Seedling Biometry of nud Knockout and win1 Knockout Barley Lines under Ionizing Radiation
Abstract
1. Introduction
2. Results
2.1. General Phenotyping of nud KO Lines
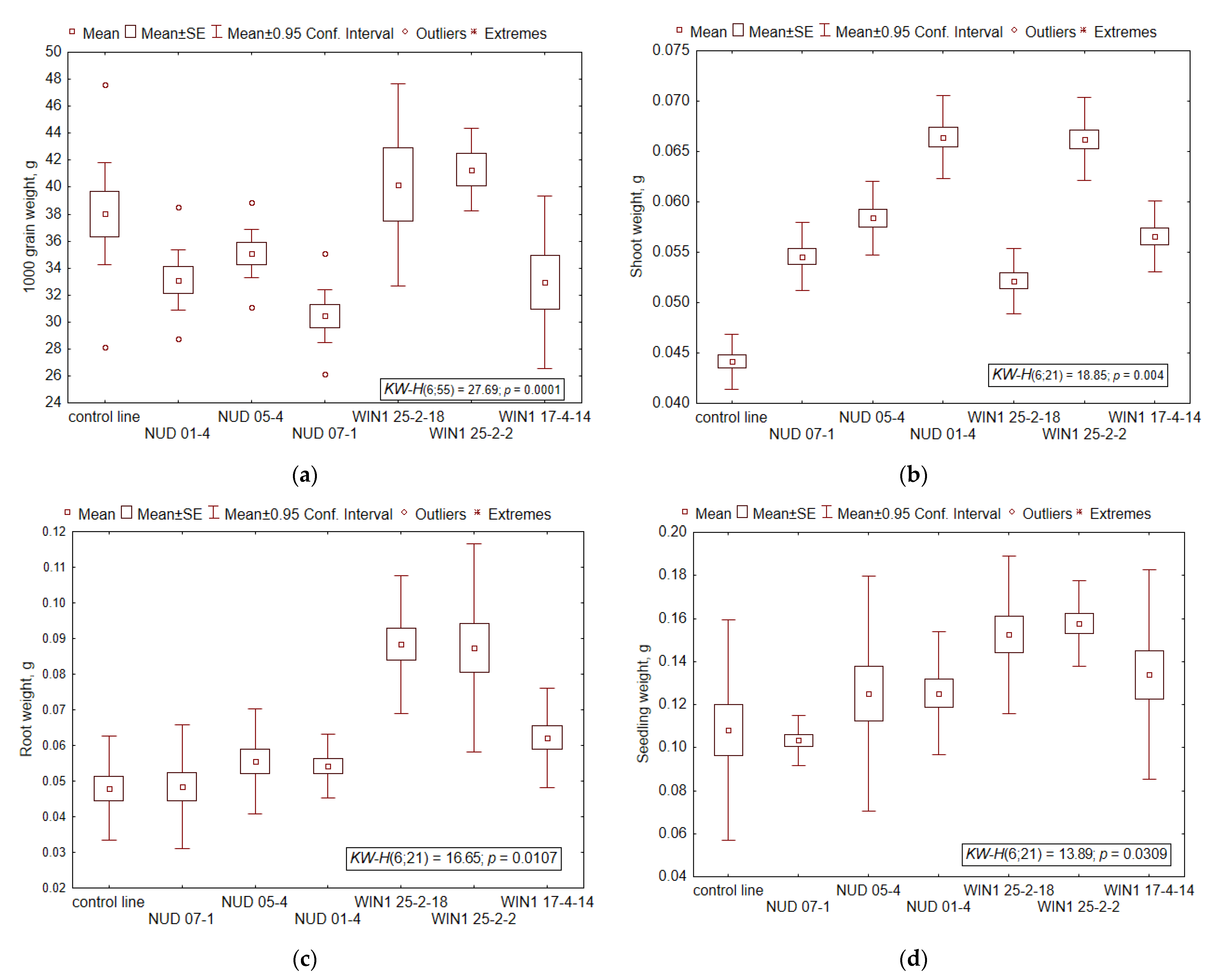
2.2. Thousand-Grain and Seedling Weights
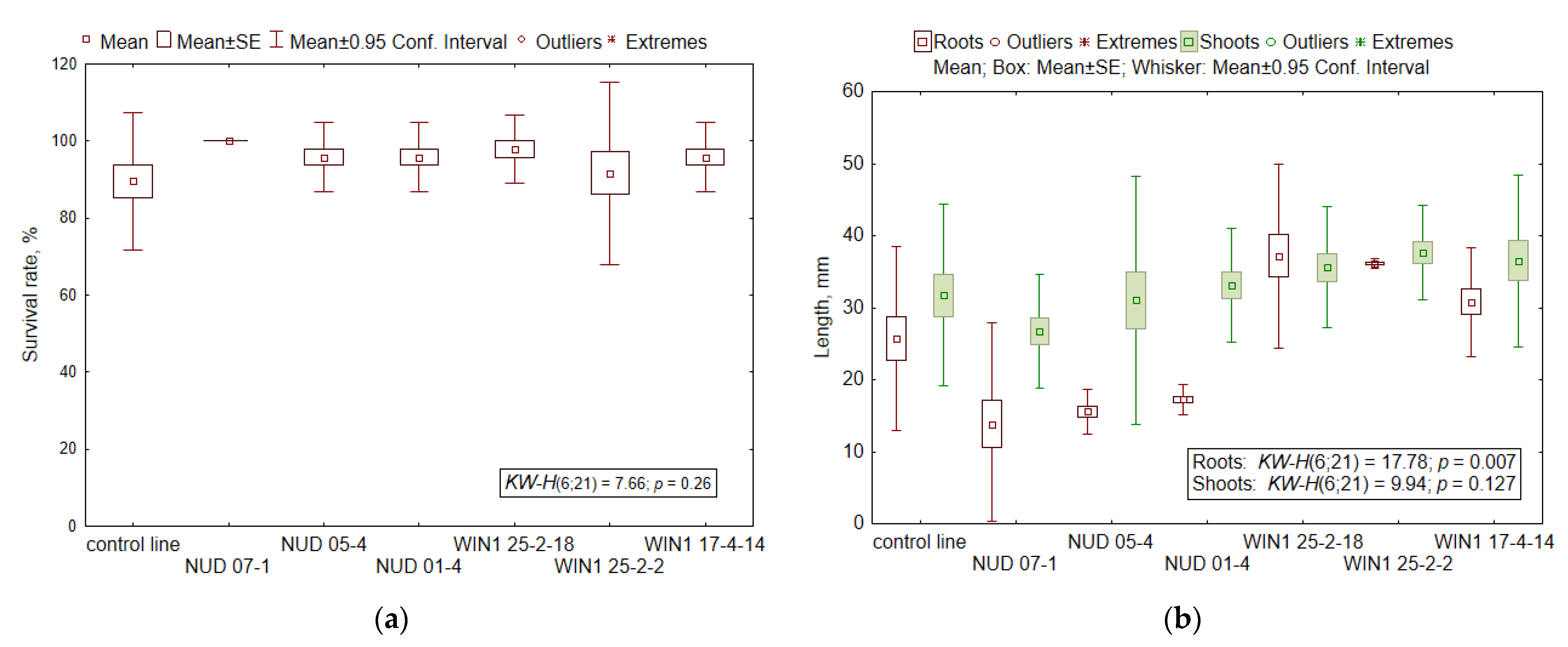
2.3. Viability of Seed Progeny
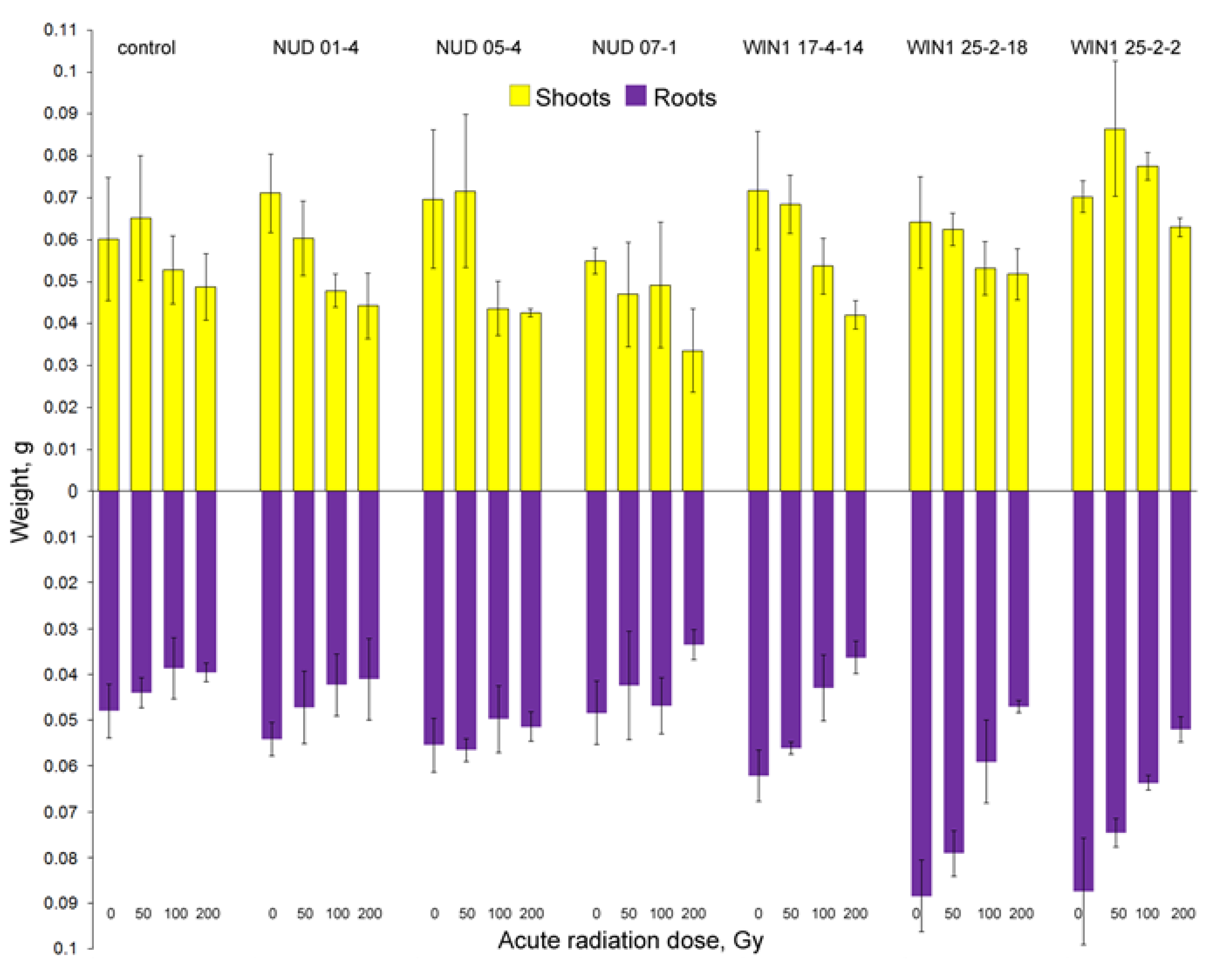
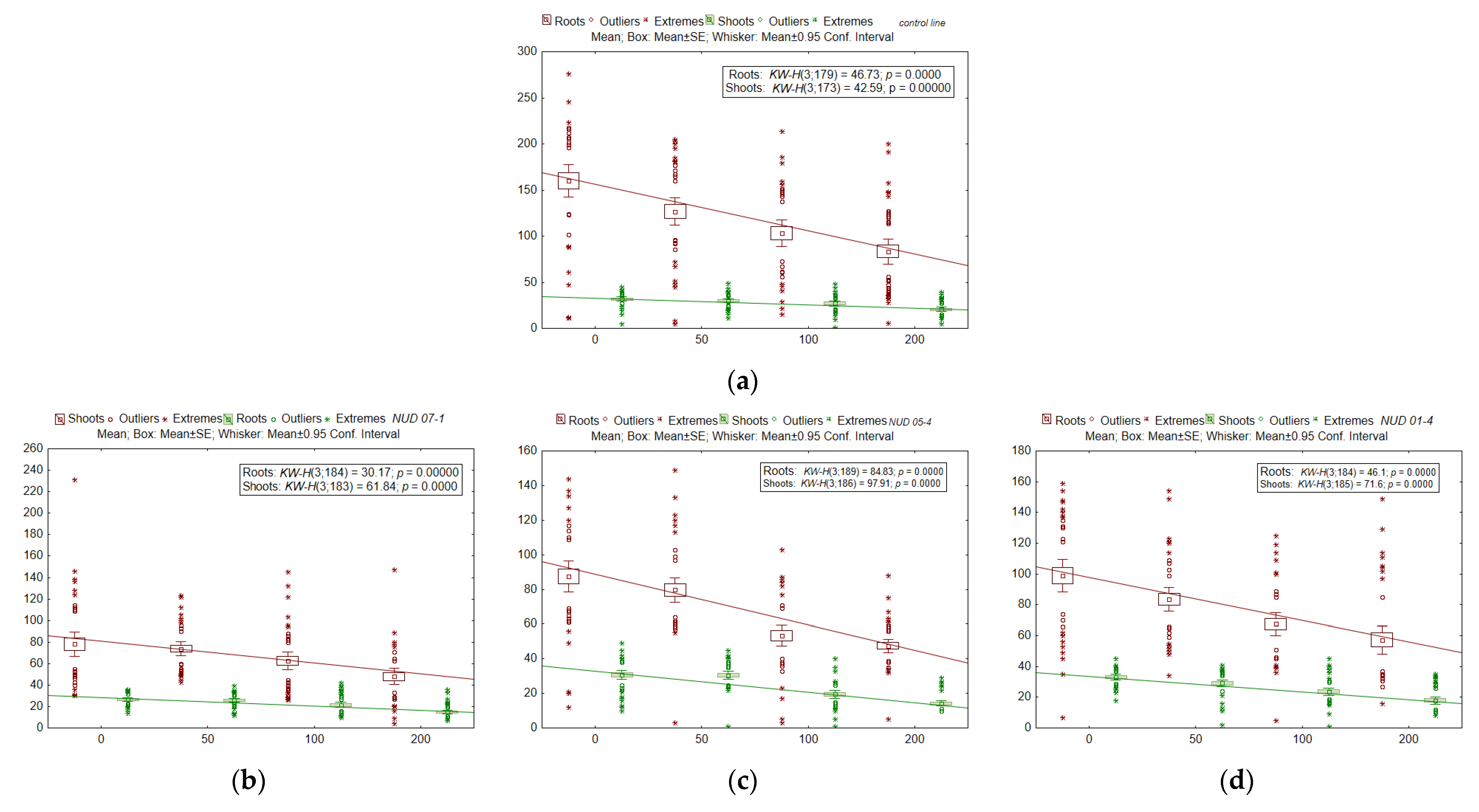
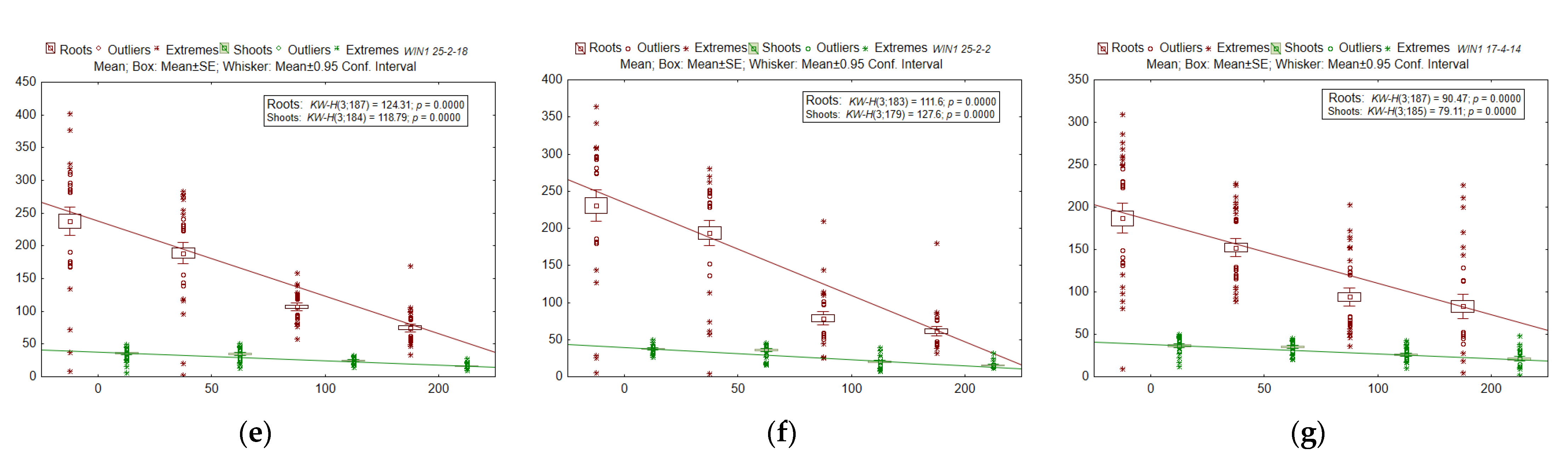
2.4. Radioresistance of the Seed Progeny
2.5. Mutability of the Seed Progeny
3. Discussion
4. Materials and Methods
4.1. Plant Models
4.2. Greenhouse Growth Conditions
4.3. General Phenotyping of nud KO Lines
4.4. Pre-Sowing Seed Treatment
4.5. Experimental Design
4.6. Seedling Emergence Rates and Growth Characteristics
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karam, P.A.; Leslie, S.A. Changes in terrestrial natural radiation levels over the history of life. In Radioactivity in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; Volume 7, pp. 107–117. [Google Scholar]
- Pozolotina, V.N. Remote Consequences of Radiation Effect on Plants; Goschitsky: Ekaterinburg, Russia, 2003; 244p. (In Russian) [Google Scholar]
- Castillo, H.; Schoderbek, D.; Dulal, S.; Escobar, G.; Wood, J.; Nelson, R.; Smith, G. Stress induction in the bacteria Shewanella oneidensis and Deinococcus radiodurans in response to below-background ionizing radiation. Int. J. Radiat. Biol. 2015, 91, 749–756. [Google Scholar] [CrossRef]
- Castillo, H.; Li, X.; Schilkey, F.; Smith, G.B. Transcriptome analysis reveals a stress response of Shewanella oneidensis deprived of background levels of ionizing radiation. PLoS ONE 2018, 13, e0196472. [Google Scholar] [CrossRef]
- Satta, L.; Augusti-Tocco, G.; Ceccarelli, R.; Esposito, A.; Fiore, M.; Paggi, P.; Poggesi, I.; Ricordy, R.; Scarsella, G.; Cundari, E. Low environmental radiation background impairs biological defence of the yeast Saccharomyces cerevisiae to chemical radiomimetic agents. Mutat. Res. Lett. 1995, 347, 129–133. [Google Scholar] [CrossRef]
- Fratini, E.; Carbone, C.; Capece, D.; Esposito, G.; Simone, G.; Tabocchini, M.A.; Tomasi, M.; Belli, M.; Satta, L. Low-radiation environment affects the development of protection mechanisms in V79 cells. Radiat. Environ. Biophys. 2015, 54, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Alves dos Reis, A.; Ansari, A.; Bertelli, L.; Carr, Z.; Dainiak, N.; Degteva, M.; Efimov, A.; Kalinich, J.; Kryuchkov, V.; et al. Public health response and medical management of internal contamination in past radiological or nuclear incidents: A narrative review. Environ. Int. 2022, 163, 107222. [Google Scholar] [CrossRef]
- Schober, A. Ein Versuch mit Röntgenschen Strahlen auf einige Pflanzen. Ber. Dtsch. Bot. Ges. 1896, 14, 108–110. [Google Scholar]
- Maldiney, E.; Thouvenin, S. De l’influence des rayons X sur la germination. Rev. Gen. Bot. 1898, 10, 81–86. [Google Scholar]
- Röntgen, W.C. Uber Eine Neue Art von Strahlen; Stahel’sche K. Hof- und Universitätsbuch- und Kunsthandlung: Würzburger, Germany, 1895. [Google Scholar]
- Breslavets, L.P. Plants and X-rays; Publishing House of the USSR Academy of Sciences: Moscow, Russia, 1946; 194p. (In Russian) [Google Scholar]
- Preobrazhenskaya, E.I. Radioresistance of Plant Seeds; Atomizdat Moscow: Moscow, Russia, 1971; 232p. (in Russian) [Google Scholar]
- Sparrow, R.C.; Sparrow, A.H. Relative Radiosensitivities of Woody and Herbaceous Spermatophytes. Science 1965, 147, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.-Y.; Forster, B.P.; Nakagawa, H.; Nakagawa, H. Plant Mutation Breeding and Biotechnology; CABI: Wallingford, UK, 2012; 616p. [Google Scholar]
- Amano, E.; Yamaguchi, T.; Yatou, O. Radiation sensitivity of plants. In Research Note; Fukui Prefectural University: Eiheiji, Japan, 1989; 18p. [Google Scholar]
- Sarapultsev, B.I.; Geras’kin, S.A. Genetic Basis of Radioresistance and Evolution; Enegroatomizdat: Moscow, Russia, 1993; 209p. (In Russian) [Google Scholar]
- Aleksakhin, R.M.; Vasilyev, A.V.; Dikarev, V.G. Agricultural Radioecology; Ecology: Moscow, Russia, 1991. (In Russian) [Google Scholar]
- Ciciani, L.; Rizzo, A. Radiological protection for the use of radiation and radioisotopes in agricultural research. In Radioisotopes in Weed Research; CRC Press: Boca Raton, FL, USA, 2020; 155p. [Google Scholar]
- Volkova, P.Y.; Bondarenko, E.V.; Kazakova, E.A. Radiation Hormesis in Plants. Curr. Opin. Toxicol. 2022, 30, 100334. [Google Scholar] [CrossRef]
- Pozolotina, V.N.; Antonova, E.V.; Karimullina, E.M. Assessment of radiation impact on Stellaria graminea cenopopulations in the zone of the Eastern Ural Radioactive Trace. Rus. J. Ecol. 2010, 41, 459–468. [Google Scholar] [CrossRef]
- Geras’kin, S.A. Ecological effects of exposure to enhanced levels of ionizing radiation. J. Environ. Radioact. 2016, 162, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Volkova, P.Y.; Geras’kin, S.A.; Horemans, N.; Makarenko, E.S.; Saenen, E.; Duarte, G.T.; Nauts, R.; Bondarenko, V.S.; Jacobs, G.; Voorspoels, S.; et al. Chronic radiation exposure as an ecological factor: Hypermethylation and genetic differentiation in irradiated Scots pine populations. Environ. Pollut. 2018, 232 (Suppl. S3), 105–112. [Google Scholar] [CrossRef]
- Kal’chenko, V.A.; Kalabushkin, B.A.; Rubanovich, A.V. Chronic Irradiation as an Ecological Factor Affecting the Genetic Structure of Populations. Rus. J. Genet. 1991, 27, 676–684. [Google Scholar]
- Caldwell, M.M. Solar Ultraviolet Radiation as an Ecological Factor for Alpine Plants. Ecol. Monogr. 1968, 38, 243–268. [Google Scholar] [CrossRef]
- Antonova, E.V.; Khlestkina, E.K. Radiosensitivity and mutability of wheat seed progeny cultivated under adverse environments. Plant Physiol. Biochem. 2019, 137, 162–168. [Google Scholar]
- Antonova, E.V.; Pozolotina, V.N.; Karimullina, E.M. Variation in the seed progeny of smooth brome grass, Bromus inermis Leyss., under conditions of chronic irradiation in the zone of the Eastern Ural Radioactive Trace. Rus. J. Ecol. 2014, 45, 508–516. [Google Scholar] [CrossRef]
- Pozolotina, V.N.; Antonova, E.V. Temporal variability of the quality of Taraxacum officinale seed progeny from the East-Ural Radioactive Trace: Is there an interaction between low level radiation and weather conditions? Int. J. Radiat. Biol. 2017, 93, 330–339. [Google Scholar] [CrossRef]
- Geras’kin, S.; Churyukin, R.; Volkova, P. Radiation exposure of barley seeds can modify the early stages of plants’ development. J. Environ. Radioact. 2017, 177 (Suppl. S3), 71–83. [Google Scholar] [CrossRef]
- Mikaelsen, K.; Brunner, H. Effect of fast neutrons and gamma radiation on seedling and root growth of barley varieties. Neutron Irradiat. Seeds II Tech. Rep. Ser. 1968, 92, 79–82. [Google Scholar]
- Gorbatova, I.V.; Kazakova, E.A.; Podlutskii, M.S.; Pishenin, I.A.; Bondarenko, V.S.; Dontsova, A.A.; Dontsov, D.P.; Snegirev, A.S.; Makarenko, E.S.; Bitarishvili, S.V.; et al. Studying Gene Expression in Irradiated Barley Cultivars: PM19L-like and CML31-like Expression as Possible Determinants of Radiation Hormesis Effect. Agronomy 2020, 10, 1837. [Google Scholar] [CrossRef]
- Volkova, P.Y.; Duarte, G.T.; Soubigou-Taconnat, L.; Kazakova, E.A.; Pateyron, S.; Bondarenko, V.S.; Bitarishvili, S.V.; Makarenko, E.S.; Churyukin, R.S.; Lychenkova, M.A.; et al. Early response of barley embryos to low- and high-dose gamma irradiation of seeds triggers changes in the transcriptional profile and an increase in hydrogen peroxide content in seedlings. J. Agron. Crop Sci. 2020, 206, 277–295. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection. Environmental Protection—The Concept and Use of Reference Animals and Plants; ICRP Publication: Ottawa, ON, Canada, 2008; Volume 38, 251p. [Google Scholar]
- Long, L.M.; Patel, H.P.; Cory, W.C.; Stapleton, A.E. The maize epicuticular wax layer provides UV protection. Funct. Plant Biol. 2003, 30, 75–81. [Google Scholar] [CrossRef]
- Shepherd, T.; Wynne Griffiths, D. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kong, L.; Zhi, P.; Chang, C. Update on Cuticular Wax Biosynthesis and Its Roles in Plant Disease Resistance. Int. J. Mol. Sci. 2020, 21, 5514. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yogendra, K.N.; Karre, S.; Kushalappa, A.C.; Dion, Y.; Choo, T.M. WAX INDUCER1 (HvWIN1) transcription factor regulates free fatty acid biosynthetic genes to reinforce cuticle to resist Fusarium head blight in barley spikelets. J. Exp. Bot. 2016, 67, 4127–4139. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Davies, N.W.; Shabala, L.; Zhou, M.; Brodribb, T.J.; Shabala, S. Residual transpiration as a component of salinity stress tolerance mechanism: A case study for barley. BMC Plant Biol. 2017, 17, 107. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, C.-M. Cuticular wax biosynthesis as a way of inducing drought resistance. Plant Signal. Behav. 2011, 6, 1043–1045. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.-H. Molecular and Evolutionary Mechanisms of Cuticular Wax for Plant Drought Tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- González, A.; Ayerbe, L. Effect of terminal water stress on leaf epicuticular wax load, residual transpiration and grain yield in barley. Euphytica 2010, 172, 341–349. [Google Scholar] [CrossRef]
- Gerasimova, S.V.; Hertig, C.; Korotkova, A.M.; Kolosovskaya, E.V.; Otto, I.; Hiekel, S.; Kochetov, A.V.; Khlestkina, E.K.; Kumlehn, J. Conversion of hulled into naked barley by Cas endonuclease-mediated knockout of the NUD gene. BMC Plant Biol. 2020, 20, 255. [Google Scholar] [CrossRef]
- Broun, P.; Poindexter, P.; Osborne, E.; Jiang, C.-Z.; Riechmann, J.L. WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 4706–4711. [Google Scholar] [CrossRef] [PubMed]
- Gerasimova, S.; Kolosovskaya, E.; Vikhorev, A.; Korotkova, A.; Hertig, C.; Genaev, M.; Domrachev, D.; Morozov, S.; Chernyak, E.; Shmakov, N.; et al. Apetala2/Ethylene responsive factor WAX INDUCER 1 encoded at the barley Cer-x locus regulates β-diketone biosynthesis by activating the cer-cqu gene cluster. in prep.
- Kannangara, R.; Branigan, C.; Liu, Y.; Penfield, T.; Rao, V.; Mouille, G.; Hofte, H.; Pauly, M.; Riechmann, J.L.; Broun, P. The transcription factor WIN1/SHN1 regulates cutin biosynthesis in Arabidopsis thaliana. Plant Cell 2007, 19, 1278–1294. [Google Scholar] [CrossRef] [PubMed]
- Capo-chichi, L.; Kenward, K.; Nyachiro, J.; Anyia, A. Nud locus and the effects on seedling vigour related traits for genetic improvement of hulless barley. J. Plant Sci. Mol. Breed. 2012, 1, 1–9. [Google Scholar] [CrossRef]
- Choo, T.-M.; Ho, K.M.; Martin, R.A. Genetic Analysis of a Hulless × Covered Cross of Barley Using Doubled-Haploid Lines. Crop Sci. 2001, 41, 1021–1026. [Google Scholar] [CrossRef]
- Ktitorova, I.N.; Skobeleva, O.V.; Kanash, E.V.; Bilova, T.E.; Sharova, E.I. Causes of root growth retardation induced by ultraviolet-B irradiation of shoots in barley seedlings. Russ. J. Plant. Phys. 2006, 53, 85–95. [Google Scholar] [CrossRef]
- GBIF Backbone Taxonomy. Checklist Dataset; GBIF Secretariat: Copenhagen, Denmark, 2021. [Google Scholar] [CrossRef]
- STATISTICA (Data Analysis Software System); Version 10, New features and enhancements; StatSoft Inc.: Tulsa, OK, USA, 2011.
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]

| Trait | Control Line | nud50 KO Lines | ||
|---|---|---|---|---|
| WT | nud 01-4 | nud 05-4 | nud 07-1 | |
| Spikelet number per spike | 11.8 ± 0.63 | 12.2 ± 0.63 | 11.6 ± 1.27 | 12.4 ± 1.27 |
| Spike length, cm | 8.1 ± 0.53 | 7.3 ± 0.40 * | 7.3 ± 0.33 * | 7.3 ± 0.96 |
| Main spike length, cm | 8.7 ± 0.94 | 8.0 ± 0.78 | 8.7 ± 0.63 | 8.3 ± 1.44 |
| Grain number per main spike | 15 ± 3.68 | 14.8 ± 5.55 | 17.6 ± 1.5 | 20.3 ± 3.6 * |
| Grain number per plant | 111.3 ± 33.8 | 119.1 ± 61.8 | 123.5 ± 67.9 | 109.4 ± 59.2 |
| Grain weight per main spike, g | 0.67 ± 0.19 | 0.52 ± 0.14 | 0.70 ± 0.17 | 0.71 ± 0.18 |
| Grain weight per plant, g | 4.12 ± 0.81 | 3.87 ± 1.80 | 4.35 ± 2.42 | 3.42 ± 1.96 |
| 1000-grain weight, g | 38.0 ± 5.31 | 33.1 ± 3.14 | 35.1 ± 2.52 | 30.4 ± 2.73 * |
| Plant height, cm | 62.5 ± 4.60 | 57.6 ± 3.27 | 60.4 ± 5.32 | 59.5 ± 5.38 |
| Total number of tillers | 9.1 ± 2.69 | 8.6 ± 4.27 | 9.2 ± 3.79 | 6.2 ± 2.97 |
| Number of fertile tillers | 8.0 ± 1.70 | 8.0 ± 3.65 | 7.8 ± 3.97 | 5.9 ± 2.96 |
| Awn length, cm | 9.3 ± 0.68 | 8.9 ± 0.81 | 9.05 ± 0.73 | 9.1 ± 0.84 |
| Line | Index | b0 | b1 | RD50, * Gy |
|---|---|---|---|---|
| WT | Seedling weight | 0.0075 | −9.05 × 10−6 | 414 |
| nud 07-1 | 0.0066 | −9.28 × 10−6 | 356 | |
| nud 05-4 | 0.0081 | −1.25 × 10−5 | 324 | |
| nud 01-4 | 0.0077 | −1.21 × 10−5 | 318 | |
| win1 25-2-18 | 0.0095 | −1.58 × 10−5 | 300 | |
| win1 25-2-2 | 0.0102 | −2.53 × 10−5 | 201 | |
| win1 17-4-14 | 0.0084 | −1.63 × 10−5 | 258 | |
| WT | Total root length | 150.6 | −0.37 | 206 |
| nud 07-1 | 79.4 | −0.16 | 254 | |
| nud 05-4 | 85.5 | −0.21 | 202 | |
| nud 01-4 | 94.8 | −0.21 | 231 | |
| win1 25-2-18 | 225.5 | −0.83 | 135 | |
| win1 25-2-2 | 220.1 | −0.90 | 123 | |
| win1 17-4-14 | 175.4 | −0.53 | 165 | |
| WT | Shoot length | 32.4 | −0.06 | 285 |
| nud 07-1 | 27.7 | −0.06 | 224 | |
| nud 05-4 | 31.8 | −0.09 | 173 | |
| nud 01-4 | 32.7 | −0.08 | 212 | |
| win1 25-2-18 | 36.8 | −0.10 | 178 | |
| win1 25-2-2 | 37.9 | −0.12 | 158 | |
| win1 17-4-14 | 36.9 | −0.08 | 225 |
| Parameters | Lines | Resume | |
|---|---|---|---|
| nud | win1 | ||
| Viability | |||
| 1000-grain weight | ≈WT, <win1 | >WT, >nud | >win1 |
| Root weight | ≈WT, <win1 | >WT, >nud | >win1 |
| Shoot weight | >WT, <win1 | >WT, >nud | >win1 |
| Seedling weight | ≈WT, <win1 | >WT, >nud | >win1 |
| Root length | <WT, <win1 | >WT, >nud | <nud |
| Shoot/Root length | >WT, >win1 | ≈WT, <nud | >nud |
| Radioresistance | |||
| Seedlings with leaves | <WT, ≈win1 | <WT, ≈nud | all KO lines are sensitive |
| Root weight | <WT, >win1 | <WT, <nud | win1 is more sensitive |
| Shoot weight | <WT, <win1 | <WT, >nud | nud is more sensitive |
| Seedling weight | <WT, >win1 | <WT, <nud | win1 is more sensitive |
| Shoot length | <WT, >win1 | <WT, <nud | win1 is more sensitive |
| Root number | <WT, <win1 | ≈WT, >nud | nud is more sensitive |
| Root length | >WT, >win1 | <WT, <nud | nud is more resistance |
| Shoot/Root length | >WT, >win1 | ≈WT, <nud | nud is more resistance |
| Morphological abnormalities | |||
| Root shape change | >WT, >win1 | >WT, <nud | common in nud |
| Leaf color change | >WT, <win1 | >WT, >nud | common in win1 |
| Leaf shape change | >WT, <win1 | >WT, >nud | common in win1 |
| Coleoptile shape change | >WT, ≈win1 | >WT, ≈nud | common in both KO lines |
| Hairy roots (R *) | <WT, >win1 | <WT, <nud | common in WT |
| Root necrosis (R *) | >WT, >win1 | >WT, <nud | common in nud |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, E.V.; Shimalina, N.S.; Korotkova, A.M.; Kolosovskaya, E.V.; Gerasimova, S.V.; Khlestkina, E.K. Seedling Biometry of nud Knockout and win1 Knockout Barley Lines under Ionizing Radiation. Plants 2022, 11, 2474. https://doi.org/10.3390/plants11192474
Antonova EV, Shimalina NS, Korotkova AM, Kolosovskaya EV, Gerasimova SV, Khlestkina EK. Seedling Biometry of nud Knockout and win1 Knockout Barley Lines under Ionizing Radiation. Plants. 2022; 11(19):2474. https://doi.org/10.3390/plants11192474
Chicago/Turabian StyleAntonova, Elena V., Nadezhda S. Shimalina, Anna M. Korotkova, Ekaterina V. Kolosovskaya, Sophia V. Gerasimova, and Elena K. Khlestkina. 2022. "Seedling Biometry of nud Knockout and win1 Knockout Barley Lines under Ionizing Radiation" Plants 11, no. 19: 2474. https://doi.org/10.3390/plants11192474
APA StyleAntonova, E. V., Shimalina, N. S., Korotkova, A. M., Kolosovskaya, E. V., Gerasimova, S. V., & Khlestkina, E. K. (2022). Seedling Biometry of nud Knockout and win1 Knockout Barley Lines under Ionizing Radiation. Plants, 11(19), 2474. https://doi.org/10.3390/plants11192474









