Single and Associated Effects of Drought and Heat Stresses on Physiological, Biochemical and Antioxidant Machinery of Four Eggplant Cultivars
Abstract
:1. Introduction
2. Results
2.1. Growth and Yield Evaluation
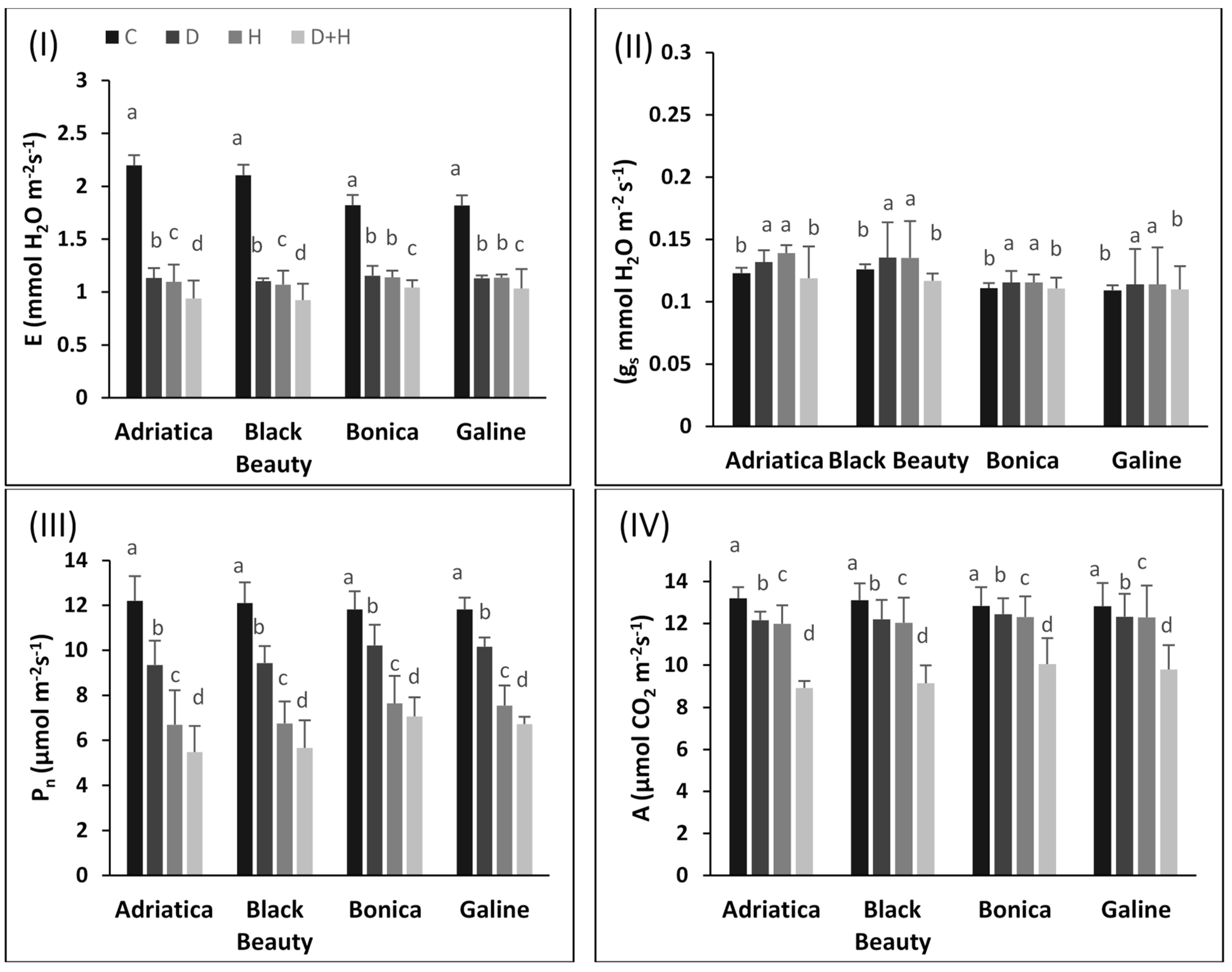
2.2. Individual and Combined Drought and Heat Stress-Induced Changes in Pigments, Chlorophyll Fluorescence, and Gas Exchange Parameters
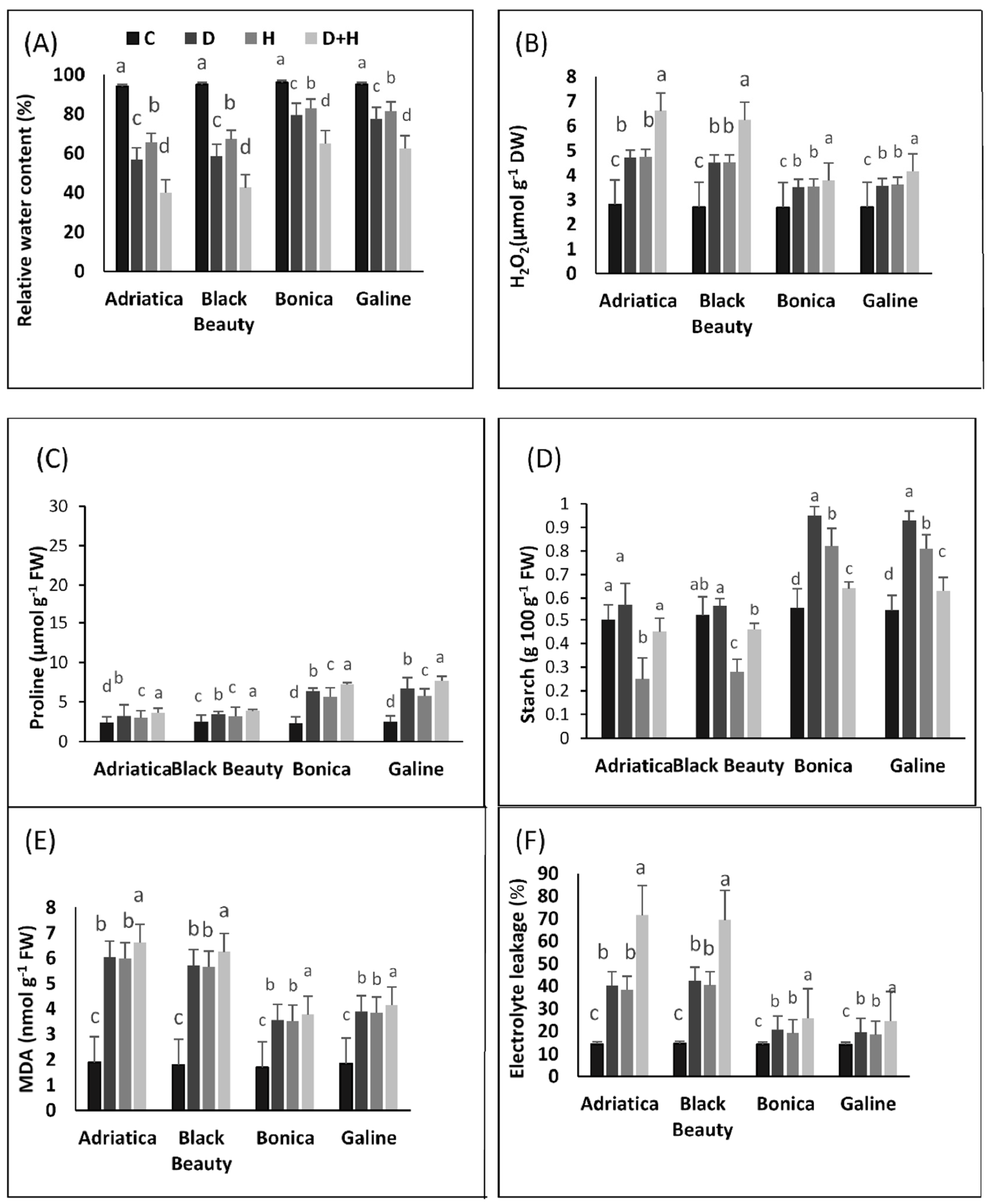
2.3. Drought-, Heat-, and Combined Drought- and Heat-Induced Changes in Relative Water Content, H2O2 Content, Malonaldehyde, Electrolyte Leakage, Starch, and Proline Concentration
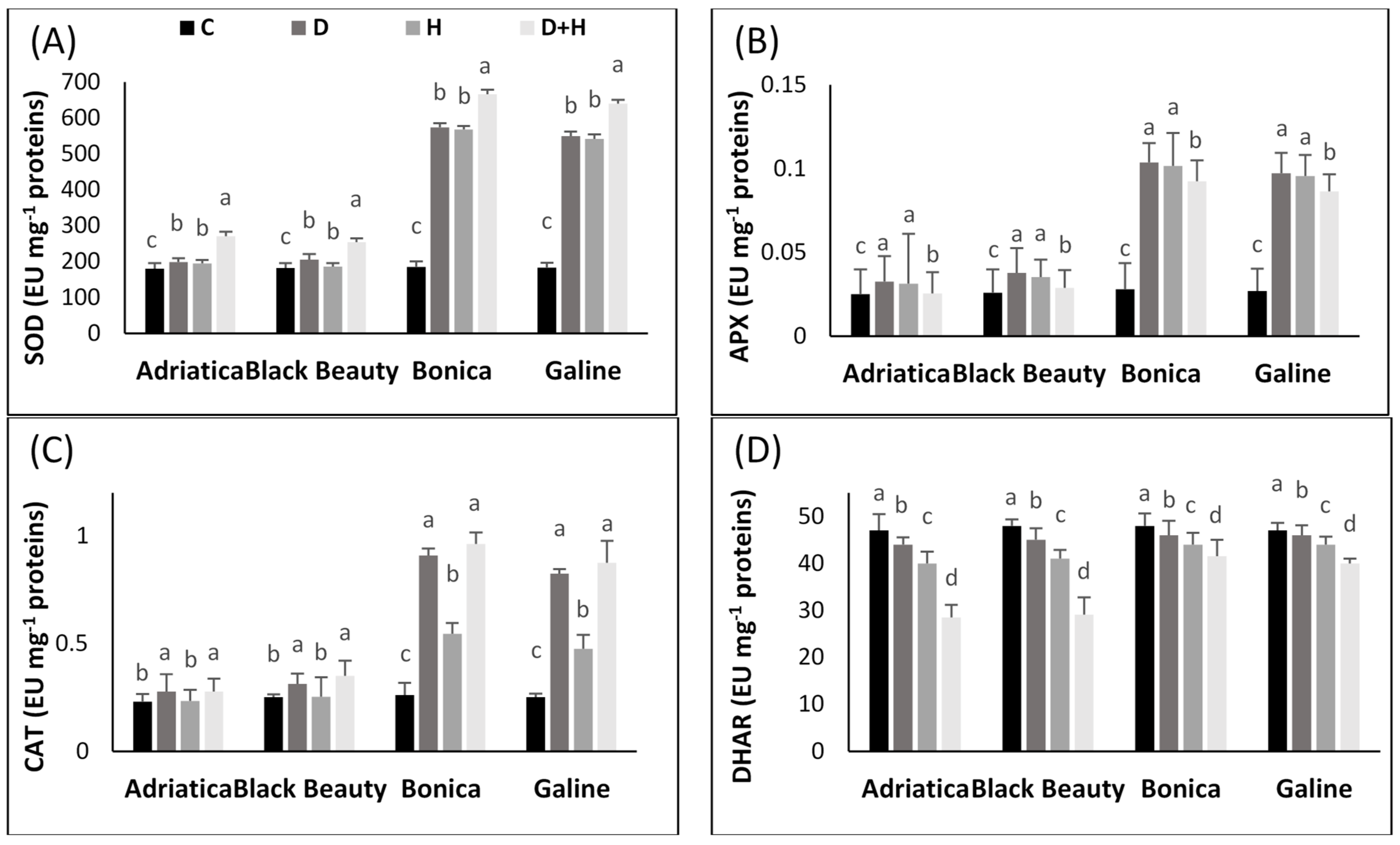
2.4. Drought-, Heat-, and Combined Drought and Heat-Induced Activity Changes in Antioxidant Enzyme Pathway
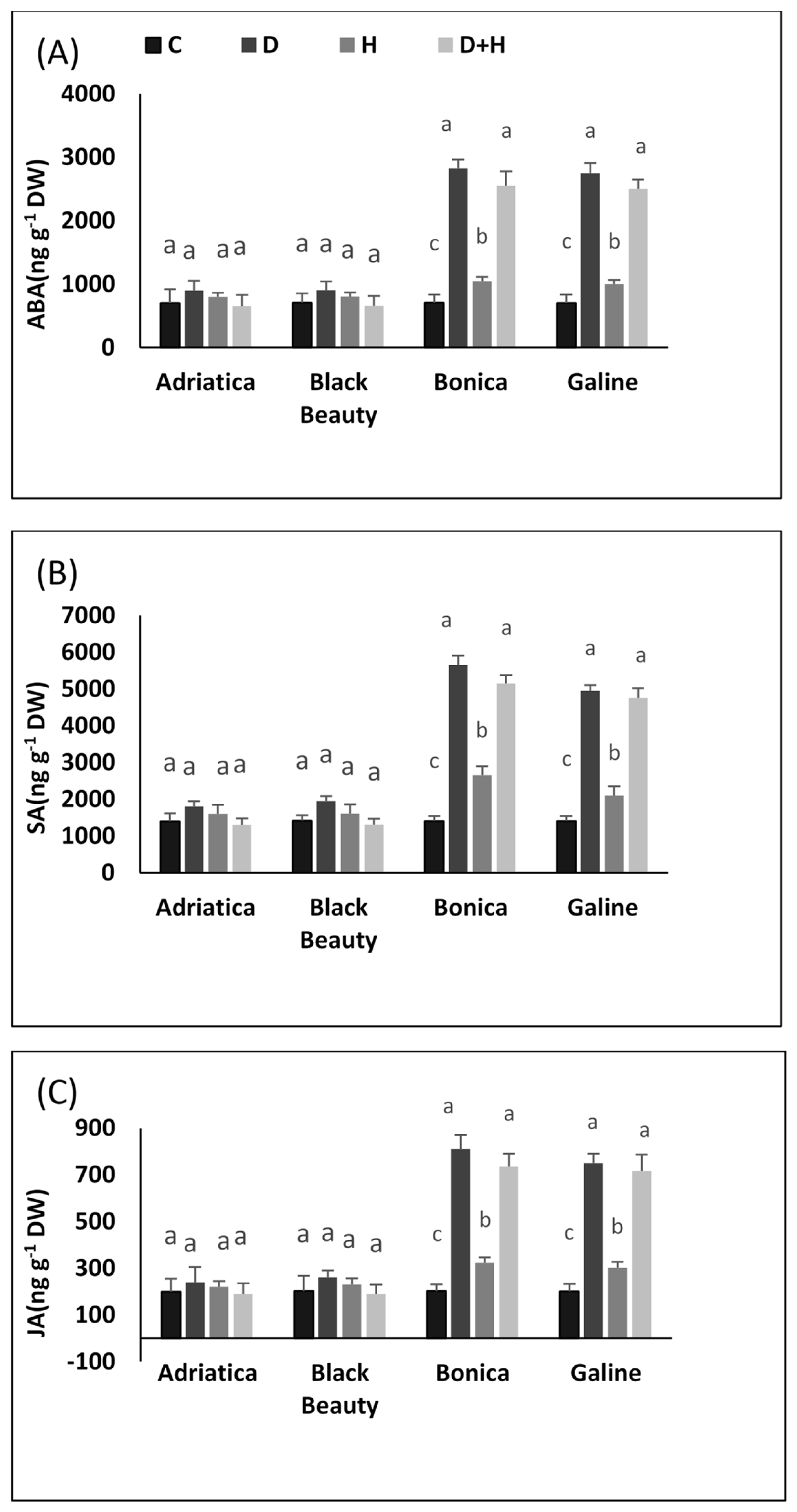
2.5. Drought-, Heat-, and Combined Drought and Heat-Induced Changes in Hormones: Abscisic Acid (ABA), Salicylic Acid (SA), and Jasmonic Acid (JA)
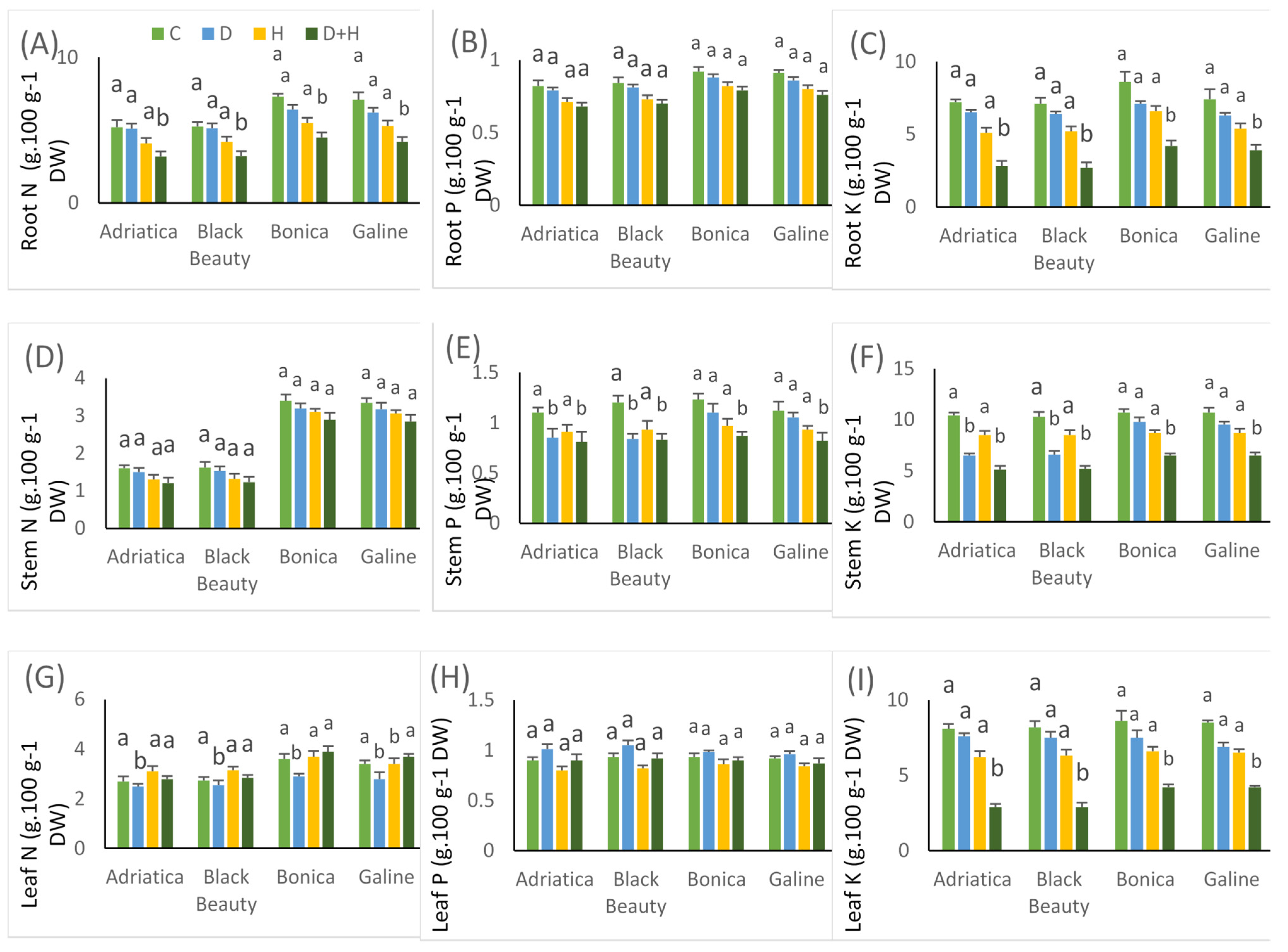
2.6. Drought-, Heat-, and Combined Drought and Heat-Induced Changes in Nutrient Uptake
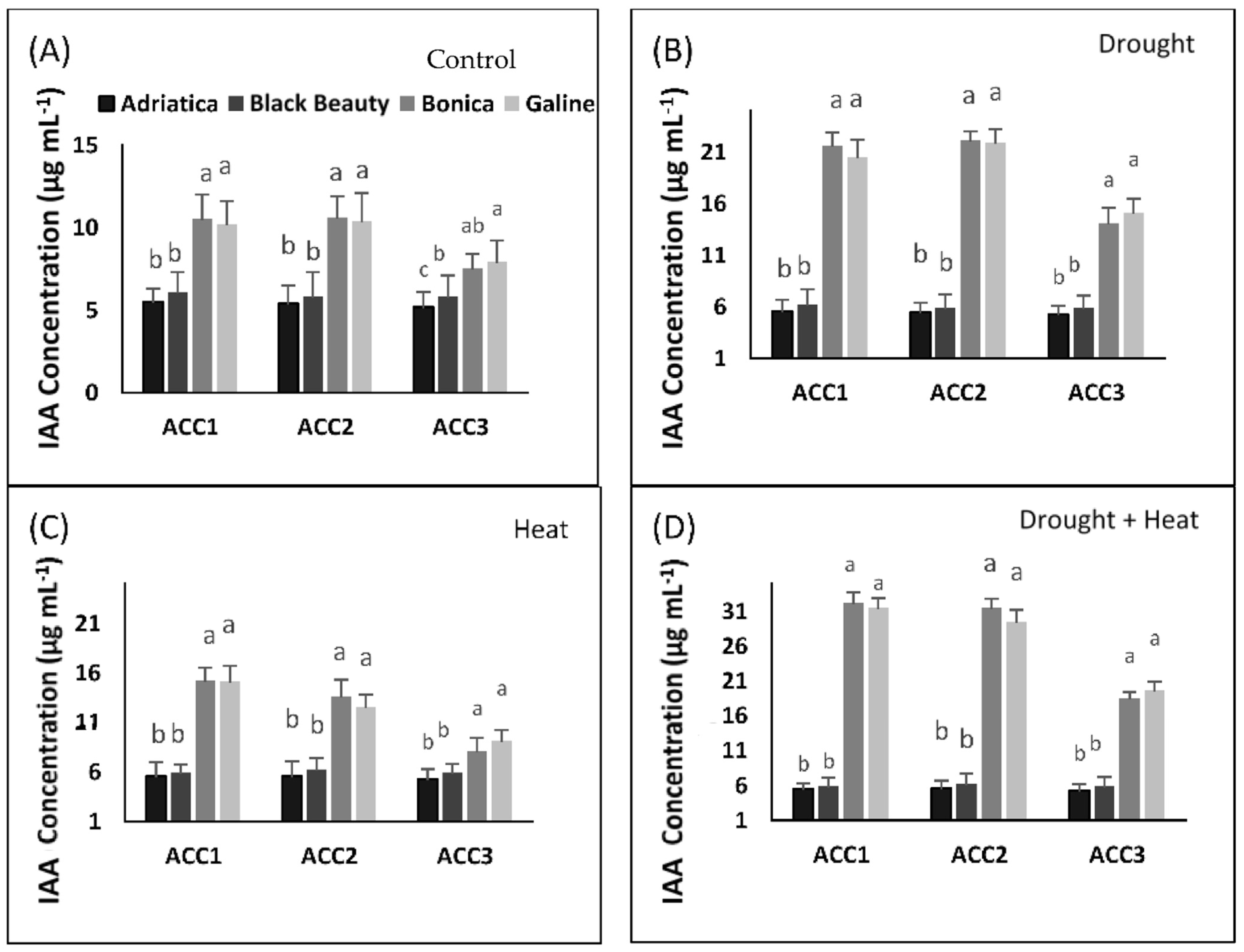
2.7. Drought-, Heat-, and Combined Drought and Heat-Induced Changes in ACC Deaminase-Producing Bacteria Activity
2.7.1. Quantitative Estimation of ACC Deaminase Activity
2.7.2. Estimation of Produced Indole Acetic Acid
3. Discussion
4. Materials and Methods
4.1. Experimental Designs and Stress Treatments
4.2. Growth and Yield Parameters Assessment
4.3. Relative Water Content and H2O2 Level
4.4. Pigments and Metabolites Analysis
4.5. Electrolyte Leakage (EL)
4.6. Gas Exchange Measurements
4.7. Enzymatic Assays
4.8. Non-Enzymatic Antioxidants Assay
4.9. Hormone Analysis
4.10. ACC Deaminase-Producing Bacteria Assay
4.10.1. Collection of Rhizospheric Soil Sample
4.10.2. Isolation of Rhizobacteria with ACC Deaminase Activity
4.10.3. Quantification of ACC Deaminase Activity
4.10.4. Indole Acetic Acid Production by Bacterial Isolates
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Sales, C.; Beltran, J.; Gómez-Cadenas, A.; Arbona, V. Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front. Plant Sci. 2017, 7, 1954. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Wang, W.; Xin, H.; Wang, M.; Ma, Q.; Wang, L.; Kaleri, N.A.; Li, X. Transcriptomic analysis reveals the molecular mechanisms of drought-stress-induced decreases in Camellia sinensis leaf quality. Front. Plant Sci. 2016, 7, 385. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Wang, L.C. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant Productivity and Environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Li, J.; Yu, J. Effects of low temperature and poor light on physiological characteristics of eggplant seedlings. J. Gansu Agric. Univ. 2004, 4, 011. [Google Scholar]
- Inthichack, P.; Nishimura, Y.; Fukumoto, Y. Diurnal Temperature Alternations on Plant Growth and Mineral Absorption in Eggplant, Sweet Pepper, and Tomato. Hortic. Environ. Biotechnol. 2013, 54, 37–43. [Google Scholar] [CrossRef]
- Barnabás, B.; Jager, K.; Feher, A. Te efect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar]
- Lawas, L.; Zuther, E.; Jagadish, S.K.; Hincha, D.K. Molecular mechanisms of combined heat and drought stress resilience in cereals. Curr. Opin. Plant Biol. 2018, 45, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.M.; Ghassemian, M.; Kwak, C.M.; McCourt, P.; Schroeder, J.I. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 1998, 282, 287–290. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tao, Y.; Hussain, S.; Jiang, Q.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Seed priming in dry direct-seeded rice: Consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul. 2016, 78, 167–178. [Google Scholar] [CrossRef]
- Alghabari, F.; Ihsan, M.Z.; Hussain, S.; Aishia, G.; Daur, I. Efect of rht alleles on wheat grain yield and quality under high temperature and drought stress during booting and anthesis. Environ. Sci. Pollut. Res. 2015, 22, 15506–15515. [Google Scholar] [CrossRef]
- Alghabari, F.; Ihsan, M.Z.; Khaliq, A.; Hussain, S.; Daur, I.; Fahad, S.; Nasim, W. Gibberellin-sensitive rht alleles confer tolerance to heat and drought stresses in wheat at booting stage. J. Cereal Sci. 2016, 70, 72–78. [Google Scholar] [CrossRef]
- Shah, N.H.; Paulsen, G.M. Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 2003, 257, 226. [Google Scholar] [CrossRef]
- Sekara, A.; Baczek-Kwinta, R.; Kalisz, A.; Cebula, S. Tolerance of eggplant (Solanum melongena L.) seedlings to stress factors. Acta Agrobot. 2012, 65, 83–91. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Luo, S.; Sun, B. Effects of heat stress on gene expression in eggplant Solanum melongema L. seedlings. Afr. J. Biotechnol. 2011, 10, 18078–18084. [Google Scholar]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant. Sci. 2018, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Khan, F.; Cao, W.; Wu, L.; Geng, M. Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front. Plant Sci. 2016, 7, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.A.; Tanveer, M.; Ashraf, U.; Hussain, S.; Shahzad, B.; Khan, I.; Wang, L. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ. Sci. Pollut. Res. 2016, 23, 17132–17141. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.; Ferreira–Silva, S.L.; de Vasconcelos Fontenele, A.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Dobra, J.; Motyka, V.; Dobrev, P.; Malbeck, J.; Prasil, I.T.; Haisel, D.; Gaudinova, A.; Havlova, M.; Gubis, J.; Vankova, R. Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J. Plant Physiol. 2010, 167, 1360–1370. [Google Scholar] [CrossRef]
- Duan, H.; Duursma, R.A.; Huang, G.; Smith, R.A.; Choat, B.; O’Grady, A.P.; Tissue, D.T. Elevated [CO 2] does not ameliorate the negative effects of elevated temperature on drought-induced mortality in E ucalyptus radiata seedlings. Plant Cell Environ. 2014, 37, 1598–1613. [Google Scholar] [CrossRef]
- Wang, C.J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.D.; Liu, H.X.; Wang, Y.P.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 2012, 7, e52565. [Google Scholar]
- Mukhtar, T.; Rehman, S.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar] [CrossRef]
- Kalozoumis, P.; Savvas, D.; Aliferis, K.; Ntatsi, G.; Marakis, G.; Simou, E.; Tampakaki, A.; Karapanos, I. Impact of plant growth-promoting rhizobacteria inoculation and grafting on tolerance of tomato to combined water and nutrient stress assessed via metabolomics analysis. Front. Plant Sci. 2021, 12, 670236. [Google Scholar] [CrossRef]
- Duan, B.; Li, L.; Chen, G.; Su-Zhou, C.; Li, Y.; Merkeryan, H.; Liu, W.; Liu, X. 1-Aminocyclopropane-1-Carboxylate Deaminase-Producing Plant Growth-Promoting Rhizobacteria Improve Drought Stress Tolerance in Grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 12, 706990. [Google Scholar] [CrossRef]
- Singh, R.P.; Shelke, G.M.; Kumar, A.; Jha, P.N. Biochemistry and genetics of ACC deaminase: A weapon to “stress ethylene” produced in plants. Front. Microbiol. 2015, 6, 937. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Duraivadivel, P.; Sharma, S.; Hariprasad, P. 1-Aminocyclopropane-1-carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. By mitigating drought and salt stress. Sci. Rep. 2018, 8, 17513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Spaepen, S.; Dobbelaere, S.; Croonenborghs, A.; Vanderleyden, J. Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil 2008, 312, 15–23. [Google Scholar] [CrossRef]
- Naveed, M.; Qureshi, M.A.; Zahir, Z.A.; Hussain, M.B.; Sessitsch, A.; Mitter, B. L-tryptophan-dependent biosynthesis of indole-3-acetic acid (IAA) improves plant growth and colonization of maize by Burkholderia phytofirmans PsJN. Ann. Microbiol. 2015, 65, 1381–1389. [Google Scholar] [CrossRef]
- Peck, S.C.; Teisberg, T.J. CETA: A model for carbon emissions trajectory assessment. Energy J. 1992, 13, 55–77. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Chang, X.; Li, R.; Jing, R. Efects of favorable alleles for water-soluble carbohydrates at grain flling on grain weight under drought and heat stresses in wheat. PLoS ONE 2014, 9, e102917. [Google Scholar] [CrossRef]
- Aref, I.M.; Khan, P.R.; Khan, S.; El-Atta, H.; Ahmed, A.I.; Iqbal, M. Modulation of antioxidant enzymes in Juniperus procera needles in relation to habitat environment and dieback incidence. Trees 2016, 30, 1669–1681. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Upreti, K.; Murti, G.; Bhatt, R. Response of pea cultivars to water stress: Changes in morphophysiological characters, endogenous hormones and yield. Veg. Sci. 2000, 27, 57–61. [Google Scholar]
- Joshi, P.; Swami, A. Air pollution induced changes in the photosynthetic pigments of selected plant species. J. Environ. Biol. 2009, 30, 295–298. [Google Scholar]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypiński, P.; Samborska, I.A.; et al. Prompt chlorophyll fuorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Digrado, A.; Bachy, A.; Mozaffar, A.; Schoon, N.; Bussotti, F.; Amelynck, C.; Dalcq, A.-C.; Fauconnier, M.; Aubinet, M.; Heinesch, B.; et al. Long-term measurements of chlorophyll a fuorescence using the JIP-test show that combined abiotic stresses influence the photosynthetic performance of the perennial ryegrass (Lolium perenne) in a managed temperate grassland. Physiol. Plant 2017, 161, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.P.; Tripathi, B.D. Biochemical responses in tree foliage exposed to coal-fred power plant emission in seasonally dry tropical environment. Environ. Monit. Assess 2008, 158, 197–212. [Google Scholar] [CrossRef]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Correlation between strawberry (Fragaria ananassa Duch.) productivity and photosynthesisrelated parameters under various growth conditions. Front. Plant Sci. 2016, 7, 1607. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, S.; Van Labeke, M.C.; Mehouachi, T. Application of chlorophyll fluorescence to screen eggplant (Solanum melongena L.) cultivars for salt tolerance. Photosynthetica 2014, 52, 57–62. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Chlorophyll a fuorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses. Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Khatri, K.; Rathore, M.S. Photosystem photochemistry, prompt and delayed fuorescence, photosynthetic responses and electron fow in tobacco under drought and salt stress. Photosynthetica 2019, 57, 61–74. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specifc photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M. PSII fuorescence techniques for measurement of drought and high temperature stress signal in crop plants: Protocols and applications. In Molecular Stress Physiology of Plants; Springer: New Delhi, India, 2013; pp. 87–131. [Google Scholar]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.-O.; Wu, Z. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ. Exp. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Dreesen, F.E.; De Boeck, H.J.; Janssens, I.A.; Nijs, I. Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ. Exp. Bot. 2012, 79, 21–30. [Google Scholar] [CrossRef]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; von Korf, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Baker, N.R. A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr. Opin. Plant Biol. 2002, 5, 193–198. [Google Scholar] [CrossRef]
- Sainz, M.; Díaz, P.; Monza, J.; Borsani, O. Heat stress results in loss of chloroplast Cu/Zn superoxide dismutase and increased damage to Photosystem II in combined drought-heat stressed Lotus japonicus. Physiol. Plant 2010, 140, 46–56. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Physiological responses to heat stress alone or in combination with drought: A comparison between tall fescue and perennial ryegrass. HortScience 2001, 36, 682–686. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Diferential responses to changes in growth temperature between trees from diferent functional groups and biomes: A review and synthesis of data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- Arend, M.; Brem, A.; Kuster, T.M.; Günthardt-Goerg, M.S. Seasonal photosynthetic responses of European oaks to drought and elevated daytime temperature. Plant Biol. 2013, 15, 169–176. [Google Scholar] [CrossRef]
- Demirevska, K.; Simova-Stoilova, L.; Fedina, I.; Georgieva, K.; Kunert, K. Response of oryzacystatin I transformed tobacco plants to drought heat and light stress. J. Agron. Crop Sci. 2010, 196, 90–99. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant efect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in perennial ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ. 1993, 16, 461–467. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.-O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant 2015, 153, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Asrar, H.; Hussain, T.; Hadi, S.M.S.; Gul, B.; Nielsen, B.L.; Khan, M.A. Salinity induced changes in light harvesting and carbon assimilating complexes of Desmostachya bipinnata (L.) Staph. Environ. Exp. Bot. 2017, 135, 86–95. [Google Scholar] [CrossRef]
- Rizhsky, L. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002, 130, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.M.; Nayyar, H. Individual and combined efects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Sun, X.; Lin, H.; Chen, J.; Ren, J.; Hu, X.; Yang, Y. Comparative physiological and proteomic analyses of poplar (Populus yunnanensis) plantlets exposed to high temperature and drought. PLoS ONE 2014, 9, e107605. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z.; Ahuja, L.R.; Reddy, V.R.; Saseendran, S.A.; Yu, Q. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. Am. Soc. Agron. Crop Sci. 2008, 1, 301–355. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant 2004, 120, 179–186. [Google Scholar] [CrossRef]
- Yang, X.; Wen, X.; Gong, H.; Lu, Q.; Yang, Z.; Tang, Y.; Liang, Z.; Lu, C. Genetic engineering of the biosynthesis of glycinebetaine enhances thermotolerance of photosystem II in tobacco plants. Planta 2006, 225, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, S.; Werbrouck, S.; Bahrini, I.; Abdelgadir, A.; Affan Siddiqui, H. Agronomical, Physiological and Biochemical Characterization of In Vitro Selected Eggplant Somaclonal Variants under NaCl Stress. Plants 2021, 10, 2544. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Liu, P.; Li, G.; Dong, S.T.; Wang, F.H.; Kong, L.A.; Zhang, J.W. Efect of heat stress on the photosynthetic characteristics in fag leaves at the grain-flling stage of diferent heat-resistant winter wheat varieties. J. Agron. Crop Sci. 2014, 200, 143–155. [Google Scholar] [CrossRef]
- Faseela, P.; Puthur, J.T. The imprints of the high light and UV-B stresses in Oryza sativa L. ‘Kanchana’ seedlings are diferentially modulated. J. Photochem. Photobiol. B Biol. 2018, 178, 551–559. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fuorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism oxydative stress and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant Responses to Drought, Acclimation, and Stress Tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Moller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Bhatt, D.; Prasad, R.; Gill, S.S.; Anjum, N.A.; Tuteja, N. Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 2016, 7, 1574. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative efect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined efect of salinity and heat reveals a specifc physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Correia, B.; Hancock, R.D.; Amaral, J.; Gomez-Cadenas, A.; Valledor, L.; Pinto, G. Combined drought and heat activates protective responses in Eucalyptus globulus that are not activated when subjected to drought or heat stress alone. Front. Plant Sci. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, N.A.; Iqbal, M.; Muhammad, A.; Ashraf, M.; Al-Qurainy, F.; Shafq, S. Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 2017, 255, 163–174. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Kuşvuran, S.; Rady, M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the feld environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide the response of arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef]
- Cvikrová, M.; Gemperlová, L.; Martincová, O.; Vanková, R. Efect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiol. Biochem. 2013, 73, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Moreno-Galván, A.E.; Cortés-Patiño, S.; Romero-Perdomo, F.; Uribe-Vélez, D.; Bashan, Y.; Bonilla, R.R. Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in Guinea grass. Appl. Soil. Ecol. 2020, 147, 103367. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Nurdiani, D.; Widyajayantie, D.; Nugroho, S. OsSCE1 encoding SUMO E2-conjugating enzyme involves in drought stress response of Oryza sativa. Rice Sci. 2018, 25, 73–81. [Google Scholar] [CrossRef]
- Petrov, P.; Petrova, A.; Dimitrov, I.; Tashev, T.; Olsovska, K.; Brestic, M.; Misheva, S. Relationships between leaf morphoanatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J. Agron. Crop Sci. 2018, 204, 219–227. [Google Scholar] [CrossRef]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, J.; Achary, V.M.M.; Reddy, M.K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015, 3, 25. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Carvalho, L.S.C.; Vidigal, P.C.; Amancio, S. Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Front. Environ. Sci. 2015, 3, 20. [Google Scholar] [CrossRef]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, Y.; Chen, Y.; Gao, M.; Zhao, Y.; Wu, L. Effects of Drought Stress and Rehydration on Physiological and Biochemical Properties of Four Oak Species in China. Plants 2022, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ahammed, G.J.; Li, C.; Bao, X.; Yu, J.; Huang, C.; Yin, H.; Zhou, J. Brassinosteroid ameliorates zinc oxide nanoparticles induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant Sci. 2016, 7, 615. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of brassinosteroids in alleviation of phenanthrene– cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef]
- Hayat, S.; Hasan, S.A.; Yusuf, M.; Hayat, Q.; Ahmad, A. Efect of 28-homobrassinolide on photosynthesis, fuorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiate. Environ. Exp. Bot. 2010, 69, 105–112. [Google Scholar] [CrossRef]
- Yuan, L.-Y.; Du, J.; Yuan, Y.-H.; Shu, S.; Sun, J.; Guo, S.-R. Efects of 24-epibrassinolide on ascorbate–glutathione cycle and polyamine levels in cucumber roots under Ca(NO3)2 stress. Acta Physiol. Plant 2013, 35, 253–262. [Google Scholar] [CrossRef]
- Batth, R.; Singh, K.; Kumari, S.; Mustafz, A. Transcript profling reveals the presence of abiotic stress and developmental stage specifc ascorbate oxidase genes in plants. Front. Plant Sci. 2017, 8, 198. [Google Scholar] [CrossRef]
- Arbona, V.; Hossain, Z.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant 2008, 132, 452–466. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Liao, Y.-C.; Zhang, Z.; Liu, J.; Sun, P.-W.; Gao, Z.-H.; Sui, C.; Wei, J.-H. Jasmonic acid is a crucial signal transducer in heat shock induced sesquiterpene formation in Aquilaria sinensis. Sci. Rep. 2016, 6, 21843. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hou, P.; Su, X.; Zhao, P.; Zhao, H.; Liu, S. Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Growth Regul. 2014, 73, 289–297. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Yang, C.S.; Wang, Q.; Liu, T.X.; Li, C.H. Effects of phosphorus placement depth on yield and nutrient uptake of summer maize. Sci. Agric. Sinica. 2010, 43, 4805–4813. [Google Scholar]
- Yan, Q.; Duan, Z.; Jingdong, M.; Xun, L.; Fei, D. Efects of rootzone temperature and N, P, and K supplies on nutrient uptake of cucumber (Cucumis sativus L.) seedlings in hydroponics. Soil Sci. Plant Nutr. 2012, 58, 707–717. [Google Scholar] [CrossRef]
- Tingey, D.T.; McKane, R.B.; Olszyk, D.M.; Johnson, M.G.; Rygiewicz, P.T.; Henry Lee, E. Elevated CO2 and temperature alter nitrogen allocation in douglas-fr. Glob. Change Biol. 2003, 9, 1038–1050. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Efects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Suriyagoda, L.; De Costa, W.A.J.M.; Lambers, H. Growth and phosphorus nutrition of rice when inorganic fertilizer application is partly replaced by straw under varying moisture availability in sandy and clay soils. Plant Soil 2014, 384, 53–68. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water defcits in leaves. Aust. J. Biol. Sci. 1962, 15, 413. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Keller, C.; Monnet, F.; Sallanon, H. Acclimation to drought stress enhances oxidative stress tolerance in Solanum lycopersicum L. fruits. Plant Stress 2008, 2, 145–151. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta BBA Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Van Kooten, O.; Snel, J.F.H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Bilger, W. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Guo, Z.; Ou, W.; Lu, S.; Zhong, Q. Diferential responses of antioxidative system to chilling and drought in four rice cultivars difering in sensitivity. Plant Physiol. Biochem. 2006, 44, 828–836. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Hossain, Z.; López-Climent, M.F.; Arbona, V.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage. J. Plant Physiol. 2009, 166, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C. Responses of photosynthesis and the xanthophyll and ascorbate-glutathione cycles to changes in irradiance, photoinhibition and recovery. Plant Physiol. Biochem. 1989, 27, 751–760. [Google Scholar]
- Durgbanshi, A.; Arbona, V.; Pozo, O.; Miersch, O.; Sancho, J.V.; Gómez Cadenas, A. Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 8437–8442. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.; Foster, J. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [Green Version]




| Variety | Treatments | Height (%) | FW (%) | DW (%) |
|---|---|---|---|---|
| ‘Adriatica’ | C | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a |
| D | 56.4 ± 6.8 c | 56.3 ± 4.5 b | 72.4 ± 9.2 b | |
| H | 82.8 ± 5.2 b | 68.3 ± 7.3 b | 86.6 ± 6.5 b | |
| H + D | 46.9 ± 7.3 d | 11.7 ± 4.3 c | 30.5 ± 5.4 c | |
| ‘Black Beauty’ | C | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a |
| D | 59.4 ± 8.2 c | 58 ± 5.5 b | 79.1 ± 6.7 b | |
| H | 83.4 ± 5.2 b | 70.8 ± 4.7 b | 92.8 ± 8.1 b | |
| H + D | 47.2 ± 6.3 d | 13.7 ± 4.2 c | 26.4 ± 4.8 c | |
| ‘Bonica’ | C | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a |
| D | 78.2 ± 5.3 b | 73.4 ± 9.1 c | 74 ± 5.8 c | |
| H | 95 ± 7.2 b | 91.8 ± 5.2 b | 89.7 ± 4.2 b | |
| H + D | 58.9 ± 3.9 c | 52.4 ± 6.3 d | 53.7 ± 4.7 d | |
| ‘Galine’ | C | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a |
| D | 78.1 ± 7.1 b | 73.4 ± 6.1 c | 73.9 ± 3.9 c | |
| H | 94.7 ± 6.4 b | 91.8 ± 4.1 b | 89.7 ± 4.7 b | |
| H + D | 58.8 ± 5.3 c | 52.4 ± 5.2 d | 53.7 ± 6.5 d |
| Parameter | Treatment | ‘Adriatica’ | ‘Black Beauty’ | ‘Bonica’ | ‘Galine’ |
|---|---|---|---|---|---|
| Fruit number | C | 6 ± 1.9 a | 6 ± 1.7 a | 7 ± 1.6 a | 6 ± 0.5 a |
| D | 2 ± 1.2 b | 2 ± 1.3 b | 3 ± 1.2 b | 3 ± 1.1 b | |
| H | 3 ± 1.5 b | 3 ± 1.2 b | 5 ± 1.2 b | 4 ± 2.3 b | |
| D + H | 1 ± 1.3 c | 1 ± 0.9 c | 2 ± 1.7 c | 2 ± 3.2 c | |
| Fruit weight | C | 180 ± 1.2 a | 182 ± 1.6 a | 210 ± 1.1 a | 250 ± 2.8 a |
| D | 80 ± 3.6 c | 79 ± 2.3 c | 130 ± 2.2 c | 124 ± 3.1 c | |
| H | 150 ± 0.9 b | 155 ± 1.9 b | 160 ± 2.9 b | 180 ± 1.8 b | |
| D + H | 40 ± 3.2 d | 38 ± 2.1 d | 80 ± 0.8 d | 73 ± 2.1 d |
| cv | Treatments | Chla (µg g−1 FW) | Chlb (µg g−1 FW) | Chla/b | Chla+b (µg g−1 FW) | Carotenoids (µg g−1 FW) |
|---|---|---|---|---|---|---|
| ‘Adriatica’ | C | 764.9 ± 2.1 a | 351.2 ± 4.5 a | 2.17 ± 4.5 a | 1116.1 ± 2.5 a | 269.3 ± 5.9 a |
| D | 695.8 ± 2.7 b | 346.2 ± 2.1 b | 2.00 ± 3.3 b | 1042.0 ± 2.7 b | 237.5 ± 2.5 b | |
| H | 332.5 ± 1.2 c | 261.3 ± 2.3 c | 1.27 ± 1.3 c | 593.8 ± 2.1 c | 149.9 ± 2.1 c | |
| H + D | 161.7 ± 2.5 d | 160.2 ± 2.3 d | 1.01 ± 4.3 d | 321.9 ± 3.2 d | 113.1 ± 3.2 d | |
| ‘Black Beauty’ | C | 766.5 ± 1.7 a | 355.3 ± 3.4 a | 2.15 ± 3.4 a | 1121.8 ± 3.4 a | 274.2 ± 2.5 a |
| D | 691.8 ± 1.4 b | 351.1 ± 3.5 b | 1.97 ± 2.2 a | 1042.9 ± 3.3 b | 242.3 ± 4.3 b | |
| H | 337.5 ± 1.5 c | 266.2 ± 1.2 c | 1.26 ± 2.1 b | 603.7 ± 1.3 c | 153.7 ± 1.3 c | |
| H + D | 156.8 ± 2.2 d | 164.5 ± 4.1 d | 0.95 ± 3.1 c | 321.3 ± 3.2 d | 118.5 ± 2.3 d | |
| ‘Bonica’ | C | 617.5 ± 2.7a | 304.1 ± 1.3 a | 2.03 ± 8.6 a | 921.6 ± 3.2 a | 147.6 ± 2.8 a |
| D | 515.3 ± 3.2 a | 280.2 ± 3.7 a | 1.83 ± 6.9 a | 795.5 ± 2.3 a | 140.8 ± 1.9 a | |
| H | 450.1 ± 3.6 a | 250.6 ± 2.6 a | 1.79 ± 3.5 a | 700.7 ± 5.2 a | 124.8 ± 1.7 a | |
| H + D | 405.1 ± 2.5 a | 202.5 ± 3.3 a | 2.00 ± 2.9 a | 607.6 ± 3.6 a | 105.7 ± 1.6 a | |
| ‘Galine’ | C | 614.4 ± 3.2 a | 298.2 ± 3.1 a | 2.06 ± 3.4 a | 912.6 ± 4.1 a | 142.5 ± 1.3 a |
| D | 511.2 ± 1.2 a | 277.1 ± 1.2 a | 1.84 ± 2.1 a | 788.3 ± 1.1 a | 132.7 ± 2.5 a | |
| H | 445.3 ± 5.2 a | 244.5 ± 3.2 a | 1.82 ± 7.5 a | 689.8 ± 3.3 a | 120.2 ± 3.2 a | |
| H + D | 400.1 ± 2.5 a | 200.5 ± 3.3 a | 1.99 ± 1.3 a | 606.6 ± 4.2 a | 103.5 ± 2.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannachi, S.; Signore, A.; Adnan, M.; Mechi, L. Single and Associated Effects of Drought and Heat Stresses on Physiological, Biochemical and Antioxidant Machinery of Four Eggplant Cultivars. Plants 2022, 11, 2404. https://doi.org/10.3390/plants11182404
Hannachi S, Signore A, Adnan M, Mechi L. Single and Associated Effects of Drought and Heat Stresses on Physiological, Biochemical and Antioxidant Machinery of Four Eggplant Cultivars. Plants. 2022; 11(18):2404. https://doi.org/10.3390/plants11182404
Chicago/Turabian StyleHannachi, Sami, Angelo Signore, Mohd Adnan, and Lassaad Mechi. 2022. "Single and Associated Effects of Drought and Heat Stresses on Physiological, Biochemical and Antioxidant Machinery of Four Eggplant Cultivars" Plants 11, no. 18: 2404. https://doi.org/10.3390/plants11182404
APA StyleHannachi, S., Signore, A., Adnan, M., & Mechi, L. (2022). Single and Associated Effects of Drought and Heat Stresses on Physiological, Biochemical and Antioxidant Machinery of Four Eggplant Cultivars. Plants, 11(18), 2404. https://doi.org/10.3390/plants11182404








