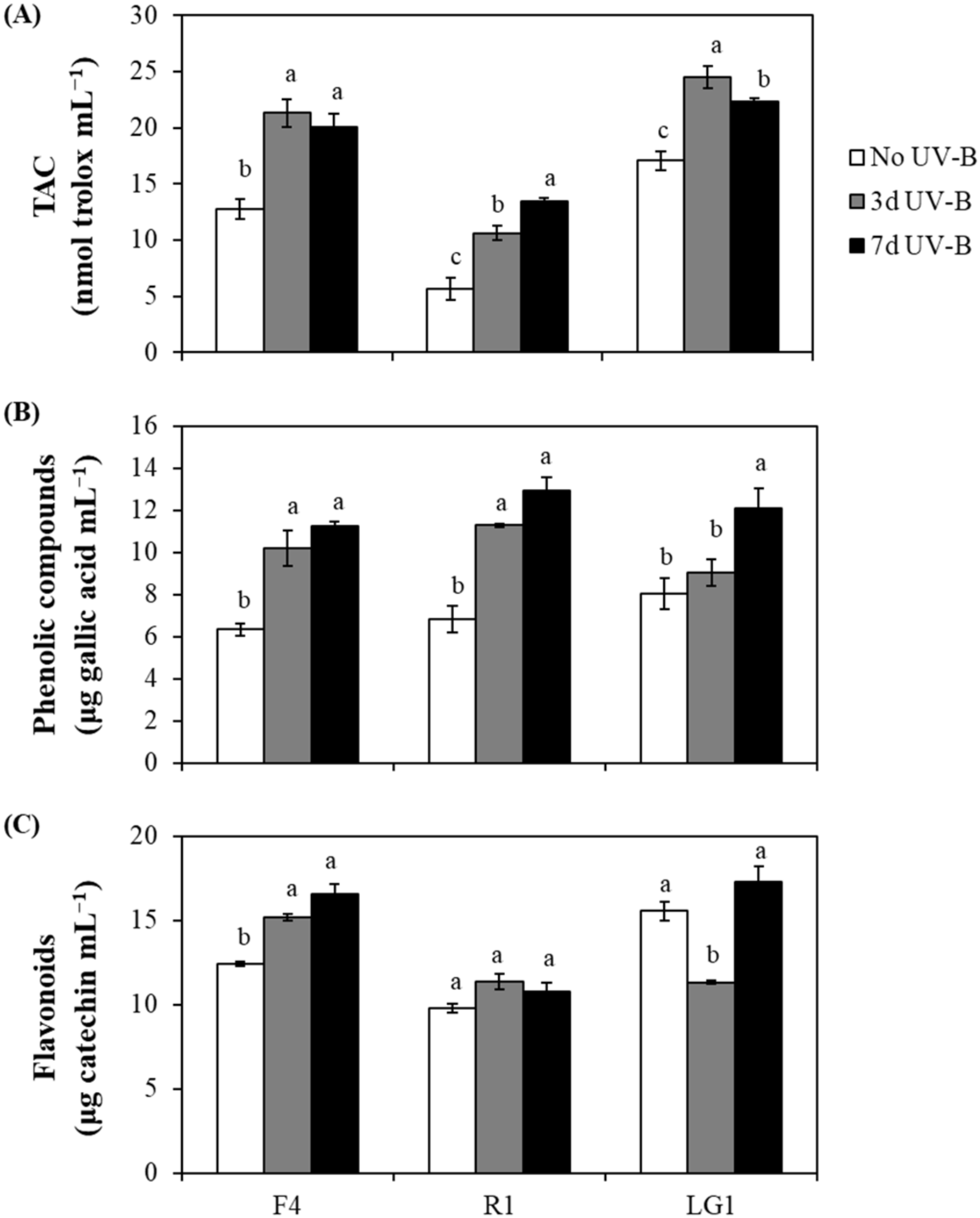
2.2. Non-Enzymatic Antioxidants in Microalgal Strains
2.3. CB Wastewater Subjected to Microalgal-Based Remediation
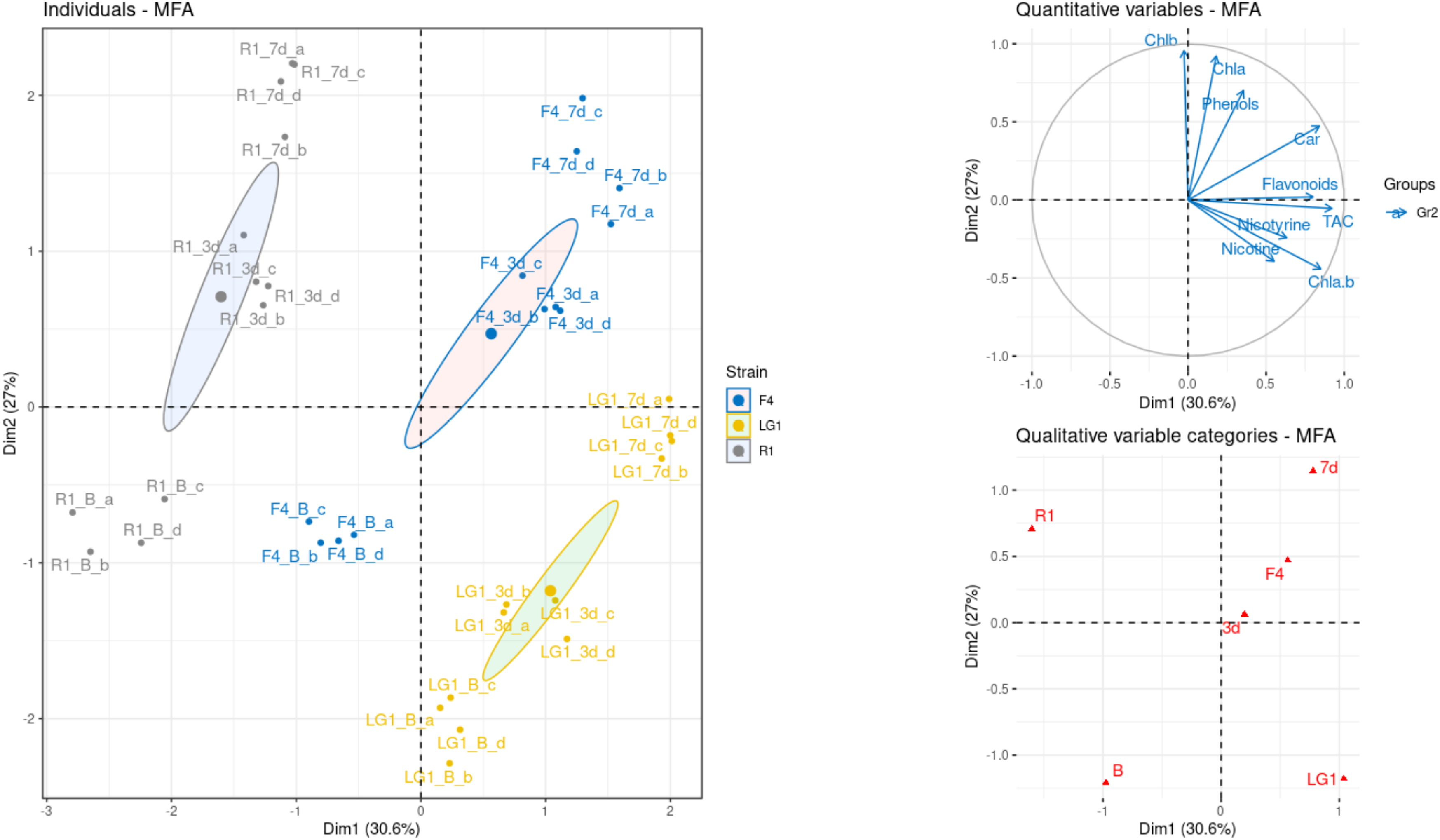
2.4. Multiple Factor Analysis
3. Discussion
4. Materials and Methods
4.1. CB Collection and Cleaning Process
4.2. Microalgal Strains and Growth Conditions
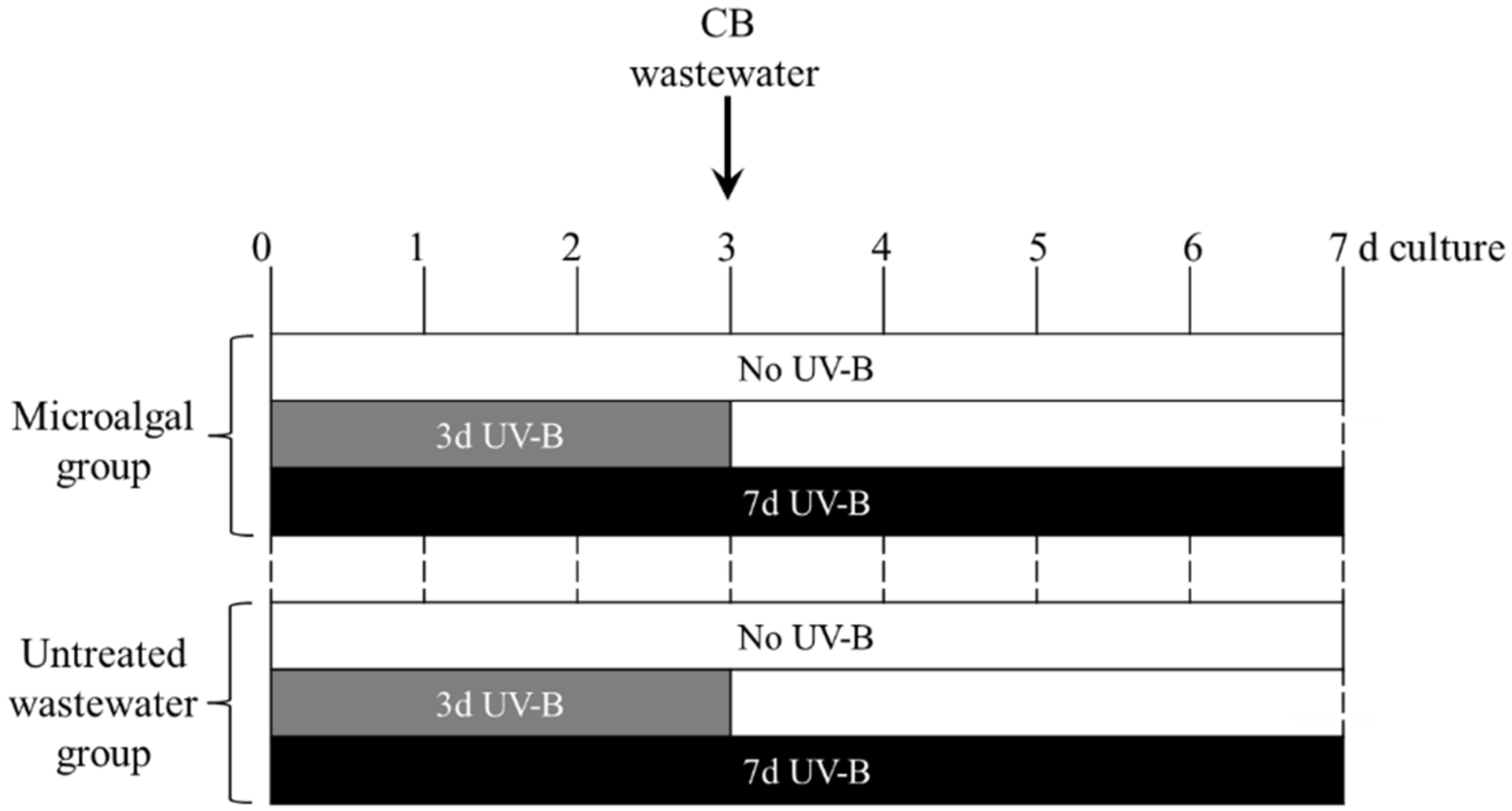
4.3. UV-B Radiation Treatment
4.4. Evaluation of Microalgal Remediation
4.5. Extraction and Determination of Photosynthetic Pigments
4.6. Extraction and Determination of Total Antioxidant Capacity, Phenolic Compounds and Flavonoids
4.7. Analytical Determinations
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurmus, H.; Mohajerani, A. The Toxicity and Valorization Options of Cigarette Butts. Waste Manag. 2020, 104, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Hoffmann, I. The Changing Cigarette, 1950–1995. J. Toxicol. Environ. Health 1997, 50, 307–364. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tobacco and Its Environmental Impact: An Overview; WHO: Geneva, Switzerland, 2017; ISBN 9789241512497. [Google Scholar]
- Oliva, M.; De Marchi, L.; Cuccaro, A.; Pretti, C. Bioassay-Based Ecotoxicological Investigation on Marine and Freshwater Impact of Cigarette Butt Littering. Environ. Pollut. 2021, 288, 117787. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Radwan, A.; Abdalla, N.; Taha, H.; Wittke, C.; El-Henawy, A.; Alshaal, T.; Amer, M.; Kleinwächter, M.; Nowak, M.; et al. Uptake of Nicotine from Discarded Cigarette Butts—A so Far Unconsidered Path of Contamination of Plant-Derived Commodities. Environ. Pollut. 2018, 238, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.J.; Gribben, P.; Parkinson, K. Impact of Cigarette Butt Leachate on Tidepool Snails. Mar. Pollut. Bull. 2015, 95, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Rebischung, F.; Chabot, L.; Biaudet, H.; Pandard, P. Cigarette Butts: A Small but Hazardous Waste, According to European Regulation. Waste Manag. 2018, 82, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Marinello, S.; Lolli, F.; Gamberini, R.; Rimini, B. A Second Life for Cigarette Butts? A Review of Recycling Solutions. J. Hazard. Mater. 2020, 384, 121245. [Google Scholar] [CrossRef]
- Mariotti, L.; Huarancca Reyes, T.; Curadi, M.; Guglielminetti, L. Recycling Cigarette Filters as Plant Growing Substrate in Soilless System. Horticulturae 2022, 8, 135. [Google Scholar] [CrossRef]
- Chiellini, C.; Mariotti, L.; Huarancca Reyes, T.; de Arruda, E.J.; Fonseca, G.G.; Guglielminetti, L. Remediation Capacity of Different Microalgae in Effluents Derived from the Cigarette Butt Cleaning Process. Plants 2022, 11, 1770. [Google Scholar] [CrossRef]
- Chiellini, C.; Guglielminetti, L.; Sarrocco, S.; Ciurli, A. Isolation of Four Microalgal Strains from the Lake Massaciuccoli: Screening of Common Pollutants Tolerance Pattern and Perspectives for Their Use in Biotechnological Applications. Front. Plant Sci. 2020, 11, 607651. [Google Scholar] [CrossRef]
- Bornman, J.F.; Barnes, P.W.; Robinson, S.A.; Ballaré, C.L.; Flint, S.D.; Caldwell, M.M. Solar Ultraviolet Radiation and Ozone Depletion-Driven Climate Change: Effects on Terrestrial Ecosystems. Photochem. Photobiol. Sci. 2015, 14, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Müller-Xing, R.; Xing, Q.; Goodrich, J. Footprints of the Sun: Memory of UV and Light Stress in Plants. Front. Plant Sci. 2014, 5, 474. [Google Scholar] [CrossRef]
- Rozema, J.; Björn, L.O.; Bornman, J.F.; Gaberščik, A.; Häder, D.P.; Trošt, T.; Germ, M.; Klisch, M.; Gröniger, A.; Sinha, R.P.; et al. The Role of UV-B Radiation in Aquatic and Terrestrial Ecosystems-An Experimental and Functional Analysis of the Evolution of UV-Absorbing Compounds. J. Photochem. Photobiol. B Biol. 2002, 66, 2–12. [Google Scholar] [CrossRef]
- Jenkins, G.I. Photomorphogenic Responses to Ultraviolet-B Light. Plant Cell Environ. 2017, 40, 2544–2557. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Esparza, E.; Crestani, G.; Limonchi, F.; Cruz, R.; Salinas, N.; Scartazza, A.; Guglielminetti, L.; Cosio, E. Physiological Responses of Maca (Lepidium meyenii Walp.) Plants to UV Radiation in Its High-Altitude Mountain Ecosystem. Sci. Rep. 2020, 10, 2654. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Yu, N.; Li, W.; Vanhaelewyn, L.; Hamshou, M.; Van Der Straeten, D.; Smagghe, G. An Ultraviolet B Condition That Affects Growth and Defense in Arabidopsis. Plant Sci. 2018, 268, 54–63. [Google Scholar] [CrossRef]
- Dahms, H.U.; Lee, J.S. UV Radiation in Marine Ectotherms: Molecular Effects and Responses. Aquat. Toxicol. 2010, 97, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Madamwar, D.; Nakamoto, H.; Incharoensakdi, A. Resilience and Self-Regulation Processes of Microalgae under UV Radiation Stress. J. Photochem. Photobiol. C Photochem. Rev. 2020, 43, 100322. [Google Scholar] [CrossRef]
- Favory, J.-J.; Stec, A.; Gruber, H.; Rizzini, L.; Oravecz, A.; Funk, M.; Albert, A.; Cloix, C.; Jenkins, G.I.; Oakeley, E.J.; et al. Interaction of COP1 and UVR8 Regulates UV-B-Induced Photomorphogenesis and Stress Acclimation in Arabidopsis. EMBO J. 2009, 28, 591–601. [Google Scholar] [CrossRef]
- Rizzini, L.; Favory, J.-J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 Protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Tilbrook, K.; Dubois, M.; Crocco, C.D.; Yin, R.; Chappuis, R.; Allorent, G.; Schmid-Siegert, E.; Goldschmidt-Clermont, M.; Ulma, R. UV-B Perception and Acclimation in Chlamydomonas reinhardtii. Plant Cell 2016, 28, 966–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, C.; Zhang, S.; Shi, C.; Cheng, H.; Liu, H.; Zhong, B. Origin and Adaptive Evolution of UV RESISTANCE LOCUS 8-Mediated Signaling during Plant Terrestrialization. Plant Physiol. 2022, 188, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic Stresses as Tools for Metabolites in Microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, C.; Serra, V.; Gammuto, L.; Ciurli, A.; Longo, V.; Gabriele, M. Evaluation of Nutraceutical Properties of Eleven Microalgal Strains Isolated from Different Freshwater Aquatic Environments: Perspectives for Their Application as Nutraceuticals. Foods 2022, 11, 654. [Google Scholar] [CrossRef]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B Exposure, ROS, and Stress: Inseparable Companions or Loosely Linked Associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Kovac, D.; Navratil, M.; Malenovsk, Z.; Stroch, M.; Spunda, V.; Urban, O. Reflectance Continuum Removal Spectral Index Tracking the Xanthophyll Cycle Photoprotective Reactions in Norway Spruce Needles. Funct. Plant Biol. 2012, 39, 987–998. [Google Scholar] [CrossRef]
- Hamed, S.M.; Zinta, G.; Klöck, G.; Asard, H.; Selim, S.; AbdElgawad, H. Zinc-induced differential oxidative stress and antioxidant responses in Chlorella sorokiniana and Scenedesmus acuminatus. Ecotoxicol. Environ. Saf. 2017, 140, 256–263. [Google Scholar] [CrossRef]
- Abbew, A.W.; Qiu, S.; Amadu, A.A.; Qasim, M.Z.; Chen, Z.; Wu, Z.; Wang, L.; Ge, S. Insights into the Multi-Targeted Effects of Free Nitrous Acid on the Microalgae Chlorella sorokiniana in Wastewater. Bioresour. Technol. 2022, 347, 126389. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Y.; Zhang, T.; An, L.; Wang, X. Effects of Enhanced Ultraviolet-B Radiation on Algae and Cyanobacteria. Crit. Rev. Microbiol. 2005, 31, 79–89. [Google Scholar] [CrossRef]
- Tian, J.; Yu, J. Changes in Ultrastructure and Responses of Antioxidant Systems of Algae (Dunaliella salina) during Acclimation to Enhanced Ultraviolet-B Radiation. J. Photochem. Photobiol. B Biol. 2009, 97, 152–160. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Zhou, B.; Hu, S.; Wang, Y. Effect of Enhanced UV-B Radiation on Photosynthetic Characteristics of Marine Microalgae Dunaliella salina (Chlorophyta, Chlorophyceae). J. Exp. Mar. Bio. Ecol. 2015, 469, 27–35. [Google Scholar] [CrossRef]
- Mátai, A.; Nagy, D.; Hideg, É. UV-B Strengthens Antioxidant Responses to Drought in Nicotiana benthamiana Leaves Not Only as Supplementary Irradiation but Also as Pre-Treatment. Plant Physiol. Biochem. 2019, 134, 9–19. [Google Scholar] [CrossRef]
- Malerba, M.E.; Palacios, M.M.; Palacios Delgado, Y.M.; Beardall, J.; Marshall, D.J. Cell Size, Photosynthesis and the Package Effect: An Artificial Selection Approach. New Phytol. 2018, 219, 449–461. [Google Scholar] [CrossRef]
- Hao, T.-B.; Balamurugan, S.; Zhang, Z.-H.; Liu, S.-F.; Wang, X.; Li, D.-W.; Yang, W.-D.; Li, H.-Y. Effective Bioremediation of Tobacco Wastewater by Microalgae at Acidic PH for Synergistic Biomass and Lipid Accumulation. J. Hazard. Mater. 2022, 426, 127820. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Czégény, G.; Mátai, A.; Hideg, É. UV-B Effects on Leaves-Oxidative Stress and Acclimation in Controlled Environments. Plant Sci. 2016, 248, 57–63. [Google Scholar] [CrossRef]
- Malanga, G.; Calmanovici, G.; Puntarulo, S. Oxidative Damage to Chloroplasts from Chlorella vulgaris Exposed to Ultraviolet-B Radiation. Physiol. Plant. 1997, 101, 455–462. [Google Scholar] [CrossRef]
- Estevez, M.S.; Malanga, G.; Puntarulo, S. UV-B Effects on Antarctic Chlorella sp Cells. J. Photochem. Photobiol. B Biol. 2001, 62, 19–25. [Google Scholar] [CrossRef]
- Al-Rashed, S.A.; Ibrahim, M.M.; El-Gaaly, G.A.; Al-Shehri, S.; Mostafa, A. Evaluation of Radical Scavenging System in Two Microalgae in Response to Interactive Stresses of UV-B Radiation and Nitrogen Starvation. Saudi J. Biol. Sci. 2016, 23, 706–712. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, J.; Zhao, X.; Qu, T.; Guan, C.; Hou, C.; Tang, X.; Wang, Y. Balancing Damage via Non-Photochemical Quenching, Phenolic Compounds and Photorespiration in Ulva prolifera Induced by Low-Dose and Short-Term UV-B Radiation. Int. J. Mol. Sci. 2022, 23, 2693. [Google Scholar] [CrossRef]
- Fernández, M.B.; Tossi, V.; Lamattina, L.; Cassia, R. A Comprehensive Phylogeny Reveals Functional Conservation of the UV-B Photoreceptor UVR8 from Green Algae to Higher Plants. Front. Plant Sci. 2016, 7, 1698. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K.; Bilger, W.; Hideg, É.; Strid, Å.; Aphalo, P.; Brelsford, C.; Klem, K.; Mátai, A.; Llorens, L.; Nezval, J.; et al. Editorial: Interactive Effects of UV-B Radiation in a Complex Environment. Plant Physiol. Biochem. 2019, 134, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of Temperature and Nitrogen Concentration on the Growth and Lipid Content of Nannochloropsis oculata and Chlorella vulgaris for Biodiesel Production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Pompeiano, A.; Ranieri, A.; Volterrani, M.; Guglielminetti, L.; Scartazza, A. Photosynthetic Performance of Five Cool-Season Turfgrasses under UV-B Exposure. Plant Physiol. Biochem. 2020, 151, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Moles, T.M.; Guglielminetti, L.; Huarancca Reyes, T. Differential Effects of Sodium Chloride on Germination and Post-Germination Stages of Two Tomato Genotypes. Sci. Hortic. 2019, 257, 108730. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Scartazza, A.; Castagna, A.; Cosio, E.G.; Ranieri, A.; Guglielminetti, L. Physiological Effects of Short Acute UVB Treatments in Chenopodium quinoa Willd. Sci. Rep. 2018, 8, 371. [Google Scholar] [CrossRef]
- Mariotti, L.; Huarancca Reyes, T.; Ramos-Diaz, J.M.; Jouppila, K.; Guglielminetti, L. Hormonal Regulation in Different Varieties of Chenopodium quinoa Willd. Exposed to Short Acute UV-B Irradiation. Plants 2021, 10, 858. [Google Scholar] [CrossRef]
- Pompeiano, A.; Huarancca Reyes, T.; Moles, T.M.; Guglielminetti, L.; Scartazza, A. Photosynthetic and Growth Responses of Arundo donax L. Plantlets under Different Oxygen Deficiency Stresses and Reoxygenation. Front. Plant Sci. 2019, 10, 408. [Google Scholar] [CrossRef] [Green Version]
- Team R Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. 2013. Available online: https://www.r-project.org/ (accessed on 20 April 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huarancca Reyes, T.; Mariotti, L.; Chiellini, C.; Guglielminetti, L.; Fonseca, G.G. UV-B Irradiation Effect on Microalgae Performance in the Remediation of Effluent Derived from the Cigarette Butt Cleaning Process. Plants 2022, 11, 2356. https://doi.org/10.3390/plants11182356
Huarancca Reyes T, Mariotti L, Chiellini C, Guglielminetti L, Fonseca GG. UV-B Irradiation Effect on Microalgae Performance in the Remediation of Effluent Derived from the Cigarette Butt Cleaning Process. Plants. 2022; 11(18):2356. https://doi.org/10.3390/plants11182356
Chicago/Turabian StyleHuarancca Reyes, Thais, Lorenzo Mariotti, Carolina Chiellini, Lorenzo Guglielminetti, and Gustavo Graciano Fonseca. 2022. "UV-B Irradiation Effect on Microalgae Performance in the Remediation of Effluent Derived from the Cigarette Butt Cleaning Process" Plants 11, no. 18: 2356. https://doi.org/10.3390/plants11182356
APA StyleHuarancca Reyes, T., Mariotti, L., Chiellini, C., Guglielminetti, L., & Fonseca, G. G. (2022). UV-B Irradiation Effect on Microalgae Performance in the Remediation of Effluent Derived from the Cigarette Butt Cleaning Process. Plants, 11(18), 2356. https://doi.org/10.3390/plants11182356






