Impact of Biochar Application at Water Shortage on Biochemical and Physiological Processes in Medicago ciliaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Growth Parameter
2.2.1. Chlorophyll Fluorescence
2.2.2. CO2/H2O Gas Exchange
2.2.3. Chlorophyll Content
2.2.4. Proline Content
2.2.5. Lipid Peroxidation
2.2.6. Hydrogen Peroxide Content
2.3. Protein Quantification and Antioxidant Enzyme Assay
2.4. Extraction and Determination of Non-Enzymatic Antioxidant Ascorbate (AsA) and Dehydro-Ascorbate (DHAsA)
2.5. Statistics
3. Results
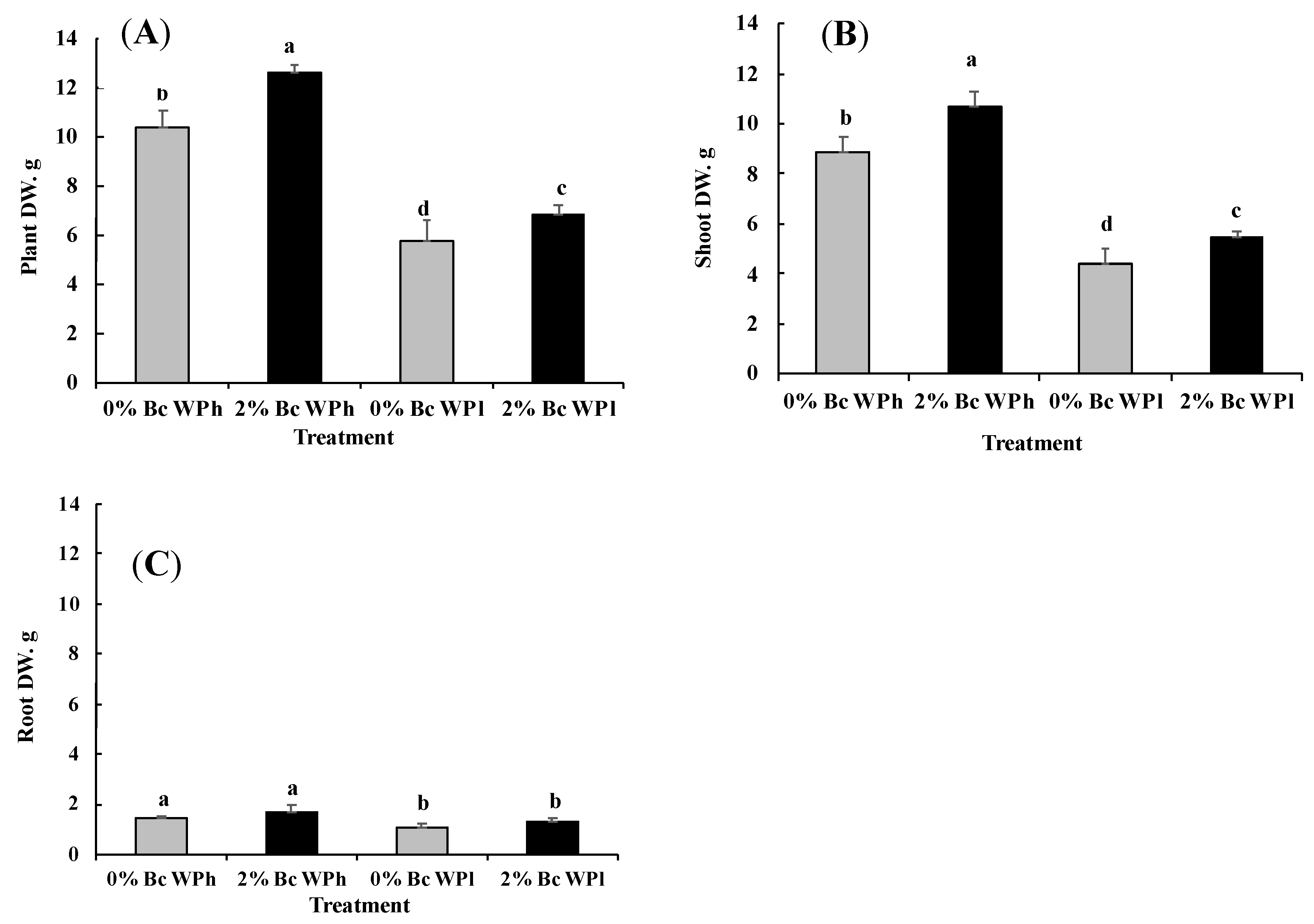
3.1. Growth
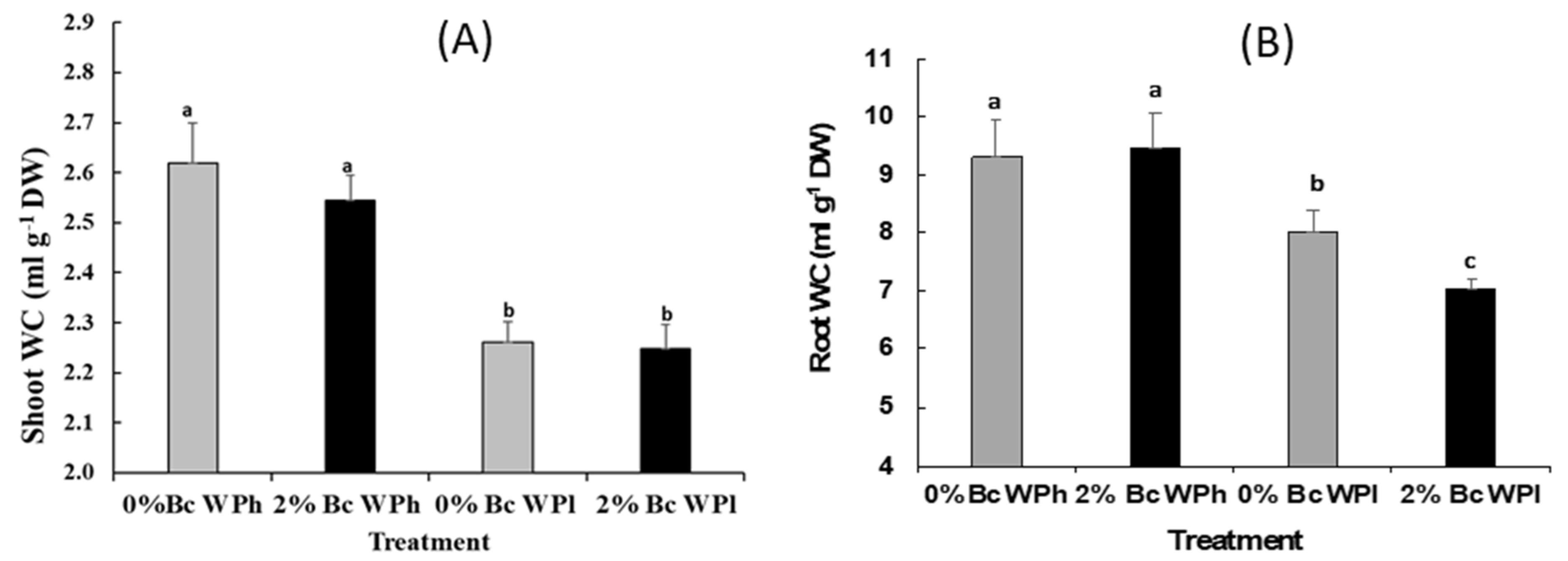
3.2. Tissue Water Status
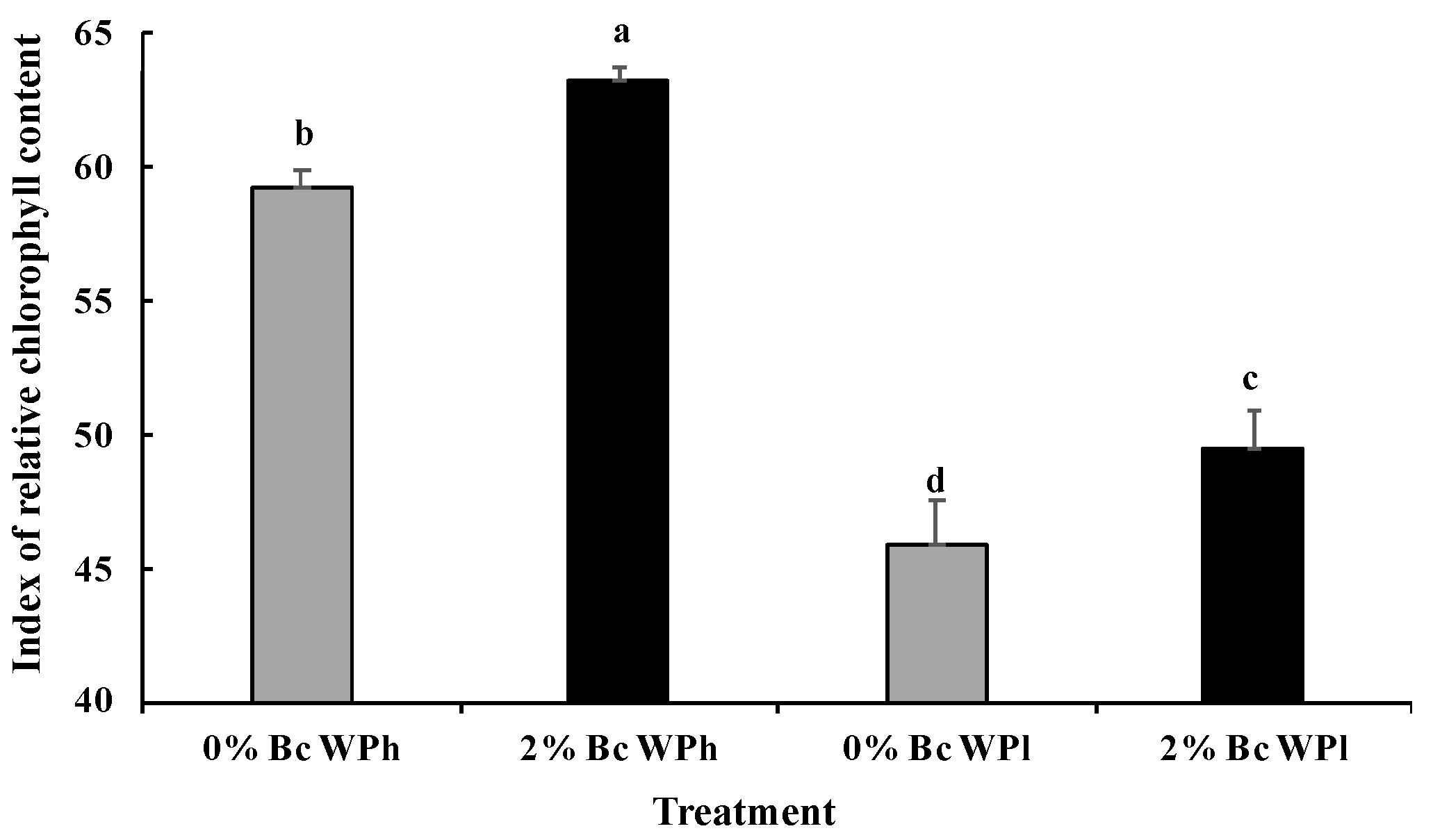
3.3. Chlorophyll and Protein Content
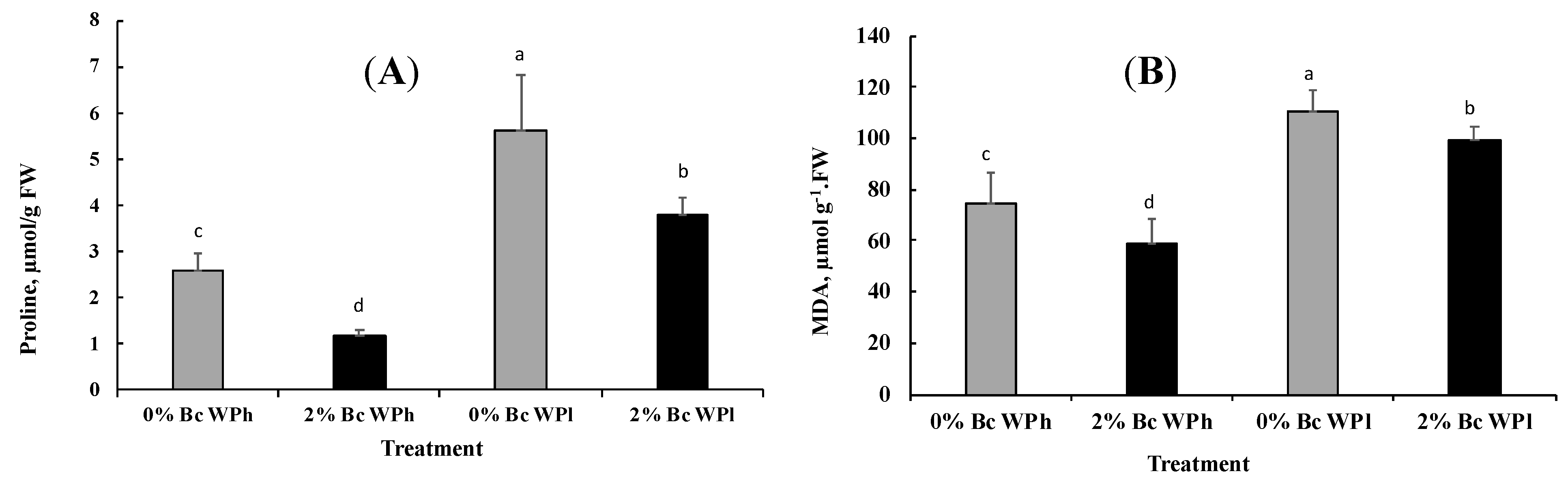
3.4. Proline and MDA Accumulation
3.5. Leaf CO2/H2O Gas Exchange
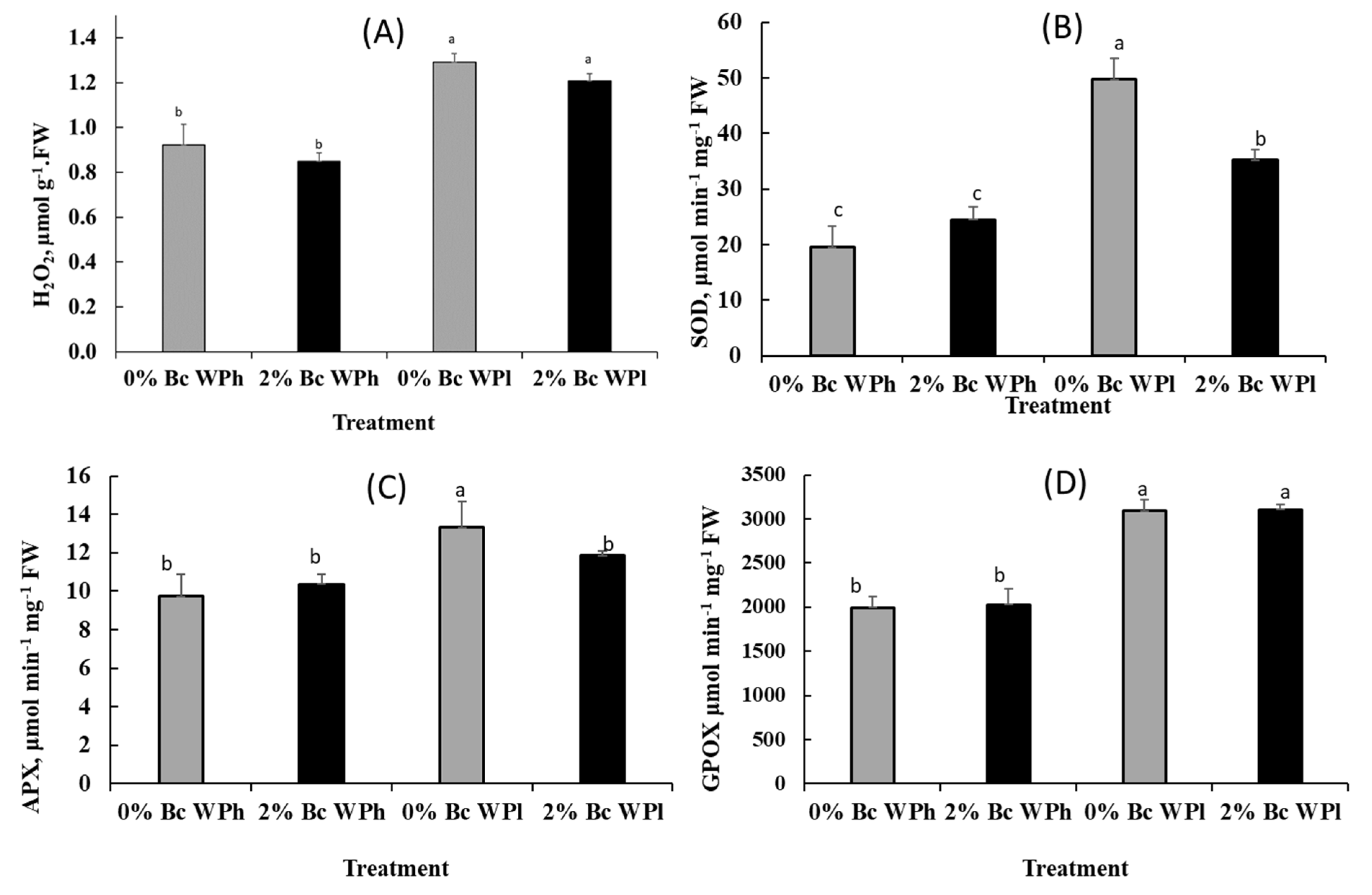
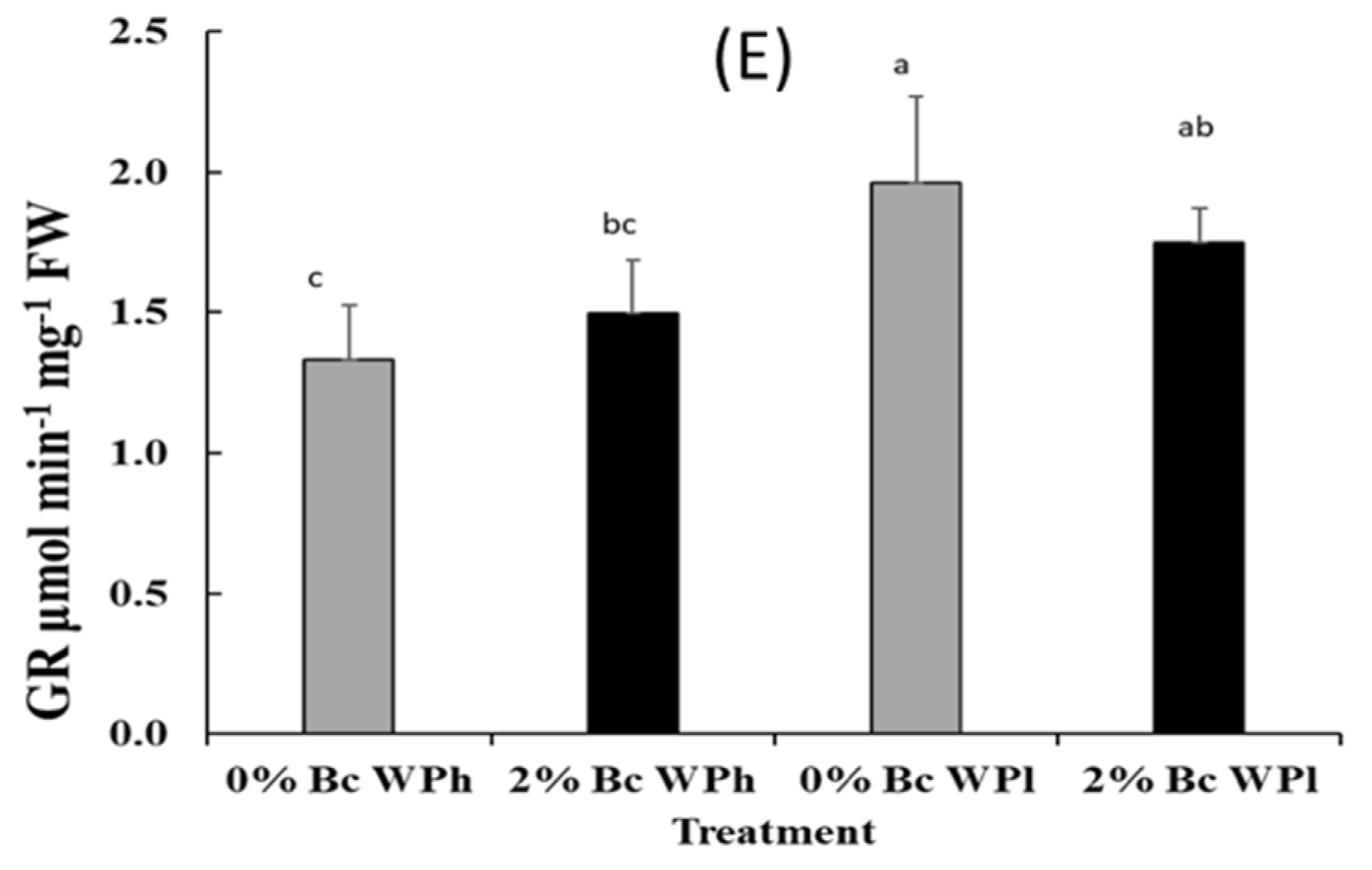
3.6. Enzymatic Antioxidant Assays
3.7. Non Enzymatic Antioxidant Assays: Ascorbate Determination
4. Discussion
4.1. Adjustment of Growth and Water Relations
4.2. Regulation of Photosynthesis
4.3. Indicators of Oxidative Stress
4.4. Photoprotective Mechanisms: Enzymatic Oxidants
4.5. Photoprotective Mechanisms: Non-Enzymatic Antioxidants
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 2391. [Google Scholar] [CrossRef]
- Chakraborty, U.; Pradhan, B. Oxidative stress in five wheat varieties (Triticum aestivum L.) exposed to water stress and study of their antioxidant enzyme defense system, water stress responsive metabolites and H2O2 accumulation. Braz. J. Plant Physiol. 2012, 24, 117–130. [Google Scholar] [CrossRef]
- Zubieta, Á.S.; Savian, J.V.; de Souza Filho, W.; Wallau, M.O.; Gómez, A.M.; Bindelle, J.; Bonnet, O.J.F.; Carvalho, P.C.d.F. Does grazing management provide opportunities to mitigate methane emissions by ruminants in pastoral ecosystems? Sci. Total Environ. 2021, 754, 142029. [Google Scholar] [CrossRef] [PubMed]
- Volaire, F. A unified framework of plant adaptive strategies to drought: Crossing scales and disciplines. Glob. Change Biol. 2018, 24, 2929–2938. [Google Scholar] [CrossRef]
- Singh, S.; Bainsla, N.K. Analysis of climate change impacts and their mitigation strategies on vegetable sector in tropical islands of Andaman and Nicobar Islands, India. J. Hortic. 2015, 2, 1–5. [Google Scholar]
- Ramazan, S.; Bhat, H.A.; Zargar, M.A.; Ahmad, P.; John, R. Combined gas exchange characteristics, chlorophyll fluorescence and response curves as selection traits for temperature tolerance in maize genotypes. Photosynth. Res. 2021, 150, 213–225. [Google Scholar] [CrossRef]
- Holman, I.P.; Hess, T.; Rey, D.; Knox, J. A multi-level framework for adaptation to drought within temperate agriculture. Front. Environ. Sci. 2021, 8, 282. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Liu, T.; Liu, Y. Impacts of heat and drought on gross primary productivity in China. Remote Sens. 2021, 13, 378. [Google Scholar] [CrossRef]
- Rozema, J.; Flowers, T. Crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef]
- Hirich, A.; Choukr-Allah, R.; Ragab, R. Emerging Research in Alternative Crops; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Elouafi, I.; Shahid, M.A.; Begmuratov, A.; Hirich, A. The Contribution of Alternative Crops to Food Security in Marginal Environments. In Emerging Research in Alternative Crops; Springer: Cham, Switzerland, 2020; pp. 1–23. [Google Scholar]
- Alandia, G.; Pulvento, C.; Sellami, M.; Hoidal, N.; Anemone, T.; Nigussie, E.; Aguero, J.J.; Lavini, A.; Jacobsen, S.-E. Grain legumes may enhance high-quality food production in Europe. In Emerging Research in Alternative Crops; Springer: Cham, Switzerland, 2020; pp. 25–53. [Google Scholar]
- Reid, J.; Berberet, R.; Caddel, J. Effects of alfalfa dormancy on egg and larval population levels of the alfalfa weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 1989, 82, 264–269. [Google Scholar] [CrossRef]
- Lodge, G. Seedling emergence and survival of annual pasture legumes in northern New South Wales. Aust. J. Agric. Res. 1996, 47, 559–574. [Google Scholar] [CrossRef]
- De Haan, R.L.; Sheaffer, C.C.; Barnes, D.K. Effect of annual medic smother plants on weed control and yield in corn. Agron. J. 1997, 89, 813–821. [Google Scholar] [CrossRef]
- Sheaffer, C.; Gunsolus, J.; Grimsbo Jewett, J.; Lee, S. Annual Medicago as a smother crop in soybean. J. Agron. Crop Sci. 2002, 188, 408–416. [Google Scholar] [CrossRef]
- Young, N.D.; Mudge, J.; Ellis, T.N. Legume genomes: More than peas in a pod. Curr. Opin. Plant Biol. 2003, 6, 199–204. [Google Scholar] [CrossRef]
- Howieson, J.; Ballard, R. Optimising the legume symbiosis in stressful and competitive environments within southern Australia—Some contemporary thoughts. Soil Biol. Biochem. 2004, 36, 1261–1273. [Google Scholar] [CrossRef]
- Echeverria, A.; Larrainzar, E.; Li, W.; Watanabe, Y.; Sato, M.; Tran, C.D.; Moler, J.A.; Hirai, M.Y.; Sawada, Y.; Tran, L.-S.P.; et al. Medicago sativa and Medicago truncatula show contrasting root metabolic responses to drought. Front. Plant Sci. 2021, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Badri, M.; Toumi, G.; Mahfoudh, S.; Hessini, K.; Abdelguerfi-Laouar, M.; Abdelguerfi, A.; Aouani, M.E.; Abdelly, C.; Djebali, N. Diversity of Response to Drought in a Collection of Lines of Medicago truncatula, M. ciliaris, and M. polymorpha. Crop Sci. 2016, 56, 3125–3132. [Google Scholar] [CrossRef]
- Du Toit, S.F.; Farrant, J.M.; Faigon, L.; Neta-Sharir, I.; Reich, Z. Physiological characterisation of tissue differentiation in response to desiccation in the homoiochlorophyllous dicot resurrection plant Craterostigma pumilum Hochst. Environ. Exp. Bot. 2021, 192, 104650. [Google Scholar] [CrossRef]
- Matos, I.S.; Eller, C.B.; Oliveras, I.; Mantuano, D.; Rosado, B.H. Three eco-physiological strategies of response to drought maintain the form and function of a tropical montane grassland. J. Ecol. 2021, 109, 327–341. [Google Scholar] [CrossRef]
- Upadhyay, P. Climate change and adaptation strategies: A study of agriculture and livelihood adaptation by farmers in Bardiya District, Nepal. Adv. Agr. Environ. Sci. 2019, 2, 47–52. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [Green Version]
- Salmon, Y.; Lintunen, A.; Dayet, A.; Chan, T.; Dewar, R.; Vesala, T.; Holtta, T. Leaf carbon and water status control stomatal and nonstomatal limitations of photosynthesis in trees. New Phytol. 2020, 226, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Koyro, H.-W.; Huchzermeyer, B. Coordinated regulation of photosynthesis in plants increases yield and resistance to different types of environmental stress. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 281–309. [Google Scholar]
- Siddique, Z.; Jan, S.; Imadi, S.R.; Gul, A.; Ahmad, P. Drought stress and photosynthesis in plants. In Water Stress and Crop Plants: A Sustainable Approach; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; Volume 2, pp. 1–11. [Google Scholar]
- Kocsy, G.; Tari, I.; Vanková, R.; Zechmann, B.; Gulyás, Z.; Poór, P.; Galiba, G. Redox control of plant growth and development. Plant Sci. 2013, 211, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Koyro, H.-W.; Hussain, T.; Huchzermeyer, B.; Khan, M.A. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Environ. Exp. Bot. 2013, 91, 22–29. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M.R.O. Oxidative Damage and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front. Plant Sci. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Jithesh, M.; Prashanth, S.; Sivaprakash, K.; Parida, A.K. Antioxidative response mechanisms in halophytes: Their role in stress defence. J. Genet. 2006, 85, 237–254. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Fry, S.C. The oxidation of dehydroascorbic acid and 2, 3-diketogulonate by distinct reactive oxygen species. Biochem. J. 2018, 475, 3451–3470. [Google Scholar] [CrossRef]
- Foyer, C.H.; Souriau, N.; Perret, S.; Lelandais, M.; Kunert, K.-J.; Pruvost, C.; Jouanin, L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995, 109, 1047–1057. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Gallie, D.R. Violaxanthin de-epoxidase is rate-limiting for non-photochemical quenching under subsaturating light or during chilling in Arabidopsis. Plant Physiol. Biochem. 2012, 58, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yan, J.; Ahammed, G.J.; Wang, X.; Bu, X.; Xiang, H.; Li, Y.; Lu, J.; Liu, Y.; Qi, H.; et al. PGR5/PGRL1 and NDH mediate far-red light-induced photoprotection in response to chilling stress in tomato. Front. Plant Sci. 2020, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Kammann, C.I.; Linsel, S.; Gößling, J.W.; Koyro, H.-W. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil 2011, 345, 195–210. [Google Scholar] [CrossRef]
- Paneque, M.; Turchetti, D.; Jackson, L.; Lunt, P.; Houwink, E.; Skirton, H. A systematic review of interventions to provide genetics education for primary care. BMC Fam. Pract. 2016, 17, 89. [Google Scholar] [CrossRef]
- Sharma, G.; Bhogal, S.; Gupta, V.K.; Agarwal, S.; Kumar, A.; Pathania, D.; Mola, G.T.; Stadler, F.J. Algal biochar reinforced trimetallic nanocomposite as adsorptional/photocatalyst for remediation of malachite green from aqueous medium. J. Mol. Liq. 2019, 275, 499–509. [Google Scholar] [CrossRef]
- Haider, I.; Raza, M.A.S.; Iqbal, R.; Aslam, M.U.; Habib-ur-Rahman, M.; Raja, S.; Khan, M.T.; Aslam, M.M.; Waqs, M.; Ahmad, S. Potential effects of biochar application on mitigating the drought stress implications on wheat (Triticum aestivum L.) under various growth stages. J. Saudi Chem. Soc. 2020, 24, 974–981. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental benefits of biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef]
- Case, S.D.; McNamara, N.P.; Reay, D.S.; Whitaker, J. The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil–the role of soil aeration. Soil Biol. Biochem. 2012, 51, 125–134. [Google Scholar] [CrossRef]
- Paneque, M.; José, M.; Franco-Navarro, J.D.; Colmenero-Flores, J.M.; Knicker, H. Effect of biochar amendment on morphology, productivity and water relations of sunflower plants under non-irrigation conditions. Catena 2016, 147, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Tayyab, M.; Noman, A.; Islam, W.; Waheed, S.; Arafat, Y.; Ali, F.; Zaynab, M.; Lin, S.; Zhang, H.; Lin, W. Bioethanol production from lignocellulosic biomass by environment-friendly pretreatment methods: A review. Appl. Ecol. Environ. Res. 2018, 16, 225–249. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Irfan, M.; Butt, M.; Abbas, T.; Hussain, S.; Abbas, A.; Ullah, M.S.; Cheema, M.A. Biochar application improves the drought tolerance in maize seedlings. Phyton 2019, 88, 379. [Google Scholar] [CrossRef]
- Mannan, M.; Mia, S.; Halder, E.; Dijkstra, F.A. Biochar application rate does not improve plant water availability in soybean under drought stress. Agric. Water Manag. 2021, 253, 106940. [Google Scholar] [CrossRef]
- Lyu, H.; Gong, Y.; Tang, J.; Huang, Y.; Wang, Q. Immobilization of heavy metals in electroplating sludge by biochar and iron sulfide. Environ. Sci. Pollut. Res. 2016, 23, 14472–14488. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and water culture methods used in the study of plant nutrition. In Sand and Water Culture Methods used in the Study of Plant Nutrition; Commonwealth Agricultural Bureaux: Wallingford, UK, 1952. [Google Scholar]
- Bouyoucos, C. Les propriétés physiques du sol dépendent de sa texture et de sa structure. Bases Prod. Végétale 1983, 1, 67–87. [Google Scholar]
- Zribi, O.T.; Labidi, N.; Slama, I.; Debez, A.; Ksouri, R.; Rabhi, M.; Smaoui, A.; Abdelly, C. Alleviation of phosphorus deficiency stress by moderate salinity in the halophyte Hordeum maritimum L. Plant Growth Regul. 2012, 66, 75–85. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Valentini, R.; Epron, D.; de Angelis, P.; Matteucci, G.; Dreyer, E. In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: Diurnal cycles under different levels of water supply. Plant Cell Environ. 1995, 18, 631–640. [Google Scholar] [CrossRef]
- Schulte, M.; Offer, C.; Hansen, U. Induction of CO2-gas exchange and electron transport: Comparison of dynamic and steady-state responses in Fagus sylvatica leaves. Trees 2003, 17, 153–163. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P.; Whaley, E. Growth environment and leaf anatomy affect nondestructive estimates of chlorophyll and nitrogen in Citrus sp. leaves. J. Am. Soc. Hortic. Sci. 2005, 130, 152–158. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Rao, K.M.; Sresty, T. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Zaharieva, T.; Yamashita, K.; Matsumoto, H. Iron deficiency induced changes in ascorbate content and enzyme activities related to ascorbate metabolism in cucumber roots. Plant Cell Physiol. 1999, 40, 273–280. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Ngumbi, E.; Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016, 105, 109–125. [Google Scholar] [CrossRef]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water scarcity and future challenges for food production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Ines, S.; Talbi, O.; Nasreddine, Y.; Rouached, A.; Gharred, J.; Jdey, A.; Hanana, M.; Abdelly, C. Drought tolerance traits in Medicago species: A review. Arid. Land Res. Manag. 2022, 36, 67–83. [Google Scholar] [CrossRef]
- Ahmed, R.; Li, Y.; Mao, L.; Xu, C.; Lin, W.; Ahmed, S.; Ahmed, W. Biochar effects on mineral nitrogen leaching, moisture content, and evapotranspiration after 15N urea fertilization for vegetable crop. Agronomy 2019, 9, 331. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Zhou, W. Assessment of future drought in Southwest China based on CMIP5 multimodel projections. Adv. Atmos. Sci. 2014, 31, 1035–1050. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, G.; Shao, H. Furfural and its biochar improve the general properties of a saline soil. Solid Earth 2014, 5, 665–671. [Google Scholar] [CrossRef]
- López-Galiano, M.J.; García-Robles, I.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Real, M.D.; Rausell, C. Expression of miR159 is altered in tomato plants undergoing drought stress. Plants 2019, 8, 201. [Google Scholar] [CrossRef]
- Naidu, B.P.; Paleg, L.G.; Jones, G.P. Nitrogenous compatible solutes in drought-stressed Medicago spp. Phytochemistry 1992, 31, 1195–1197. [Google Scholar] [CrossRef]
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef]
- Manolikaki, I.; Diamadopoulos, E. Positive effects of biochar and biochar-compost on maize growth and nutrient availability in two agricultural soils. Commun. Soil Sci. Plant Anal. 2019, 50, 512–526. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Haider, I.; Saleem, M.F.; Iqbal, R.; Aslam, M.U.; Ahmad, S.; Abbasi, S.H. Integrating biochar, rhizobacteria and silicon for strenuous productivity of drought stressed wheat. Commun. Soil Sci. Plant Anal. 2021, 52, 338–352. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Geissler, N.; Hussin, S.; El-Far, M.M.; Koyro, H.-W. Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ. Exp. Bot. 2015, 118, 67–77. [Google Scholar] [CrossRef]
- Turner, N.C.; Schulze, E.-D.; Gollan, T. The responses of stomata and leaf gas exchange to vapour pressure deficits and soil water content. Oecologia 1985, 65, 348–355. [Google Scholar] [CrossRef]
- He, T.; Fan, L.; Tarin, M.; Shen, S.; Xie, D.; Chen, L.; Rong, J.D.; Chen, L.G.; Zheng, Y.S. Physiological and proteomic responses of Dendrocalamus minor var. amoenus (ghost bamboo) under drought stress. Appl. Ecol. Environ. Res. 2020, 18, 4817–4838. [Google Scholar] [CrossRef]
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Keshavarz Afshar, R.; Hashemi, M.; DaCosta, M.; Spargo, J.; Sadeghpour, A. Biochar application and drought stress effects on physiological characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 2016, 47, 743–752. [Google Scholar] [CrossRef]
- Iqbal, M.T. Utilization of biochar in improving yield of wheat in Bangladesh. Bulg. J. Soil Sci. 2017, 2, 53–74. [Google Scholar]
- Lyu, S.; Du, G.; Liu, Z.; Zhao, L.; Lyu, D. Effects of biochar on photosystem function and activities of protective enzymes in Pyrus ussuriensis Maxim. under drought stress. Acta Physiol. Plant. 2016, 38, 220. [Google Scholar]
- Xiao, Q.; Zhu, L.-x.; Shen, Y.-f.; Li, S.-q. Sensitivity of soil water retention and availability to biochar addition in rainfed semi-arid farmland during a three-year field experiment. Field Crops Res. 2016, 196, 284–293. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, J.; Wang, Y.; Yang, Q.; Chen, T.; Chen, Y.; Chi, D.; Xia, G.; Siddique, K.H.M.; Wang, T. Photosynthesis, chlorophyll fluorescence, and yield of peanut in response to biochar application. Front. Plant Sci. 2021, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Zainul, A.; Koyro, H.-W.; Huchzermeyer, B.; Gul, B.; Khan, M.A. Impact of a biochar or a compost-biochar mixture on water relation, nutrient uptake and photosynthesis of Phragmites karka. Pedosphere 2017, 160. [Google Scholar]
- Fetjah, D.; Ainlhout, L.F.E.; Ihssane, B.; Bouqbis, L. Effect of Banana Waste Biochar on Physiological Responses and Growth of Seashore Paspalum. J. Ecol. Eng. 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Farooq, M.; Romdhane, L.; Rehman, A.; Al-Alawi, A.K.; Al-Busaidi, W.M.; Asad, S.A.; Lee, D.-J. Integration of seed priming and biochar application improves drought tolerance in cowpea. J. Plant Growth Regul. 2021, 40, 1972–1980. [Google Scholar] [CrossRef]
- Singh, M.; Saini, R.K.; Singh, S.; Sharma, S.P. Potential of Integrating Biochar and Deficit Irrigation Strategies for Sustaining Vegetable Production in Water-limited Regions: A Review. HortScience 2019, 54, 1872–1878. [Google Scholar] [CrossRef]
- Rochaix, J.-D. Regulation of photosynthetic electron transport. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 375–383. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Massacci, A.; Nabiev, S.; Pietrosanti, L.; Nematov, S.; Chernikova, T.; Thor, K.; Leipner, J. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2008, 46, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J.; Pfannschmidt, T. Novel regulators in photosynthetic redox control of plant metabolism and gene expression. Plant Physiol. 2011, 155, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Sharma, S. Reactive oxygen species, oxidative stress and ROS scavenging system in plants. J. Chem. Pharm. Res. 2016, 8, 595–604. [Google Scholar]
- Nixon, P.J.; Michoux, F.; Yu, J.; Boehm, M.; Komenda, J. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 2010, 106, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef] [PubMed]
- Anwar Hossain, M.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Chapter 16—Proline protects plants against abiotic oxidative stress: Biochemical and molecular mechanisms. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 477–522. [Google Scholar]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiq, S.; Akram, N.A.; Ashraf, M. Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci. Hortic. 2015, 185, 68–75. [Google Scholar] [CrossRef]
- Ramachandra Reddy, A.; Chaitanya, K.V.; Jutur, P.P.; Sumithra, K. Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ. Exp. Bot. 2004, 52, 33–42. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.-W.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 2020, 22, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Ekinci, M.; Turan, M. Impact of Biochar in Mitigating the Negative Effect of Drought Stress on Cabbage Seedlings. J. Soil Sci. Plant Nutr. 2021, 21, 2297–2309. [Google Scholar] [CrossRef]
- Anjum, S.; Farooq, M.; Wang, L.C.; Xue, L.; Wang, S.; Wang, L.; Zhang, S.; Chen, M. Gas exchange and chlorophyll synthesis of maize cultivars are enhanced by exogenously-applied glycinebetaine under drought conditions. Plant Soil Environ. 2011, 57, 326–331. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Ashraf, M.; Ahmad, A.; Alyemeni, M.N.; Ahmad, P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol. Plant. 2021, 172, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Sarkar, S.; Era, F.M.; Mominul Islam, A.K.M.; Anwar, P.; Fahad, S.; Datta, R.; Islam, A.K.M.A. Drought Stress in Grain Legumes: Effects, Tolerance Mechanisms and Management. Agronomy 2021, 11, 2374. [Google Scholar] [CrossRef]
- Slesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, stress, and reactive oxygen species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Chen, X.; Liu, J.; Lin, G.; Wang, A.; Wang, Z.; Lu, G. Overexpression of AtWRKY28 and AtWRKY75 in Arabidopsis enhances resistance to oxalic acid and Sclerotinia sclerotiorum. Plant Cell Rep. 2013, 32, 1589–1599. [Google Scholar] [CrossRef]
- Farooq, M.; Irfan, M.; Aziz, T.; Ahmad, I.; Cheema, S. Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop Sci. 2013, 199, 12–22. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [PubMed]
- Koyro, H.-W.; Ahmad, P.; Geissler, N. Abiotic stress responses in plants: An overview. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]
- Duarte, B.; Santos, D.; Caçador, I. Halophyte anti-oxidant feedback seasonality in two salt marshes with different degrees of metal contamination: Search for an efficient biomarker. Funct. Plant Biol. 2013, 40, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef]
- Anjum, S.; Wang, L.; Farooq, M.; Khan, I.; Xue, L. Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J. Agron. Crop Sci. 2011, 197, 296–301. [Google Scholar] [CrossRef]
- Pehlivan, F.E. Vitamin C: An antioxidant agent. Vitam. C 2017, 2, 23–35. [Google Scholar]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid—A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Miret, J.A.; Müller, M. AsA/DHA redox pair influencing plant growth and stress tolerance. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Springer: Cham, Switzerland, 2017; pp. 297–319. [Google Scholar]
- Ahmad, P.; Abd Allah, E.F.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Siddique, K.H.M. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci. Rep. 2018, 8, 13515. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Pignocchi, C.; Kiddle, G.; Hernández, I.; Foster, S.J.; Asensi, A.; Taybi, T.; Barnes, J.; Foyer, C.H. Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signaling and orchestration of defense processes in tobacco. Plant Physiol. 2006, 141, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Jubany-Marí, T.; Munné-Bosch, S.; Alegre, L. Redox regulation of water stress responses in field-grown plants. Role of hydrogen peroxide and ascorbate. Plant Physiol. Biochem. 2010, 48, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gallie, D.R. The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 2004, 16, 1143–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sensitivity Index (SI) | WPl | Bc WPh | Bc WPl |
|---|---|---|---|
| Plant | −46.81% | 17.61% | −37.2% |
| Shoot | −50.47% | 40.29% | −37.77% |
| Root | −24.34% | 16.38% | −9.00% |
| Treatment Parameter | WPh (at 1500 μE m−2 s−1 PPFD) | WPl (at 750 μE m−2 s−1 PPFD) | ||
|---|---|---|---|---|
| 0% Bc | 2% Bc | 0% Bc | 2% Bc | |
| Anet (µmol CO2 m−2 s−1) | 10.667 a ± 0.566 | 8.029 b ± 0.803 | 2.513 d ± 0.294 | 4.495 c ± 0.801 |
| Vc (µmol CO2*m−2*s−1) | 0.057 a ± 0.008 | 0.048 ac ± 0.012 | 0.030 b ± 0.001 | 0.045 bc ± 0.012 |
| SC (µmol CO2 m−2 s−1) | 0.07 a ± 0.008 | 0.05 b ± 0.004 | 0,021 c ± 0,008 | 0.03 c ± 0.007 |
| Ci/Ca ratio | 0.367 a ± 0.05 | 0.323 a ± 0.06 | 0.510 b ± 0.09 | 0.422 b ± 0.01 |
| WUE (A/E) | 9.476 b ± 1 27 | 6.595 c ± 0.21 | 6.4 d ± 1.85 | 11.04 a ± 0.61 |
| RL (µmol(CO2)*m−2*s−1) | 11.88 b ± 0.73 | 13.59 a ± 0.95 | 9.05 c ± 0.56 | 8.01 c ± 0.72 |
| RD (µmol CO2 m−2 s−1) | 1.189 ab ± 0.141 | 0.640 b ± 0.157 | 1.675 a ± 0.600 | 0.900 b ± 0.452 |
| ETR (µmol e− m−2 s−1) | 182.23 b ± 7.71 | 195.73 a ±8.15 | 112.38 c ± 3.9 | 109.6 c ± 6.42 |
| ETR/Agross ratio (e−/CO2) | 7.75 a ± 0.2 | 8.65 a ± 0.26 | 8.43 a ± 0.63 | 8.4 a ± 0.68 |
| Y(NPQ) | 0.382 a ± 0.04 | 0.301 b ± 0.06 | 0.329 a ± 0.03 | 0.371 a ± 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharred, J.; Derbali, W.; Derbali, I.; Badri, M.; Abdelly, C.; Slama, I.; Koyro, H.-W. Impact of Biochar Application at Water Shortage on Biochemical and Physiological Processes in Medicago ciliaris. Plants 2022, 11, 2411. https://doi.org/10.3390/plants11182411
Gharred J, Derbali W, Derbali I, Badri M, Abdelly C, Slama I, Koyro H-W. Impact of Biochar Application at Water Shortage on Biochemical and Physiological Processes in Medicago ciliaris. Plants. 2022; 11(18):2411. https://doi.org/10.3390/plants11182411
Chicago/Turabian StyleGharred, Jihed, Walid Derbali, Imed Derbali, Mounawer Badri, Chedly Abdelly, Inès Slama, and Hans-Werner Koyro. 2022. "Impact of Biochar Application at Water Shortage on Biochemical and Physiological Processes in Medicago ciliaris" Plants 11, no. 18: 2411. https://doi.org/10.3390/plants11182411
APA StyleGharred, J., Derbali, W., Derbali, I., Badri, M., Abdelly, C., Slama, I., & Koyro, H.-W. (2022). Impact of Biochar Application at Water Shortage on Biochemical and Physiological Processes in Medicago ciliaris. Plants, 11(18), 2411. https://doi.org/10.3390/plants11182411







