Interaction of the Nanoparticles and Plants in Selective Growth Stages—Usual Effects and Resulting Impact on Usage Perspectives
Abstract
:1. Introduction
2. Current Knowledge about the Uptake of Nanoparticles by Plants
3. Movement and Accumulation of Nanoparticles in the Plant
4. Application of Nanoparticles to Seeds
4.1. Nanopriming to Reduce/eliminate Undesirable Microbial Seed Contamination
4.2. Nanopriming to Increase Tolerance to Biotic/Abiotic Stress
4.3. Seed Nanopriming to Improve Plant Performance
5. Application of Nanoparticles to Plants
5.1. Treatment of Plants with Nanoparticles for Protection against Pathogens
5.2. Use of Nanoparticles to Increase Plant Productivity and Resistance to Stress
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zítka, O.; Brně, V.U.T.V.; Univerzita, M. Moderní Nanotechnologie na Počátku 21. Století: Kolekce Učebních Textů Projektu OPVK NANOTEAM; Vysoké Učení Technické v Brně: Brno, Czechia, 2013. [Google Scholar]
- Hasan, S. A Review on Nanoparticles: Their Synthesis and Types. Res. J. Recent. Sci. 2015, 4, 1–3. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Elmer, W.; White, J.C. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.S.N.; Gunasekara, T.; Holton, J. Antimicrobial Nanoparticles: Applications and mechanisms of action. Sri Lankan J. Infect. Dis. 2018, 8, 2–11. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in Plant Science: To Make a Long Story Short. Front Bioeng Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.; Albergaria, J.T.; Delerue-Matos, C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Total Env. 2015, 533, 76–81. [Google Scholar] [CrossRef]
- Yu, M.; Yao, J.; Liang, J.; Zeng, Z.; Cui, B.; Zhao, X.; Sun, C.; Wang, Y.; Liu, G.; Cui, H. Development of functionalized abamectin poly(lactic acid) nanoparticles with regulatable adhesion to enhance foliar retention. RSC Adv. 2017, 7, 11271–11280. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Urík, M.; Ďurišová, Ľ.; Bujdoš, M.; Šebesta, M.; Dobročka, E.; Kšiňan, S.; Illa, R.; Qian, Y. Foliar application of low concentrations of titanium dioxide and zinc oxide nanoparticles to the common sunflower under field conditions. Nanomaterials 2020, 10, 1619. [Google Scholar] [CrossRef]
- Jurkow, R.; Pokluda, R.; Sekara, A.; Kalisz, A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biol. 2020, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Env. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Env. Sci. Pollut. Res. Int. 2021, 28, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.; Abideen, Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. NanoImpact 2022, 27, 100411. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano. 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar] [CrossRef]
- Aliasghari, A.; Khorasgani, M.R.; Vaezifar, S.; Rahimi, F.; Younesi, H.; Khoroushi, M. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran. J. Microbiol. 2016, 8, 93–100. [Google Scholar]
- Kalwar, K.; Shan, D. Antimicrobial effect of silver nanoparticles (AgNPs) and their mechanism—A mini review. Micro Nano Lett. 2018, 13, 277–280. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Novotný, D.; Baránek, M.; Eichmeier, A.; Salava, J.; Peňázová, E.; Pečenka, J.; Koudela, M. Prostředky Diagnostiky A Ochrany Proti Vybraným Druhům Škodlivých Mikroorganismů Zelí; Výzkumný Ústav Rostlinné Výroby: Praha, Czech Republic, 2019. [Google Scholar]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Env. Toxicol. Pharm. 2019, 71, 103220. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.H.; Carvalho, T.C.; Souza, M.G.; Ravagnani, C.; Peitl, O.; Zanotto, E.D.; Panzeri, H.; Casemiro, L.A. Assessment of antimicrobial effect of Biosilicate(R) against anaerobic, microaerophilic and facultative anaerobic microorganisms. J. Mater. Sci. Mater. Med. 2011, 22, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Parra, A.; Toro, M.; Jacob, R.; Navarrete, P.; Troncoso, M.; Figueroa, G.; Reyes-Jara, A. Antimicrobial effect of copper surfaces on bacteria isolated from poultry meat. Braz. J. Microbiol. 2018, 49 (Suppl 1), 113–118. [Google Scholar] [CrossRef]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol Sci. 2020, 8258. [Google Scholar] [CrossRef]
- Do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Tripathi, D.K.; Prasad, S.M.; Chauhan, D.K. Plant Responses to Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Ma, X.; Geisler-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Env. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanoparticle Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants--A soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Tripathi, A.; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; Upadhyay, R.G.; et al. Uptake, Accumulation and Toxicity of Silver Nanoparticle in Autotrophic Plants, and Heterotrophic Microbes: A Concentric Review. Front. Microbiol. 2017, 8, 07. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, J.D.; Lui, A.; Landry, M.P. Multiscale and multidisciplinary approach to understanding nanoparticle transport in plants. Curr. Opin. Chem. Eng. 2020, 30, 135–143. [Google Scholar] [CrossRef]
- Committee, E.S.; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernandez-Jerez, A.; Hougaard Bennekou, S.; Koutsoumanis, K.; Lambre, C.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19, e06768. [Google Scholar] [CrossRef]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias, H.V.; Kharisov, B.I.; Perez, B.O.; Perez, V.M. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. Synthesis and modification of bio-derived antibacterial Ag and ZnO nanoparticles by plants, fungi, and bacteria. Drug Discov. Today 2021, 26, 1953–1962. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Morais, B.P.; Clement, E.T.; Rodrigues, S.M.; Lowry, G.V. Critical Review: Role of Inorganic Nanoparticle Properties on Their Foliar Uptake and in Planta Translocation. Env. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef]
- Ha, N.; Seo, E.; Kim, S.; Lee, S.J. Adsorption of nanoparticles suspended in a drop on a leaf surface of Perilla frutescens and their infiltration through stomatal pathway. Sci. Rep. 2021, 11, 11556. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Ashraf, U.; Hussain, S.; Shahzad, B.; Khan, I.; Wang, L. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Env. Sci. Pollut. Res. Int. 2016, 23, 17132–17141. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K. The formation and function of plant cuticles. Plant Physiol 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Guerrero, J.A.; Benitez, J.J.; Dominguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Guzman-Delgado, P.; Graca, J.; Cabral, V.; Gil, L.; Fernandez, V. The presence of cutan limits the interpretation of cuticular chemistry and structure: Ficus elastica leaf as an example. Physiol. Plant 2016, 157, 205–220. [Google Scholar] [CrossRef]
- Lv, G.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. In situ detection of rice leaf cuticle responses to nitrogen supplies by depth-profiling Fourier transform photoacoustic spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117759. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Lombi, E.; Cheng, M.; Tang, C.; Howard, D.L.; Menzies, N.W.; Kopittke, P.M. Absorption of foliar-applied Zn fertilizers by trichomes in soybean and tomato. J. Exp. Bot. 2018, 69, 2717–2729. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci. Nano 2020, 7, 3901–3913. [Google Scholar] [CrossRef]
- Lin, S.; Reppert, J.; Hu, Q.; Hudson, J.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 2009, 5, 1128–1132. [Google Scholar] [CrossRef]
- Uzu, G.; Sobanska, S.; Sarret, G.; Muñoz, M.; Dumat, C. Foliar Lead Uptake by Lettuce Exposed to Atmospheric Fallouts. Environ. Sci. Technol. Am. Chem. Soc. 2010, 44, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Eichert, T.; Goldbach, H.E. Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces--further evidence for a stomatal pathway. Physiol. Plant 2008, 132, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant 2008, 134, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H. Stomatal uptake of mineral particles from a sprayed suspension containing an organosilicone surfactant. J. Plant Nutr. Soil Sci. 2014, 177, 869–874. [Google Scholar] [CrossRef]
- Magniont, C.; Escadeillas, G.; Coutand, M.; Oms-Multon, C. Use of plant aggregates in building ecomaterials. Eur. J. Environ. Civ. Eng. 2012, 16, s17–s33. [Google Scholar] [CrossRef]
- Benzon, H.R.L.; Rubenecia, M.R.U.; Ultra, J.; Venecio, U.; Lee, S.C. Nano-fertilizer affects the growth, development, and chemical properties of rice. Int. J. Agri. Agri. R. 2015, 7, 105–117. [Google Scholar]
- Lawrence, M.; Fodde, E.; Paine, K.; Walker, P. Hygrothermal Performance of an Experimental Hemp-Lime Building. Key Eng. Mater. 2012, 517, 413–421. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Van Der Ent, A.; Cheng, M.; Jiang, H.; Lund Read, T.; Lombi, E.; Tang, C.; De Jonge, M.D.; Menzies, N.W. Absorption of foliar-applied Zn in sunflower (Helianthus annuus): Importance of the cuticle, stomata and trichomes. Ann. Bot. 2019, 123, 57–68. [Google Scholar] [CrossRef]
- Larue, C.; Laurette, J.; Herlin-Boime, N.; Khodja, H.; Fayard, B.; Flank, A.M.; Brisset, F.; Carriere, M. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): Influence of diameter and crystal phase. Sci. Total Env. 2012, 431, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peralta-Videa, J.R.; Ren, M.; Varela-Ramirez, A.; Li, C.; Hernandez-Viezcas, J.A.; Aguilera, R.J.; Gardea-Torresdey, J.L. Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: Electron microprobe and confocal microscopy studies. Chem. Eng. J. 2012, 184, 1–8. [Google Scholar] [CrossRef]
- Deng, Y.-Q.; White, J.C.; Xing, B.-S. Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. J. Zhejiang Univ. SCIENCE A 2014, 15, 552–572. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Hussain, H.I.; Yi, Z.; Siegele, R.; Cresswell, T.; Kong, L.; Cahill, D.M. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014, 33, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Env. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Wang, H.; Yan, B.; Zheng, H.; Jiang, Y.; Miranda, O.R.; Rotello, V.M.; Xing, B.; Vachet, R.W. Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ. Sci. Technol. 2012, 46, 12391–12398. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Larue, C.; Veronesi, G.; Flank, A.M.; Surble, S.; Herlin-Boime, N.; Carriere, M. Comparative uptake and impact of TiO(2) nanoparticles in wheat and rapeseed. J. Toxicol. Env. Health A 2012, 75, 722–734. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, S.; Luo, L.; Zhang, J.; Yang, K.; Christie, P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ. Sci. Nano 2015, 2, 68–77. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle Size and Coating Chemistry Control Foliar Uptake Pathways, Translocation, and Leaf-to-Rhizosphere Transport in Wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef]
- Cvjetko, P.; Zovko, M.; Stefanic, P.P.; Biba, R.; Tkalec, M.; Domijan, A.M.; Vrcek, I.V.; Letofsky-Papst, I.; Sikic, S.; Balen, B. Phytotoxic effects of silver nanoparticles in tobacco plants. Env. Sci. Pollut. Res. Int. 2018, 25, 5590–5602. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yan, J. Plant uptake and accumulation of engineered metallic nanoparticles from lab to field conditions. Curr. Opin. Environ. Sci. Health 2018, 6, 16–20. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fundamentals of Plant Physiology; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Noori, A.; Ngo, A.; Gutierrez, P.; Theberge, S.; White, J.C. Silver nanoparticle detection and accumulation in tomato (Lycopersicon esculentum). J. Nanoparticle Res. 2020, 22, 131. [Google Scholar] [CrossRef]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2012, 46, 7637–7643. [Google Scholar] [CrossRef]
- Servin, A.; Elmer, W.; Mukherjee, A.; la Torre-Roche, D.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanoparticle Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Saien, J.; Hasani, R. Hydrodynamics and mass transfer characteristics of circulating single drops with effect of different size nanoparticles. Separation and Purification Technology 2017, 175, 298–304. [Google Scholar] [CrossRef]
- Moghaddasi, S.; Fotovat, A.; Khoshgoftarmanesh, A.H.; Karimzadeh, F.; Khazaei, H.R.; Khorassani, R. Bioavailability of coated and uncoated ZnO nanoparticles to cucumber in soil with or without organic matter. Ecotoxicol. Environ. Saf. 2017, 144, 543–551. [Google Scholar] [CrossRef]
- Peng, C.; Tong, H.; Shen, C.; Sun, L.; Yuan, P.; He, M.; Shi, J. Bioavailability and translocation of metal oxide nanoparticles in the soil-rice plant system. Sci. Total Environ. 2020, 713, 136662. [Google Scholar] [CrossRef]
- Gubbins, E.J.; Batty, L.C.; Lead, J.R. Phytotoxicity of silver nanoparticles to Lemna minor L. Environ. Pollut. 2011, 159, 1551–1559. [Google Scholar] [CrossRef]
- Arruda, S.C.C.; Silva, A.L.D.; Galazzi, R.M.; Azevedo, R.A.; Arruda, M.A.Z. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Khalaki, M.; Moameri, M.; Asgari Lajayer, B.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2020, 93, 13–28. [Google Scholar] [CrossRef]
- Köhl, J.; van der Wolf, J. Alternaria brassicicola and Xanthomonas campestris pv. campestris in organic seed production of Brassicae: Epidemiology and seed infection. Wageningen: Plant Research International 2005, Note 363, 1-28. Available online: http://edepot.wur.nl/17130 (accessed on 30 July 2022).
- Deshmukh, R.K.; Nguyen, H.T.; Belanger, R.R. Editorial: Aquaporins: Dynamic Role and Regulation. Front. Plant Sci. 2017, 8, 1420. [Google Scholar] [CrossRef]
- Kumawat, S.; Khatri, P.; Ahmed, A.; Vats, S.; Kumar, V.; Jaswal, R.; Wang, Y.; Xu, P.; Mandlik, R.; Shivaraj, S.M.; et al. Understanding aquaporin transport system, silicon and other metalloids uptake and deposition in bottle gourd (Lagenaria siceraria). J. Hazard Mater. 2021, 409, 124598. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.J.; Brough, J.; Hunter, P.J. Modelling the spread of Xanthomonas campestris pv. campestris in module-raised brassica transplants. Plant Pathol. 2006, 56, 391–401. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2012, 35, 1381–1396. [Google Scholar] [CrossRef]
- Paparella, S.; Araujo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Pečenka, J.; Bytešníková, Z.; Kiss, T.; Peňázová, E.; Baránek, M.; Eichmeier, A.; Tekielska, D.; Richtera, L.; Pokluda, R.; Adam, V. Silver nanoparticles eliminate Xanthomonas campestris pv. campestris in cabbage seeds more efficiently than hot water treatment. Mater. Today Commun. 2021, 27, 102284. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.á.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Sarkar, R.D.; Deka, J.; Kalita, M.C. Plant extract mediated green synthesis of selenium nanoparticle and its antimicrobial activity: A brief review. Innov. Microbiol. Biotechnol. 2021, 2, 103–110. [Google Scholar]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, R.; Sidhu, A.; Bala, A. Synthesis and evaluation of iron(ii) sulfide aqua nanoparticles (FeS-NPs) against Fusarium verticillioides causing sheath rot and seed discoloration of rice. Eur. J. Plant Pathol. 2019, 155, 163–171. [Google Scholar] [CrossRef]
- Kumar, G.D.; Raja, K.; Natarajan, N.; Govindaraju, K.; Subramanian, K.S. Invigouration treatment of metal and metal oxide nanoparticles for improving the seed quality of aged chilli seeds (Capsicum annum L.). Mater. Chem. Phys. 2020, 242, 122492. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Muthukumar, S. Chitosan guar nanoparticle preparation and its in vitro antimicrobial activity towards phytopathogens of rice. Int. J. Biol. Macromol. 2020, 153, 297–304. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 2019, 127, 126–135. [Google Scholar] [CrossRef]
- Cadena, M.B.; Preston, G.M.; Van der Hoorn, R.A.L.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crops Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Shah, V.; Belozerova, I. Influence of Metal Nanoparticles on the Soil Microbial Community and Germination of Lettuce Seeds. Water Air Soil Pollut. 2008, 197, 143–148. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize Under Drought Stress Conditions. J. Plant Growth Regul. 2021, 41, 364–375. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Tomar, R.S.; Jajoo, A. H2O2 signaling regulates seed germination in ZnO nanoprimed wheat (Triticum aestivum L.) seeds for improving plant performance under drought stress. Environ. Exp. Bot. 2021, 189, 104561. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Tomar, R.S.; Jajoo, A. Seed nanopriming by silicon oxide improves drought stress alleviation potential in wheat plants. Funct. Plant Biol. 2021, 48, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Lee, J.H.J.; Jayaprakasha, G.K.; Patil, B.S. Seed Priming with Iron Oxide Nanoparticles Modulate Antioxidant Potential and Defense-Linked Hormones in Watermelon Seedlings. ACS Sustain. Chem. Eng. 2019, 7, 5142–5151. [Google Scholar] [CrossRef]
- Vanti, G.L.; Nargund, V.B.; Vanarchi, R.; Kurjogi, M.; Mulla, S.I.; Tubaki, S.; Patil, R.R. Synthesis ofGossypium hirsutum-derived silver nanoparticles and their antibacterial efficacy against plant pathogens. Appl. Organomet. Chem. 2019, 33, e4630. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles Control Salinity-Modulated Molecular Responses in Capsicum annuum L. through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Maswada, H.F.; Djanaguiraman, M.; Prasad, P.V.V. Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J. Agron. Crop Sci. 2018, 204, 577–587. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Abdelfattah, K.E. The Possible Roles of Priming with ZnO Nanoparticles in Mitigation of Salinity Stress in Lupine (Lupinus termis) Plants. J. Plant Growth Regul. 2016, 36, 60–70. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Env. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia Ur Rehman, M.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Env. Sci. Pollut. Res. Int. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Yasmeen, F.; Raja, N.I.; Razzaq, A.; Komatsu, S. Proteomic and physiological analyses of wheat seeds exposed to copper and iron nanoparticles. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, L.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different Modes of Hydrogen Peroxide Action During Seed Germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Guha, T.; Ravikumar, K.V.G.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, U.; Luo, X.; Wang, Q.; Shu, K. Are There Unidentified Factors Involved in the Germination of Nanoprimed Seeds? Front. Plant Sci. 2020, 11, 832. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef]
- Younis, M.E.; Abdel-Aziz, H.M.M.; Heikal, Y.M. Nanopriming technology enhances vigor and mitotic index of aged Vicia faba seeds using chemically synthesized silver nanoparticles. South Afr. J. Bot. 2019, 125, 393–401. [Google Scholar] [CrossRef]
- Vijai Anand, K.; Anugraga, A.R.; Kannan, M.; Singaravelu, G.; Govindaraju, K. Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour of green gram (Vigna radiata L.). Mater. Lett. 2020, 271, 127792. [Google Scholar] [CrossRef]
- Sarkar, N.; Sharma, R.S.; Kaushik, M. Innovative application of facile single pot green synthesized CuO and CuO@APTES nanoparticles in nanopriming of Vigna radiata seeds. Env. Sci. Pollut. Res. Int. 2021, 28, 13221–13228. [Google Scholar] [CrossRef]
- Pradhan, S.; Patra, P.; Mitra, S.; Dey, K.K.; Jain, S.; Sarkar, S.; Roy, S.; Palit, P.; Goswami, A. Manganese nanoparticles: Impact on non-nodulated plant as a potent enhancer in nitrogen metabolism and toxicity study both in vivo and in vitro. J. Agric. Food Chem. 2014, 62, 8777–8785. [Google Scholar] [CrossRef]
- Rahman, M.S.; Chakraborty, A.; Mazumdar, S.; Nandi, N.C.; Bhuiyan, M.N.I.; Alauddin, S.M.; Khan, I.A.; Hossain, M.J. Effects of poly(vinylpyrrolidone) protected platinum nanoparticles on seed germination and growth performance of Pisum sativum. Nano-Struct. Nano-Objects 2020, 21, 100408. [Google Scholar] [CrossRef]
- Afsheen, S.; Naseer, H.; Iqbal, T.; Abrar, M.; Bashir, A.; Ijaz, M. Synthesis and characterization of metal sulphide nanoparticles to investigate the effect of nanoparticles on germination of soybean and wheat seeds. Mater. Chem. Phys. 2020, 252, 123216. [Google Scholar] [CrossRef]
- Afzal, S.; Sharma, D.; Singh, N.K. Eco-friendly synthesis of phytochemical-capped iron oxide nanoparticles as nano-priming agent for boosting seed germination in rice (Oryza sativa L.). Env. Sci. Pollut. Res. Int. 2021, 28, 40275–40287. [Google Scholar] [CrossRef] [PubMed]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant Growth Regul. 2018, 38, 122–131. [Google Scholar] [CrossRef]
- Najafi Disfani, M.; Mikhak, A.; Kassaee, M.Z.; Maghari, A. Effects of nano Fe/SiO2fertilizers on germination and growth of barley and maize. Arch. Agron. Soil Sci. 2016, 63, 817–826. [Google Scholar] [CrossRef]
- Awasthi, A.; Bansal, S.; Jangir, L.K.; Awasthi, G.; Awasthi, K.K.; Awasthi, K. Effect of ZnO Nanoparticles on Germination of Triticum aestivum Seeds. Macromol. Symp. 2017, 376, 1700043. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, H.M.; Li, Y.W.; Huang, X.P.; Wu, X.L.; Zhai, T.; Yuan, Y.; Cai, Q.Y.; Mo, C.H. Effects of the size and morphology of zinc oxide nanoparticles on the germination of Chinese cabbage seeds. Env. Sci. Pollut. Res. Int. 2015, 22, 10452–10462. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; Kohatsu, M.Y.; Seabra, A.B.; Monteiro, L.R.; Gomes, D.G.; Oliveira, H.C.; Rolim, W.R.; de Jesus, T.A.; Batista, B.L.; Lange, C.N. Effects of copper oxide nanoparticles on growth of lettuce (Lactuca sativa L.) seedlings and possible implications of nitric oxide in their antioxidative defense. Env. Monit. Assess 2020, 192, 232. [Google Scholar] [CrossRef]
- Afrayeem, S.M.; Chaurasia, A.K. Effect of zinc oxide nanoparticles on seed germination and seed vigour in chilli (Capsicum annuum L.). J. Pharmacogn. Phytochem. 2017, 6, 1564–1566. [Google Scholar]
- Solgi, M. Evaluation of plant-mediated Silver nanoparticles synthesis and its application in postharvest Physiology of cut Flowers. Physiol. Mol. Biol. Plants 2014, 20, 279–285. [Google Scholar] [CrossRef]
- Gorczyca, A.; Pociecha, E.; Kasprowicz, M.; Niemiec, M. Effect of nanosilver in wheat seedlings and Fusarium culmorum culture systems. Eur. J. Plant Pathol. 2015, 142, 251–261. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Wu, F.; Zhang, X.; Wang, B.; Yang, Y.; Shen, G.; Liu, J.; Tao, S.; Wang, X. Application of TiO2 nanoparticles to reduce bioaccumulation of arsenic in rice seedlings (Oryza sativa L.): A mechanistic study. J. Hazard Mater. 2021, 405, 124047. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H.; Singh, S.C. Plant-nanoparticle interaction: An approach to improve agricultural practices and plant productivity. Int. J. Pharm. Sci. Invent. 2015, 4, 25–40. [Google Scholar]
- Olchowik, J.; Bzdyk, R.; Studnicki, M.; Bederska-Błaszczyk, M.; Urban, A.; Aleksandrowicz-Trzcińska, M. The Effect of Silver and Copper Nanoparticles on the Condition of English Oak (Quercus robur L.) Seedlings in a Container Nursery Experiment. Forests 2017, 8, 310. [Google Scholar] [CrossRef]
- Moussa, S.H.; Tayel, A.A.; Alsohim, A.S.; Abdallah, R.R. Botryticidal activity of nanosized silver-chitosan composite and its application for the control of gray mold in strawberry. J. Food Sci. 2013, 78, M1589–M1594. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Kim, H.-J.; Su Kim, J.; Kim, M.-S.; Yoon, B.-D.; Park, H.-J.; Kim, C.Y. A nanosized Ag–silica hybrid complex prepared by γ-irradiation activates the defense response in Arabidopsis. Radiat. Phys. Chem. 2012, 81, 180–184. [Google Scholar] [CrossRef]
- Ocsoy, I.; Paret, M.L.; Ocsoy, M.A.; Kunwar, S.; Chen, T.; You, M.; Tan, W. Nanotechnology in Plant Disease Management: DNA-Directed Silver Nanoparticles on Graphene Oxide as an Antibacterial against Xanthomonas perforans. Am. Chem. Soc. 2013, 7, 8972–8980. [Google Scholar] [CrossRef] [Green Version]
- Swingle, W.T. A List of the Kansas Species of Peronosporaceae. Trans. Annu. Meet. Kans. Acad. Sci. 1887, 11, 63. [Google Scholar] [CrossRef]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan Oligomers and Copper Sulfate Induce Grapevine Defense Reactions and Resistance to Gray Mold and Downy Mildew. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef]
- Egger, E. Prevention is essential in the fight against peronospora. Inf. Agrar. 2009, 65, 39–52. [Google Scholar]
- Jedlička, J.; Novotná, B.; Valšíková, M. Evaluation of influence of the locality, the vintage year, wine variety and fermentation process on volume of cooper and lead in wine. Potravinarstvo 2014, 8, 290–295. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ponmurugan, P.; Manjukarunambika, K.; Elango, V.; Gnanamangai, B.M. Antifungal activity of biosynthesised copper nanoparticles evaluated against red root-rot disease in tea plants. J. Exp. Nanosci. 2016, 11, 1019–1031. [Google Scholar] [CrossRef]
- Giannousi, K.; Avramidis, I.; Dendrinou-Samara, C. Synthesis, characterization and evaluation of copper based nanoparticles as agrochemicals against Phytophthora infestans. RSC Adv. 2013, 3, 21743–21752. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Application of Copper-Chitosan Nanoparticles Stimulate Growth and Induce Resistance in Finger Millet (Eleusine coracana Gaertn.) Plants against Blast Disease. J. Agric. Food Chem. 2018, 66, 1784–1790. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Physiological effects and mode of action of ZnO nanoparticles against postharvest fungal contaminants. Food Res. Int. 2017, 101, 274–279. [Google Scholar] [CrossRef]
- Wani, A.H.; Shah, M.A. A unique and profound effect of MgO and ZnO nanoparticles on some plant pathogenic fungi. J. Appl. Pharm. Sci. 2012, 2, 40–44. [Google Scholar]
- Graham, J.H.; Johnson, E.G.; Myers, M.E.; Young, M.; Rajasekaran, P.; Das, S.; Santra, S. Potential of Nano-Formulated Zinc Oxide for Control of Citrus Canker on Grapefruit Trees. Plant Dis 2016, 100, 2442–2447. [Google Scholar] [CrossRef] [Green Version]
- Luksiene, Z.; Rasiukeviciute, N.; Zudyte, B.; Uselis, N. Innovative approach to sunlight activated biofungicides for strawberry crop protection: ZnO nanoparticles. J. Photochem. Photobiol. B 2020, 203, 111656. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, e1804838. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Han, H. Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surf B Biointerfaces 2013, 103, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Han, H. A new function of graphene oxide emerges: Inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. J. Nanoparticle Res. 2013, 15, 1658. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Chen, J.; Han, H.; Yuan, Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Parveen, A.; Ahmad, L.; Hashem, A. Effects of graphene oxide and zinc oxide nanoparticles on growth, chlorophyll, carotenoids, proline contents and diseases of carrot. Sci. Hortic. 2019, 249, 374–382. [Google Scholar] [CrossRef]
- Deshpande, A.S.; Khomane, R.B.; Vaidya, B.K.; Joshi, R.M.; Harle, A.S.; Kulkarni, B.D. Sulfur Nanoparticles Synthesis and Characterization from H2S Gas, Using Novel Biodegradable Iron Chelates in W/O Microemulsion. Nanoscale Res. Lett. 2008, 3, 221–229. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Ghosh, M.; Mandal, A.; Chakravorty, D.; Pal, M.; Pradhan, S.; Goswami, A. Surface-modified sulfur nanoparticles: An effective antifungal agent against Aspergillus niger and Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2011, 90, 733–743. [Google Scholar] [CrossRef]
- Rao, K.J.; Paria, S. Use of sulfur nanoparticles as a green pesticide on Fusarium solani and Venturia inaequalis phytopathogens. RSC Adv. 2013, 3, 10471–10478. [Google Scholar] [CrossRef]
- Imada, K.; Sakai, S.; Kajihara, H.; Tanaka, S.; Ito, S. Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant Pathol. 2016, 65, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Shenashen, M.; Derbalah, A.; Hamza, A.; Mohamed, A.; El Safty, S. Antifungal activity of fabricated mesoporous alumina nanoparticles against root rot disease of tomato caused by Fusarium oxysporium. Pest Manag. Sci. 2017, 73, 1121–1126. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Zhao, P.; Zhou, Z.; Cao, C.; Li, F.; Huang, Q. Emulsion-based synchronous pesticide encapsulation and surface modification of mesoporous silica nanoparticles with carboxymethyl chitosan for controlled azoxystrobin release. Chem. Eng. J. 2018, 348, 244–254. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chen, C.; Liu, B.; Cai, D.; Wu, Z. Fabrication of a pH-Responsively Controlled-Release Pesticide Using an Attapulgite-Based Hydrogel. ACS Sustain. Chem. Eng. 2017, 6, 1192–1201. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2014, 35, 47–66. [Google Scholar] [CrossRef]
- Yusoff, S.N.M.; Kamari, A.; Aljafree, N.F.A. A review of materials used as carrier agents in pesticide formulations. Int. J. Environ. Sci. Technol. 2016, 13, 2977–2994. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chi, Y.; Cai, D.; Wu, Z. Fabrication of a controllable nanopesticide system with magnetic collectability. Chem. Eng. J. 2017, 328, 320–330. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sidhu, M.C.; Dilbaghi, N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. [Google Scholar] [CrossRef]
- Atta, S.; Bera, M.; Chattopadhyay, T.; Paul, A.; Ikbal, M.; Maiti, M.K.; Singh, N.D.P. Nano-pesticide formulation based on fluorescent organic photoresponsive nanoparticles: For controlled release of 2,4-D and real time monitoring of morphological changes induced by 2,4-D in plant systems. RSC Adv. 2015, 5, 86990–86996. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Cao, C.; Zhang, J.; Li, F.; Huang, Q. Quaternized Chitosan-Capped Mesoporous Silica Nanoparticles as Nanocarriers for Controlled Pesticide Release. Nanomaterials 2016, 6, 216. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Translocation, distribution and degradation of prochloraz-loaded mesoporous silica nanoparticles in cucumber plants. Nanoscale 2018, 10, 1798–1806. [Google Scholar] [CrossRef]
- Yu, M.; Sun, C.; Xue, Y.; Liu, C.; Qiu, D.; Cui, B.; Zhang, Y.; Cui, H.; Zeng, Z. Tannic acid-based nanopesticides coating with highly improved foliage adhesion to enhance foliar retention. RSC Adv. 2019, 9, 27096–27104. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Marzouk, N.M.; Abd-Alrahman, H.A.; El-Tanahy, A.M.M.; Mahmoud, S.H. Impact of foliar spraying of nano micronutrient fertilizers on the growth, yield, physical quality, and nutritional value of two snap bean cultivars in sandy soils. Bull. Natl. Res. Cent. 2019, 43, 84. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Pallavi; Mehta, C.M.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 2016, 6, 254. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span. J. Agric. Res. 2016, 14, e0902. [Google Scholar] [CrossRef]
- Lopes, T.; Cruz, C.; Cardoso, P.; Pinto, R.; Marques, P.; Figueira, E. A Multifactorial Approach to Untangle Graphene Oxide (GO) Nanosheets Effects on Plants: Plant Growth-Promoting Bacteria Inoculation, Bacterial Survival, and Drought. Nanomaterials 2021, 11, 771. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Sharifi, R. Effect of seed priming and foliar application with micronutrients on quality of forage corn (Zea mays). Environ. Exp. Biol. 2016, 14, 151–156. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Amanzadeh, T.; Sabaghnia, N.; Dashti, S. Impact of foliar application of nano micronutrient fertilizers and titanium dioxide nanoparticles on the growth and yield components of barley under supplemental irrigation. Acta Agric. Slov. 2016, 107, 265–276. [Google Scholar] [CrossRef]
- Sharifan, H.; Wang, X.; Guo, B.; Ma, X. Investigation on the Modification of Physicochemical Properties of Cerium Oxide Nanoparticles through Adsorption of Cd and As(III)/As(V). ACS Sustain. Chem. Eng. 2018, 6, 13454–13461. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Malik, S.; Adrees, M.; Qayyum, M.F.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 2019, 41, 35. [Google Scholar] [CrossRef]
- Bao-Shan, L.; Shao-Qi, D.; Chun-Hui, L.; Li-Jun, F.; Shu-Chun, Q.; Min, Y. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Ashkavand, P.; Tabari, M.; Aliyari, F.; Zarafshar, M.; Striker, G.G.; Shukla, P.K.; Sattarian, A.; Misra, P. Nanosilicon Particle Effects on Physiology and Growth of Woody Plants. In Phytotoxicity of Nanoparticles; Faisal, M., Saquib, Q., Alatar, A.A., Al-Khedhairy, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 285–299. [Google Scholar]
- Qi, Y.; Lian, K.; Wu, Q.; Li, Y.; Danzy, M.; Menard, R.; Chin, K.L.; Collins, D.; Oliveria, F.; Klepzig, K. Potentials of nanotechnology application in forest protection. In Proceedings of the TAPPI International Conference on Nanotechnology for Renewable Materials, Washington, DC, USA, 6–8 June 2011; TAPPI Press: Peachtree Corners, Georgia, 2013; pp. 271–278, ISBN 978-1-61839-440-8. [Google Scholar]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Raja, N. Biopesticides and Biofertilizers: Ecofriendly Sources for Sustainable Agriculture. J. Fertil. Pestic. 2013, 4, e112. [Google Scholar] [CrossRef]
- Mahil, E.I.T.; Kumar, B.N.A. Foliar application of nanofertilizers in agricultural crops—A review. J. Farm. Sci. 2019, 32, 239–249. [Google Scholar]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y. Effect of foliar spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of foxtail millet (Setaria italica L.) under field conditions. Nanomaterials 2019, 9, 1559. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Ďurišová, Ľ.; Bujdoš, M.; Černý, I.; Chlpík, J.; Juriga, M. Effects of Foliar Application of ZnO Nanoparticles on Lentil Production, Stress Level and Nutritional Seed Quality under Field Conditions. Nanomaterials 2022, 12, 310. [Google Scholar] [CrossRef]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of nanoparticles alleviates heavy metals stress and promotes plant growth: An overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Schoenfisch, M.H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [CrossRef]
- Jatav, G.K.; De, N. Application of nano-technology in soil-plant system. Asian J. Soil Sci. 2013, 8, 176–184. [Google Scholar]
- Corradini, E.; de Moura, M.R.; Mattoso, L.H.C. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Preetha, P.S.; Balakrishnan, N. A Review of Nano Fertilizers and Their Use and Functions in Soil. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3117–3133. [Google Scholar] [CrossRef]
- Lee, W.-M.; An, Y.-J.; Yoon, H.; Kweon, H.-S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Krug, H.F.; Height, M. 120 years of nanosilver history: Implications for policy makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef] [PubMed]

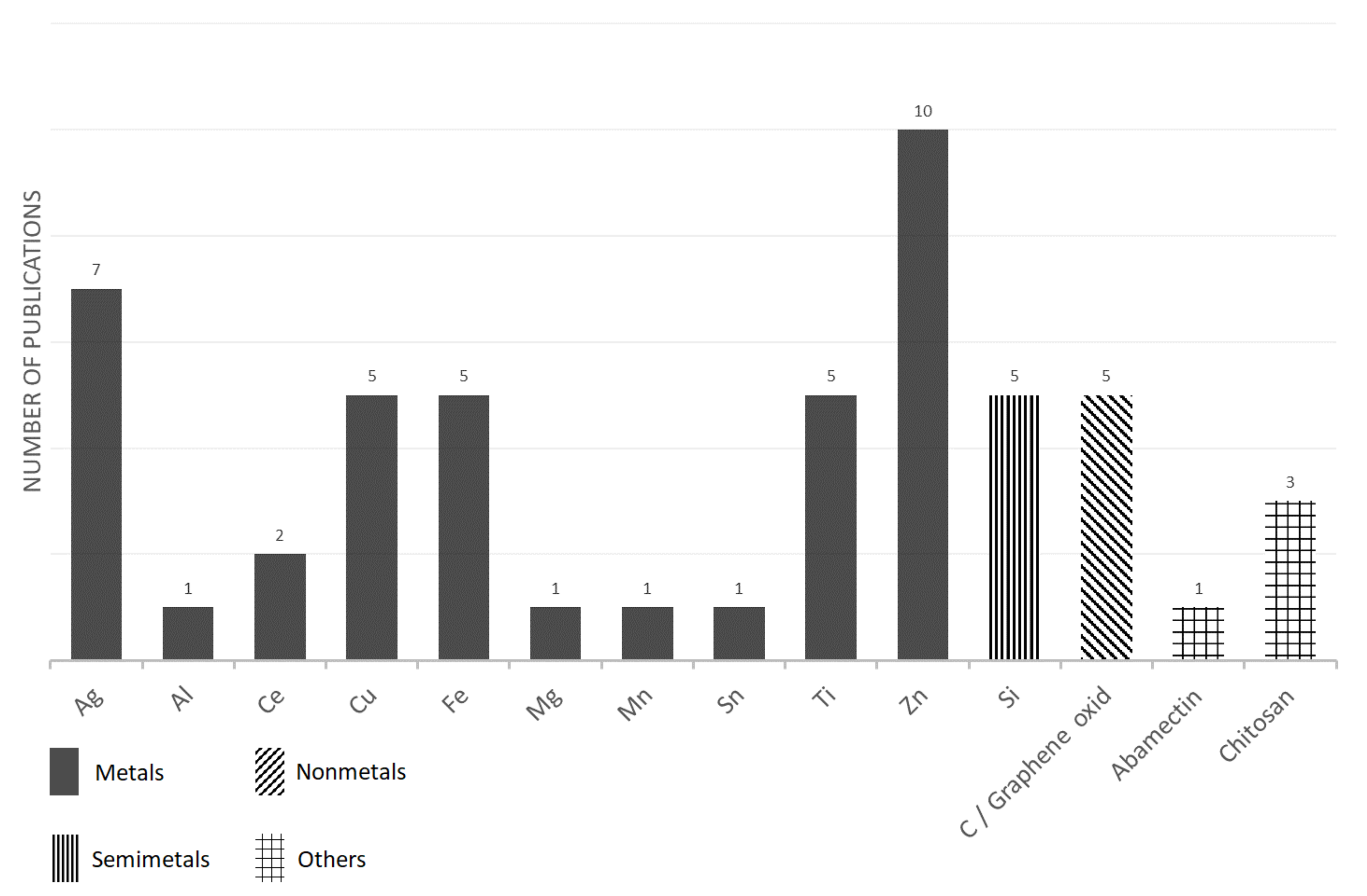
| Principal Component of Nanoparticle/Size/Other Characters * | Plant Species | Effect on Seedlings | Reference |
|---|---|---|---|
| CuO/6.6 nm /NA | Lactuca sativa L. | Lower concentrations (up to 40 μg × ml−1) slightly increase plant germination | [132] |
| ZnO/NA/NA | Chili pepper | Significant effect on seed germination growth—higher concentrations support germination, root elongation, length of the aerial part and overall plant growth | [133] |
| AgNPs/2, 22, 29 nm/spherical | Brassica oleracea L. | Xanthomonas campestris pv. campestris elimination—nanoparticles were more effective than standard hot water treatment | [93] |
| FeS/6–20 nm/spherical with slight agglomeration | Oryza sativa L. | Fusarium verticillioides elimination effect on the integrity of the cell membrane of the pathogen, along with adverse effects on the reproduction of the pathogen | [98] |
| ZnO/35–40 nm/rod morphology; TiO2/100 nm/spherical; Ag/85 nm/needle morphology | Capsicum annum L. | Aspergillus flavus, Aspergillus niger, Aspergillus fumigatus and Colletotrichum capsici elimination, improved germination by increasing nitrate reductase, antioxidant activity and reactivity of phytohormones | [99] |
| Chitosan-guar/<100 nm/spherical in agglomerates | Oryza sativa L. | Pyricularia grisea and Xanthomonas oryzae elimination, support for germination and plant growth—faster germination, greater root growth, significant increase in chlorophyll | [100] |
| Zn-chitosan/200–300 nm/spherical | Zea mays L. | Curvularia lunata elimination, positive effect on plant growth—higher percentage of chlorophyll, speed of root and plant growth, earlier maturity, spike length | [101] |
| Cinnamaldehyde encapsulated in alginate/50–300 nm/mostly spherical | Pisum sativum L. | Pseudomonas syringae pv. pisi elimination, significantly faster seed germination, development of stronger plant parts, longer pods with more seeds | [102] |
| Pt/3.2 nm/Face-Centered Cubic crystal structure; Ag/3.4 nm/NA; Au/2.6 nm/NA | Pisum sativum L. | Inhibition of rhizobial colonization and arbuscular mycorrhizal fungi, decrease in germination, significant increase in yield—number of fruits and seeds, the fatal toxic effect of Pt on Peperomia pellucida (L.) | [125] |
| SiO2/NA/NA; Pd/NA/NA; Au/NA/NA; Cu/NA/NA | Lactuca sativa L. | The antimicrobial effect on soil microflora, decrease in root length, increase in stem length | [103] |
| ZnO/NA/NA | Triticum aestivum L. | Reducing drought stress—increase in the percentage of chlorophyll; increased content of carotenoids—increased photoprotection of plants | [106] |
| SiO2/NA/NA | Triticum aestivum L. | Reducing drought stress—higher number of active reaction centers, high absorbance, trapping, and electron transport | [107] |
| Cu0/30–40 nm/NA | Zea mays L. | Mitigation of the physiological effects of drought, chlorophyll and carotenoid content and increased activity of antioxidant enzymes | [105] |
| Fe2O3/19–30 nm/spherical | Citrullus lanatus (Thunb.) | Influencing the production of phytohormones—increased synthesis of 12-oxo-phytodienoic acid (cis-OPDA) and jasmonic acid | [108] |
| Mn/50 nm/spherical | Capsicum anuum L. | Reduction in the effect of soil salinity—specific redistribution of elements in the plant body, higher roots elongation, regulation of manganese superoxide dismutase production | [110] |
| Fe2O3/<50 nm/specific surface area of 180 m2/g | Sorghum bicolor (L.) Moench | Reduction in the effect of soil salinity—the highest increase in stomatal conductance and transpiration rate, increased chlorophyll a, b, carotenoids and relative water content | [111] |
| ZnO/21 nm/crystalline | Lupinus albus L. | Reduction in the effect of soil salinity—improving photosynthesis by increasing chlorophyll a, chlorophyll b and carotenoids, increased activity of antioxidant enzymes | [112] |
| ZnO/25 nm/spherical and hexagonal shapes; Se/10–55 nm/spherical | Brassica napus L. | Reduction in the effect of soil salinity—shortening the germination time, increased activity of metabolites, increased activity of antioxidant enzymes | [113] |
| ZnO/20-30 nm/NA; Fe/50–100 nm/NA | Triticum aestivum L. | Reduction in Cd uptake from soil—increased elongation of plants and roots, the significant increase in the dry weight of shoots, roots, cobs and grains, significant influence on photosynthetic parameters such as chlorophyll a, chlorophyll b, carotenoids | [114] |
| Si/NA/NA | Triticum aestivum L. | Reduction in Cd uptake from soil—growth improvement, shoot and root dry weight, shoot length, grain weight and ear length and ear dry weight, improved photosynthesis and increased chlorophyll content, reduction of reactive oxygen species values | [115] |
| Fe/20–30 nm/NA; Cu/15–30 nm/NA | Triticum aestivum L. | Increased germination of three varieties—activated proteins involved in the process of seed germination were detected | [116] |
| Ag/6–36 nm/spherical and ellipsoidal; Ag/40 nm/spherical | Oryza sativa L. | Improvement germination and starch metabolism of aged rice seeds—acceleration of water intake, increase in α-amylase activity | [85] |
| Vicia faba L. | Reduction in the genotoxic effects—plumule fresh and dry weight and water content were non-significant, significant root elongation, the significant increase in vitality index | [121] | |
| MgO/12 nm/Face Centered Cubic structure | Vigna radiata L. | Increase in germination, % germination and elongation of seedlings and roots, increased chlorophyll content | [122] |
| Mn/21 nm/Cubic-shaped with hydrophilic character | Vigna radiata L. | Positive effect on nitrate intake—increases in both Nitrate reductase and Nitrite reductase activities in root and leaf (testing NPs on mice has not shown a danger of manganism to mammals) | [124] |
| CuO/8–9 nm/spherical; CuO coated with APTES/10–12 nm/spherical in agglomerates | Coriandrum sativum L. | Positive effect on germination with increasing amount of absorbed NPs | [123] |
| Ag/29 nm/spherical and ellipsoidal | Citrullus lanatus (Thunb.) | Increased germination—monitoring of carbohydrate metabolism confirmed the beneficial effect of Nanopriming, increase in the content of photosynthetic pigments, larger stem diameter, longer shoot length, and higher fruit yield, Ag was detected in the seeds of the fruit | [120] |
| ZnO/13 nm/spherical in agglomerates | Triticum aestivum L. | Increased germination and growth—significant increase in the length of roots, shoots and leaves, no significant effect on the number of roots | [130] |
| Zn-30/30 nm /spherical; Zn-50/50 nm /spherical; Zn-90/90 nm /columnar; Zn-150/150 nm /hexagonal rod-like | Brassica pekinensis L. | Germination not affected, significant inhibition of root growth, less inhibition of shoot growth, smaller NPs showed greater phytotoxicity | [131] |
| Ag2S/10–50 nm/spherical in agglomerates; ZnS/5–80 nm/spherical, rod, bean like | Glycine max L., Triticum aestivum L. | Increased germination but slowing plant growth, longer root and shoot length, longer soaking time and higher concentration caused inhibition of germination and growth | [126] |
| FeO/20–50 nm/irregular surfaces | Oryza sativa L. | Increased germination—faster water absorption, increased % germination, shorter germination time, significant stimulation of α-amylase and antioxidant enzymes | [127] |
| Fe2O3/80 nm/irregular | Triticum aestivum L. | Increased germination—significantly higher shoot growth, higher chlorophyll formation, nanopriming caused significant accumulation of Fe in harvested seeds | [128] |
| Fe/SiO/30–40 nm/spherical | Zea mays L., Hordeum vulgare | Increased germination—faster germination and plant growing | [129] |
| Principal Component of Nanoparticle/Size/Other Characters * | Plant Species | Effect on Seedlings | Effect on Plant Physiology | Reference |
|---|---|---|---|---|
| Ag/15-100 nm/spherical | Triticum aestivum L. | Reducing the infestation of seedlings by Fusarium culmorum, inhibiting plant growth | Induction of photosynthesis | [135] |
| Cu/NA/NA; Ag/NA/NA | Quercus robur L. | Support of ectomycorrhizal colonization, inhibition of Erysiphe alphitoides | Change of plastids shape and starch content | [138] |
| Ag/20–100 nm/ spherical | Gossypium hirsutum L. | Xanthomonas axonopodis pv. Malvacearum and Xanthomonas campestris pv. Campestris elimination | NA, but no phytotoxicity was observed | [109] |
| Ag-chitosan/≤100 nm/nano composite | Fragaria × ananassa Duchesne ex Rozier | Botritis cinerea elimination | Coated strawberry with nano CTS-Ag had a fresh-like appearance after storage. | [139] |
| Ag-Si/30 nm/spherical | Arabidopsis thaliana L. | Pseudomonas syringae pv. Tomato elimination | NP regulated the expression of SAR marker genes such as PR1, PR2 and PR5 in plants | [140] |
| Ag grown on graphene oxide/± 18 nm ± 5 nm/spherical on the surface of the GO layer | Lycopersicon esculentum Mill. | Xanthomonas perforans elimination | NA, but no phytotoxicity was observed | [141] |
| Cu/5–50 nm/spherical in aggregates | Camellia sinensis Kuntze | Poria hypolateritia elimination | A significant increase in the yield of tea leaves | [147] |
| Cu, CuO, Cu2O Cu/Cu2O/11–55 nm/spherical | Lycopersicon esculentum Mill. | Phytophthora infestans elimination | NA, but no phytotoxicity was observed | [148] |
| Cu-chitosan/88 nm/spherical | Eleusine coracana Gaertn. | Pyricularia grisea suppression | CuChNp treatment interferes with the action of endogenous plant hormones and induces changes in the growth profile of treated plants | [149] |
| Zinkicide SG4/0.2–0.5 nm/ plate-like;Zinkicide SG6/4–6 nm/gel-like structure | Citrus × paradisi Macfad. | Xanthomonas citri subsp. Citri, Escherichia coli and Xanthomonas alfalfae subsp. Citrumelonis elimination | No specific risk of the zinc to plants was observed | [153] |
| ZnO/25–1500 nm/agglomerated | Fragaria × ananassa Duchesne ex Rozier | Botritis cinerea elimination | Increased flower’s production, reduced growth of runners | [154] |
| GO/0.76 nm/layer; rGO/1,59 nm/layer | Oryza sativa L. | Xanthomonas oryzae pv. oryzae elimination | NA | [157] |
| GO/NA/layer, ZnO/≤ 40 nm/NA | Daucus carota L. | Pectobacterium carotovorum, Xanthomonas campestris pv. carotae, Meloidogyne javanica, Alternaria dauci and Fusarium solani elimination | Significant increase in the content of chlorophyll, carotenoids, proline and overall plant growth | [159] |
| MgO/20–200 nm/crystalline | Lycopersicon esculentum Mill. | Ralstonia solanacearum elimination | Resistance induced by NPs by activation of SA-, JA- and ET-signaling pathways and with accumulation of β-1,3-glucanase and tylose | [163] |
| Al2O3/100–250 nm/spherical | Lycopersicon esculentum Mill. | Fusarium oxysporium elimination | Increase of plant height, fresh weight and dry weight | [164] |
| CH3CO-PLA-NS/543 nm/agglomerate; HOOC-PLA-NS/456 nm/agglomerate; H2N-PLA-NS/429 nm/agglomerate | Cucumis sativus L. | Streptomyces avermitilis elimination | The efficient deposition and strong adhesion of pesticides on the leaf surface | [10] |
| ZnO/15–137 nm/mostly spherical | Coffea arabica L. | Increased growth and biomass production | Increase in fresh weight of roots and leaves | [175] |
| ZnO/1.2–6.8 nm/oblate spherical and hexagonal | Cyamopsis tetragonoloba L. | Increased growth and biomass production | Improvement in shoot length, root length, root area, chlorophyll content, total soluble leaf protein, rhizospheric microbial population, acid phosphatase, alkaline phosphatase and phytase | [176] |
| ZnO/16–30 nm/spherical | Cicer arietinum L. | Effect on biomass production | Improving total dry matter accumulation | [24] |
| Zn/NA/NA; Fe/NA/NA; Mn/NA/NA | Phaseolus vulgaris L. | Increased growth and biomass production | NA | [177] |
| TiO2/25 nm/; ZnO/28 nm/ | Lycopersicon esculentum Mill. | Increased growth and biomass production | TiO2 and ZnO (>250 mg.kg−1) caused root reduction | [178] |
| Ag/35–40 nm/NA | Triticum aestivum L.,Vigna sinensis (L.) Endl. ex Hassk., Brassica juncea L. | Increased growth and biomass production | Increased root nodulation (Vigna) | [179] |
| chitosan-NPK/26–30 nm/NA | Triticum aestivum L. | Increased growth and biomass production, higher and earlier yield | NA | [180] |
| GO/300 nm–5µm/multilayers nanolayers | Zea mays L. | Effect on biomass production; without effect on bacteria | A significant negative effect on root growth was observed in watered plants | [181] |
| Fe2O3/10–50 nm/spherical | Arachis hypogaea L. | Increasing plant height and iron content in plants | Regulation of phytohormone content and antioxidant enzymatic activity | [182] |
| Fe/NA/NA; Zn/NA/NA | Zea mays L. | Increased growth and biomass production | Increasing the phosphorus, leaf chlorophyll, crude protein andsoluble carbohydrate concentration compared to chemicalforms | [183] |
| TiO2/30 nm/oblate spherical; ZnO/20 nm/spherical; Fe2O3/80 nm/spherical | Hordeum vulgare L. | Increase in growth properties and greater grain production | The chlorophyll content was significantly increased—increased photosynthesis—due to the increased formation of assimilates, the number and weight of seeds increased | [184] |
| TiO2/NA/anatase and rutile structures | Oryza sativa L. | Significant reduction in intake of As plant roots | Prevention of penetration of As into the root, restriction of movement and isolation in vacuoles in the root cells | [136] |
| CeO2 + polyvinilpyrolidon/30–50 nm/spherical | synthetic root environment | Suppression of Cd and As uptake from soil solution in the presence of synthetic root exudates (SRE) | NA | [185] |
| Si/NA/NA; TiO2/NA/NA | Oryza sativa L. | Increase in biomass production and a lower accumulation of cadmium in plants | Increase in dry weight, increase in chlorophyll content—acceleration of photosynthesis, stomatal conductance, transpiration rate and activity of anti-oxidative enzymes | [186] |
| SiO2/NA/NA | Larix sp. | Increased growth | Increase in chlorophyll content | [187] |
| SiO2/10–15 nm/amorphous | Prunus mahaleb L., Crataegus azarolus L. | Increasing the growth and efficiency of photosynthesis | Slight improvement in leaf physiological performance and root elongation | [188] |
| Cu encapsulated in a carbon shell/50 nm/spherical | Taxodium distichum | Wood protection against Trametes versicolor L., Ophiostoma minus Syd and P.Syd and Coptotermes formosanus Shiraki | Facilitate copper transport by plant roots and increased Cu uptake | [189] |
| CeO2 NPs/4 nm/specific surface area; Fe2O3 NPs/6 nm/hematite phase;SnO2 NPs/6 nm/NA; TiO2 NPs/6 nm/anatase phaseSiO2 NPs/10 nm/specific surface area | Lactuca sativa L. var. foliosa | An effect on the metabolism and modification of the physiological functions of the plants was observed | Influence on the amount and activity of APX, GPOX, CAT, GSH, carotenoids, chlorophyll A + B and on the amount of dry matter | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohlmuth, J.; Tekielska, D.; Čechová, J.; Baránek, M. Interaction of the Nanoparticles and Plants in Selective Growth Stages—Usual Effects and Resulting Impact on Usage Perspectives. Plants 2022, 11, 2405. https://doi.org/10.3390/plants11182405
Wohlmuth J, Tekielska D, Čechová J, Baránek M. Interaction of the Nanoparticles and Plants in Selective Growth Stages—Usual Effects and Resulting Impact on Usage Perspectives. Plants. 2022; 11(18):2405. https://doi.org/10.3390/plants11182405
Chicago/Turabian StyleWohlmuth, Jan, Dorota Tekielska, Jana Čechová, and Miroslav Baránek. 2022. "Interaction of the Nanoparticles and Plants in Selective Growth Stages—Usual Effects and Resulting Impact on Usage Perspectives" Plants 11, no. 18: 2405. https://doi.org/10.3390/plants11182405
APA StyleWohlmuth, J., Tekielska, D., Čechová, J., & Baránek, M. (2022). Interaction of the Nanoparticles and Plants in Selective Growth Stages—Usual Effects and Resulting Impact on Usage Perspectives. Plants, 11(18), 2405. https://doi.org/10.3390/plants11182405






