Biological Activity of Bark Extracts from Northern Red Oak (Quercus rubra L.): An Antioxidant, Antimicrobial and Enzymatic Inhibitory Evaluation
Abstract
:1. Introduction
2. Results
2.1. Polyphenol and Tannin Contents
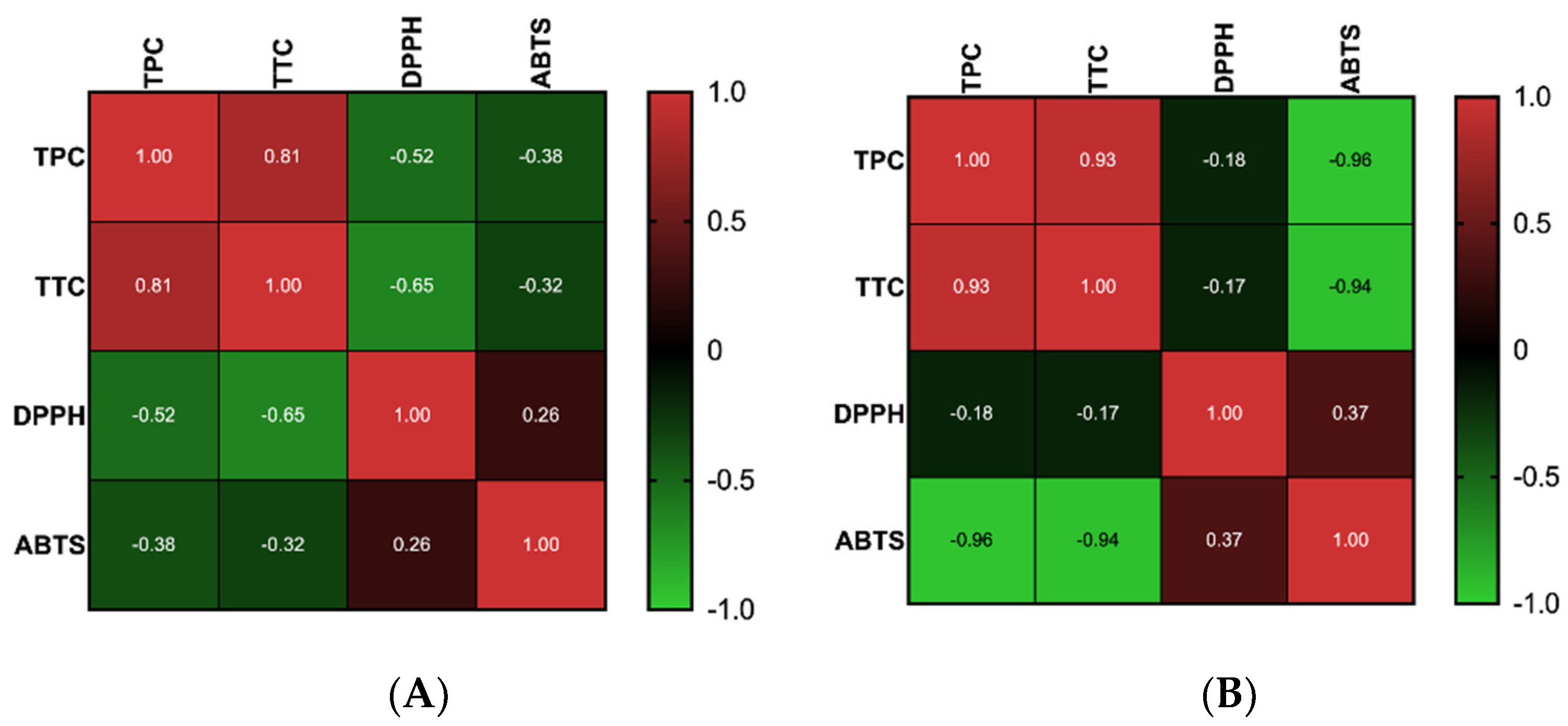
2.2. Antioxidant Activity
2.3. Antimicrobial Activity
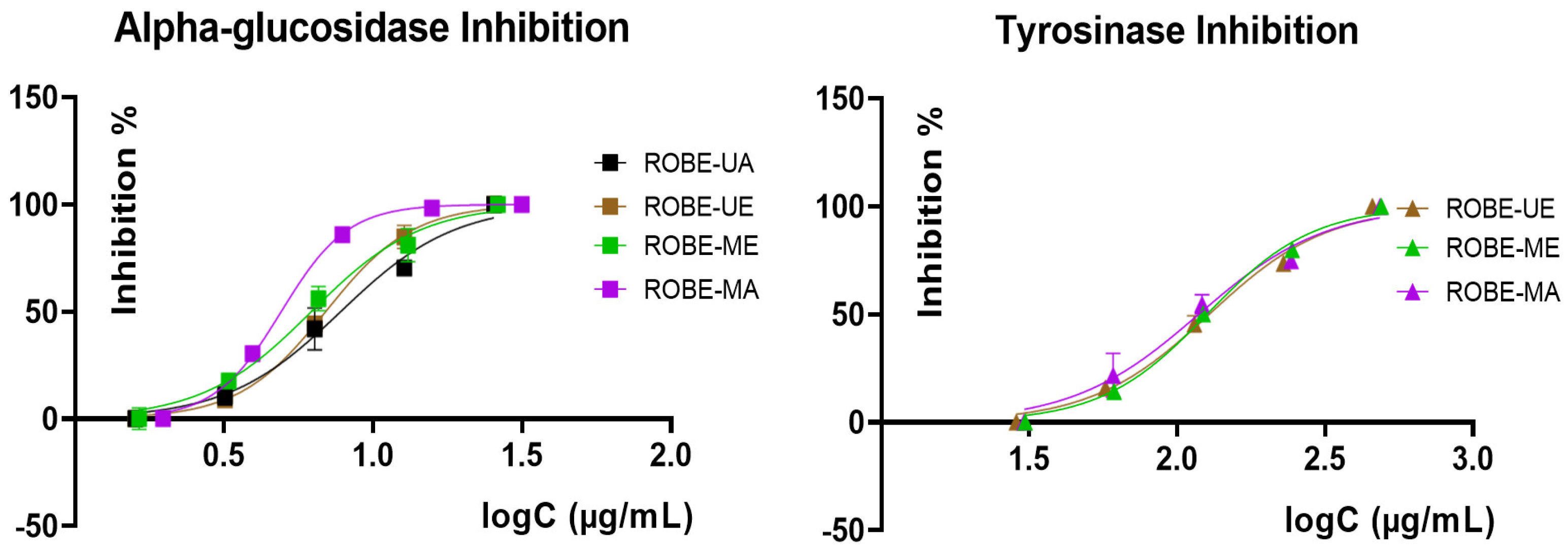
2.4. Enzyme Inhibitory Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Bacterial Strains
4.2. Plant Sample
4.3. Extraction
4.4. Quantification of Total Phenolics
4.5. Quantification of Tannins
4.6. Antioxidant In Vitro Assays
4.6.1. Determination of DPPH Radical Scavenging Activity
4.6.2. Determination of ABTS Free Radical-Scavenging Activity
4.7. Assay of the Antimicrobial Activity
4.7.1. Microdilution Method
4.7.2. The Checkerboard Method–Gentamicin Synergy Test
4.7.3. The Effect on the Formation of Bacterial Biofilm
4.8. Enzyme Inhibitory Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolescu, V.-N.; Vor, T.; Mason, W.L.; Bastien, J.-C.; Brus, R.; Henin, J.-M.; Kupka, I.; Lavnyy, V.; La Porta, N.; Mohren, F.; et al. Ecology and management of northern red oak (Quercus rubra L. syn. Q. borealis F. Michx.) in Europe: A review. For. Int. J. For. Res. 2020, 93, 481–494. [Google Scholar] [CrossRef]
- Sofletea, N.; Curtu, A.L. Dendrology; Transilvania University: Brasov, Romania, 2007; pp. 15–35. [Google Scholar]
- Brust, R. Dendrology for Foresters; Univerza v Ljubljani, Biotehniška Fakulteta: Ljubljana, Slovenia, 2011; pp. 24–88. [Google Scholar]
- Woziwoda, B.; Potocki, M.; Sagan, J.; Zasada, M.; Tomusiak, R.; Wilczyński, S. Commercial forestry as a vector of alien tree species-the case of Quercus rubra L. introduction in Poland. Balt. For. 2014, 20, 131–141. [Google Scholar]
- Woziwoda, B.; Kałucka, I.; Ruszkiewicz-Michalska, M.; Sławska, M.; Sławski, M.; Tołoczko, W.; Hachułka, M.; Kopeć, D.; Rosadziński, S.; Witkowski, J. Interdisciplinary research of ecological effects of Northern Red oak Quercus rubra L. introduction in forest ecosystems (central Poland)–the principles and aims of study. SiM CEPL 2012, 33, 181–192. [Google Scholar]
- Stanek, M.; Piechnik, Ł.; Stefanowicz, A.M. Invasive red oak (Quercus rubra L.) modifies soil physicochemical properties and forest understory vegetation. For. Ecol. Manag. 2020, 472, 118253. [Google Scholar] [CrossRef]
- Morales, D.; Miguel, M.; Garcés-Rimón, M. Pseudocereals: A novel source of biologically active peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 1537–1544. [Google Scholar] [CrossRef]
- Morales, D. Oak trees (Quercus spp.) as a source of extracts with biological activities: A narrative review. Trends Food Sci. Technol. 2021, 109, 116–125. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Lee, S.; Kang, S.-S.; Shin, H.-S. Selected commercial plants: A review of extraction and isolation of bioactive compounds and their pharmacological market value. Trends Food Sci. Technol. 2018, 82, 89–109. [Google Scholar] [CrossRef]
- Costa, R.; Lourenço, A.; Oliveira, V.; Pereira, H. Chemical characterization of cork, phloem and wood from different Quercus suber provenances and trees. Heliyon 2019, 5, e02910. [Google Scholar] [CrossRef]
- Skrypnik, L.; Grigorev, N.; Michailov, D.; Antipina, M.; Danilova, M.; Pungin, A. Comparative study on radical scavenging activity and phenolic compounds content in water bark extracts of alder (Alnus glutinosa (L.) Gaertn.), oak (Quercus robur L.) and pine (Pinus sylvestris L.). Eur. J. Wood Wood Prod. 2019, 77, 879–890. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Li, C.; Wang, X.; He, X. Triterpenes isolated from acorns of Quercus serrata var. brevipetiolata exert anti-inflammatory activity. Ind. Crops Prod. 2016, 91, 302–309. [Google Scholar] [CrossRef]
- Mai, Y.; Wang, Z.; Wang, Y.; Xu, J.; He, X. Anti-neuroinflammatory triterpenoids from the seeds of Quercus serrata Thunb. Fitoterapia 2020, 142, 104523. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Fernandes, I.; Cruz, L.; Mateus, N.; Cabral, M.; de Freitas, V. Antioxidant and Biological Properties of Bioactive Phenolic Compounds from Quercus suber L. J. Agric. Food Chem. 2009, 57, 11154–11160. [Google Scholar] [CrossRef] [PubMed]
- Quercus Cortex Monograph-European Pharmacopoeia 10.8. Available online: https://pheur.edqm.eu/app/10-8/content/10-8/1887E.htm?highlight=on&terms=quercus&terms=quercus (accessed on 20 July 2022).
- Final Community Herbal Monograph on Quercus robur L., Quercus petraea. (Matt.) Liebl., Quercus pubescens Willd., Cortex. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-quercus-robur-l-quercus-petraea-matt-liebl-quercus-pubescens-willd_en.pdf (accessed on 20 July 2022).
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Moldovan, C.; Mocan, A.; Mare, A.D.; Ciurea, C.N.; Man, A.; Muntean, D.-L.; Tanase, C. Optimization of Microwave Assisted Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant and Anti-Microbial Activity in Quercus cerris Bark Extracts. Plants 2022, 11, 240. [Google Scholar] [CrossRef]
- Tian, H.; Zhai, W.; Sun, K.; Zhu, Y.; Zhou, H.; Wan, P. Chemical composition and potential bioactivities of essential oil from Quercus mongolica bark. Arab. J. Chem. 2022, 15, 104076. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Gâvan, A.; Iacoviță, C.; Pinela, J.; Barros, L.; Ferreira, I.C.F.R.; Zhang, L.; Lucini, L.; Rocchetti, G.; et al. Optimized ultrasound-assisted extraction of phenolic compounds from Thymus comosus Heuff. ex Griseb. et Schenk (wild thyme) and their bioactive potential. Ultrason. Sonochem. 2022, 84, 105954. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Pinto, P.C.R.O.; Silvestre, A.J.D.; Neto, C.P. Chemical composition and antioxidant activity of phenolic extracts of cork from Quercus suber L. Ind. Crops Prod. 2010, 31, 521–526. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and Antiviral Activity of Hydrolysable Tannins. Mini-Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Sari, S.; Barut, B.; Özel, A.; Kuruüzüm-Uz, A.; Şöhretoğlu, D. Tyrosinase and α-glucosidase inhibitory potential of compounds isolated from Quercus coccifera bark: In vitro and in silico perspectives. Bioorg. Chem. 2019, 86, 296–304. [Google Scholar] [CrossRef]
- Semwal, P.; Painuli, S.; Badoni, H.; Bacheti, R.K. Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin. Phytosci. 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Valencia-Avilés, E.; Martínez-Flores, H.-E.; García-Pérez, M.; Meléndez-Herrera, E.; García-Pérez, M.-E. Investigation of the Antibacterial Activity and Subacute Toxicity of a Quercus crassifolia Polyphenolic Bark Extract for its Potential Use in Functional Foods. J. Food Sci. 2019, 84, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- Andrenšek, S.; Simonovska, B.; Vovk, I.; Fyhrquist, P.; Vuorela, H.; Vuorela, P. Antimicrobial and antioxidative enrichment of oak (Quercus robur) bark by rotation planar extraction using ExtraChrom®. Int. J. Food Microbiol. 2004, 92, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Cristo, J.S.; Matias, E.F.F.; Figueredo, F.G.; Santos, J.F.S.; Pereira, N.L.F.; Junior, J.G.A.S.; Aquino, P.E.A.; Nogueira, M.N.F.; Ribeiro-Filho, J.; Cunha, F.A.B.; et al. HPLC profile and antibiotic-modifying activity of Azadirachta indica A. Juss, (Meliaceae). Ind. Crops Prod. 2016, 94, 903–908. [Google Scholar] [CrossRef]
- Sahra, O.D.S.E. Synergistic Activity of Antibiotics and Bioactive Plant Extracts: A Study Against Gram-Positive and Gram-Negative Bacteria. In Bacterial Pathogenesis and Antibacterial Control; IntechOpen: Rijeka, Croatia, 2017; Chapter 2; ISBN 978-1-78923-161-8. [Google Scholar]
- Dias, M.I.; Barros, L.; Morales, P.; Cámara, M.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Wild Fragaria vesca L. fruits: A rich source of bioactive phytochemicals. Food Funct. 2016, 7, 4523–4532. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Mocan, A.; Fernandes, Â.; Calhelha, R.C.; Gavrilaş, L.; Ferreira, I.C.F.R.; Ivanov, M.; Sokovic, M.; Barros, L.; Babotă, M. Bioactive Compounds and Functional Properties of Herbal Preparations of Cystus creticus L. Collected From Rhodes Island. Front. Nutr. 2022, 9, 881210. [Google Scholar] [CrossRef]
- Chusri, S.; Phatthalung, P.N.; Voravuthikunchai, S.P. Anti-biofilm activity of Quercus infectoria G. Olivier against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2012, 54, 511–517. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. UHPLC-QToF-MS characterization of bioactive metabolites from Quercus robur L. grown in South Africa for antioxidant and antidiabetic properties. Arab. J. Chem. 2021, 14, 102970. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. The polyphenolic profile of Oak (Quercus) species: A phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Tachibana, S.; Dewi, R.T.; Itoh, K. Antioxidant and α-glucosidase inhibitor activities of natural compounds isolated from Quercus gilva Blume leaves. Asian Pac. J. Trop. Biomed. 2015, 5, 748–755. [Google Scholar] [CrossRef]
- Custódio, L.; Patarra, J.; Alberício, F.; da Rosa Neng, N.; Nogueira, J.M.F.; Romano, A. Phenolic composition, antioxidant potential and in vitro inhibitory activity of leaves and acorns of Quercus suber on key enzymes relevant for hyperglycemia and Alzheimer’s disease. Ind. Crops Prod. 2015, 64, 45–51. [Google Scholar] [CrossRef]
- Yin, P.; Yang, L.; Xue, Q.; Yu, M.; Yao, F.; Sun, L.; Liu, Y. Identification and inhibitory activities of ellagic acid- and kaempferol-derivatives from Mongolian oak cups against α-glucosidase, α-amylase and protein glycation linked to type II diabetes and its complications and their influence on HepG2 cells’ viabil. Arab. J. Chem. 2018, 11, 1247–1259. [Google Scholar] [CrossRef]
- Barrett, A.H.; Farhadi, N.F.; Smith, T.J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins— A review of efficacy and mechanisms. LWT 2018, 87, 394–399. [Google Scholar] [CrossRef]
- Di Stefano, E.; Oliviero, T.; Udenigwe, C.C. Functional significance and structure–activity relationship of food-derived α-glucosidase inhibitors. Curr. Opin. Food Sci. 2018, 20, 7–12. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Jamshidzadeh, A.; Shokri, Y.; Ahmadi, N.; Mohamadi, N.; Sharififar, F. Quercus infectoria and Terminalia chebula decrease melanin content and tyrosinase activity in B16/F10 cell lines. J. Pharm. Pharmacogn. Res. 2017, 5, 270–277. [Google Scholar]
- Tanase, C.; Domokos, E.; Coşarcă, S.; Miklos, A.; Imre, S.; Domokos, J.; Dehelean, C.A. Study of the ultrasound-assisted extraction of polyphenols from beech (Fagus sylvatica L.) bark. BioResources 2018, 13, 2247–2267. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Tannins in Herbal Drugs (Ph.Eur 10.8). Available online: https://pheur.edqm.eu/app/10-8/content/default/20814E.htm (accessed on 20 July 2022).
- Laczkó-Zöld, E.; Komlósi, A.; Ülkei, T.; Fogarasi, E.; Croitoru, M.; Fülöp, I.; Domokos, E.; Ştefănescu, R.; Varga, E. Extractability of polyphenols from black currant, red currant and gooseberry and their antioxidant activity. Acta Biol. Hung. 2018, 69, 156–169. [Google Scholar] [CrossRef]
- Tănase, C.; Coşarcă, S.; Toma, F.; Mare, A.; Man, A.; Miklos, A.; Imre, S.; Boz, I. Antibacterial activities of beech bark (Fagus sylvatica L.) polyphenolic extract. Environ. Eng. Manag. J. 2018, 17, 877–884. [Google Scholar] [CrossRef]
- Mocan, A.; Babotă, M.; Pop, A.; Fizeșan, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical constituents and biologic activities of sage species: A comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex Griseb. & Schenk) Schur. Antioxidants 2020, 9, 480. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Zhang, L.; Bunea, C.I.; Gavrilaș, L.; Vodnar, D.C.; Mocan, A.; Crișan, G.; Rocchetti, G. Optimized Ultrasound-Assisted Enzymatic Extraction of Phenolic Compounds from Rosa canina L. Pseudo-Fruits (Rosehip) and Their Biological Activity. Antioxidants 2022, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Păltinean, R.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Angenot, L.; Tits, M.; Mocan, A.; Babotă, M.; Frumuzachi, O.; et al. Biological Activities of Some Isoquinoline Alkaloids from Fumaria schleicheri Soy. Will. Plants 2022, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
| Sample | TPC (mg GAE/g dw) | TTC (%) | IC50 DPPH (µg/mL) | IC50 ABTS (µg/mL) |
|---|---|---|---|---|
| ROBE-UA | 203.58 ± 3.25 d | 37.96 ± 1.01 b | 3.54 ± 0.48 c,d | 7.41 ± 0.09 b |
| ROBE-UE | 226.79 ± 1.54 b | 44.75 ± 2.63 a | 3.32 ± 0.13 e | 6.07 ± 0.22 c |
| ROBE-MA | 216.47 ± 1.19 c | 24.67 ± 1.53 c | 6.91 ± 0.39 a | 8.28 ± 0.13 a |
| ROBE-ME | 321.08 ± 3.23 a | 37.82 ± 7.57 b | 6.24 ± 0.66 b | 7.89 ± 0.75 b |
| ROBE-UA | ROBE-UE | ROBE-MA | ROBE-ME | |||||
|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | ||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. aureus | 0.3 | 1.25 | 0.3 | 1.25 | 0.6 | 5 | 0.3 | 0.3 |
| MRSA | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 0.6 | 0.6 |
| Gram-negative bacteria | ||||||||
| E. coli | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| K. pneumoniae | 0.6 | 2.5 | 0.6 | 5 | 0.6 | 0.6 | 1.25 | 5 |
| P. aeruginosa | 2.5 | 5 | 2.5 | 5 | >5 | >5 | 2.5 | 5 |
| C. albicans | C. parapsilosis | C. krusei | |
|---|---|---|---|
| ROBE-UA | >5 | 5 | 5 |
| ROBE-UE | >5 | >5 | 5 |
| ROBE-MA | >5 | 0.3 | 0.02 |
| ROBE-ME | >5 | 5 | 2.5 |
| MRSA | K. pneumoniae | ||||
|---|---|---|---|---|---|
| Sample | Concentration (%) | BII (%) | SD | BII (%) | SD |
| ROBE-UA | 3 | −40.40 | 0.00 | 21.00 | 0.00 |
| 1.5 | −41.60 | 0.00 | 14.40 | 0.00 | |
| 0.75 | −41.60 | 0.00 | 12.60 | 0.00 | |
| ROBE-UE | 3 | −40.40 | 0.00 | 10.80 | 0.01 |
| 1.5 | −41.30 | 0.00 | 15.60 | 0.00 | |
| 0.75 | −44.50 | 0.00 | 10.80 | 0.00 | |
| ROBE-MA | 3 | 11.53 | 0.60 | 19.72 | 0.06 |
| 1.5 | 25.73 | 0.01 | 35.78 | 0.01 | |
| 0.75 | 3.49 | 0.05 | 77.53 | 0.09 | |
| ROBE-ME | 3 | −51.00 | 0.00 | 3.60 | 0.00 |
| 1.5 | −49.90 | 0.00 | 0.60 | 0.00 | |
| 0.75 | −43.10 | 0.01 | 1.20 | 0.00 | |
| α-Glucosidase | Tyrosinase | Acetylcholinesterase | |
|---|---|---|---|
| ROBE-UA | 7.79 | ND | 2978.00 |
| ROBE-UE | 6.94 | 128.48 | 1162.00 |
| ROBE-MA | 4.92 | 118.42 | 1348.00 |
| ROBE-ME | 6.21 | 126.28 | 863.20 |
| Positive control | Acarbose: 122.27 | Kojic acid: 4.44 | Galantamine: 1.85 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, C.; Nicolescu, A.; Nisca, A.; Ștefănescu, R.; Babotă, M.; Mare, A.D.; Ciurea, C.N.; Man, A. Biological Activity of Bark Extracts from Northern Red Oak (Quercus rubra L.): An Antioxidant, Antimicrobial and Enzymatic Inhibitory Evaluation. Plants 2022, 11, 2357. https://doi.org/10.3390/plants11182357
Tanase C, Nicolescu A, Nisca A, Ștefănescu R, Babotă M, Mare AD, Ciurea CN, Man A. Biological Activity of Bark Extracts from Northern Red Oak (Quercus rubra L.): An Antioxidant, Antimicrobial and Enzymatic Inhibitory Evaluation. Plants. 2022; 11(18):2357. https://doi.org/10.3390/plants11182357
Chicago/Turabian StyleTanase, Corneliu, Alexandru Nicolescu, Adrian Nisca, Ruxandra Ștefănescu, Mihai Babotă, Anca Delia Mare, Cristina Nicoleta Ciurea, and Adrian Man. 2022. "Biological Activity of Bark Extracts from Northern Red Oak (Quercus rubra L.): An Antioxidant, Antimicrobial and Enzymatic Inhibitory Evaluation" Plants 11, no. 18: 2357. https://doi.org/10.3390/plants11182357
APA StyleTanase, C., Nicolescu, A., Nisca, A., Ștefănescu, R., Babotă, M., Mare, A. D., Ciurea, C. N., & Man, A. (2022). Biological Activity of Bark Extracts from Northern Red Oak (Quercus rubra L.): An Antioxidant, Antimicrobial and Enzymatic Inhibitory Evaluation. Plants, 11(18), 2357. https://doi.org/10.3390/plants11182357












