Qualitative and Quantitative Anatomical Analysis of the Constitutive Bark of Q. ilex x Q. suber Hybrids
Abstract
:1. Introduction
2. Results and Discussion
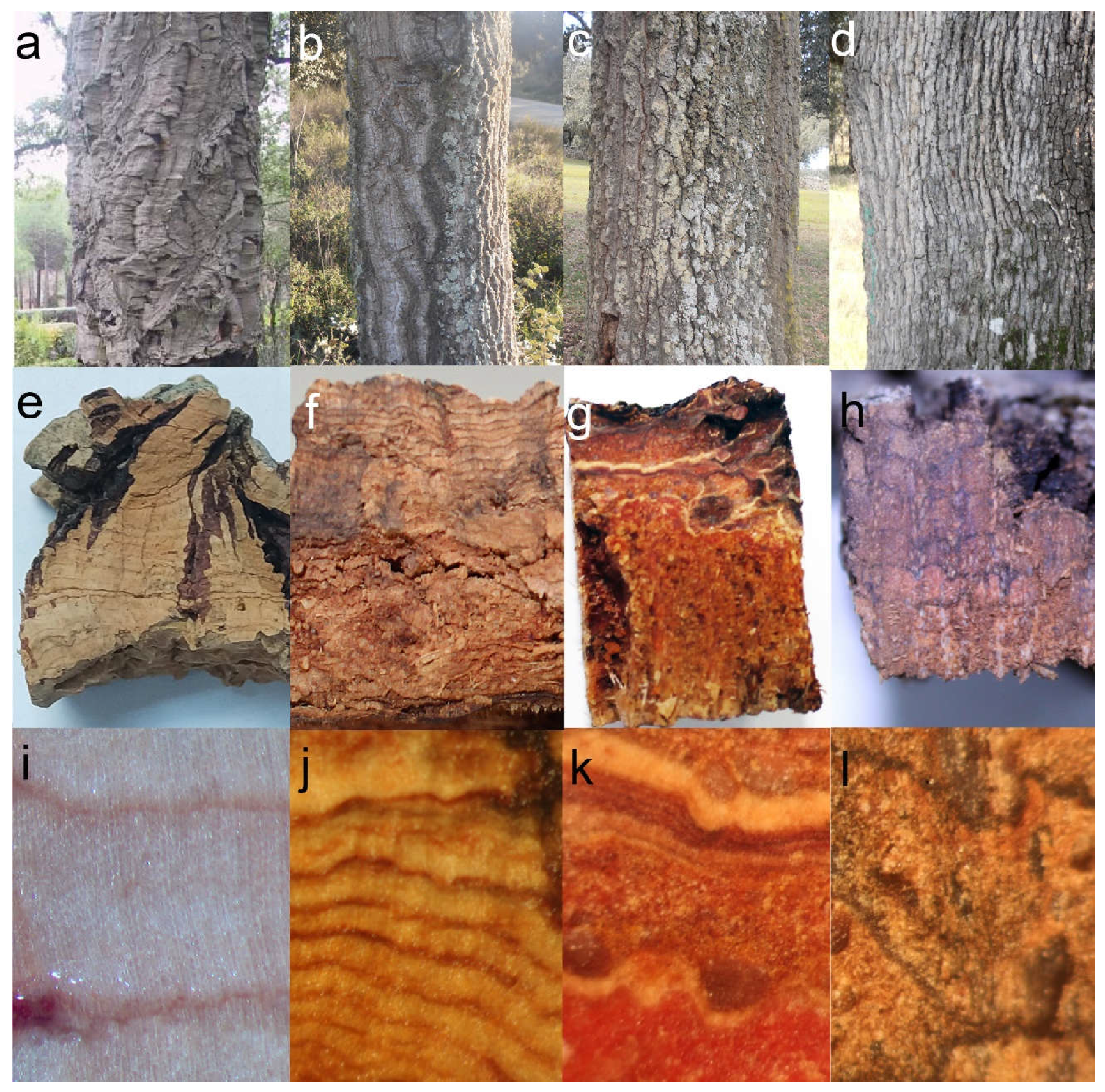
2.1. Preliminary Observations
2.2. Systematic Measurements and Analysis

| Variable | Factor | df | SS | MS | F | Pr (>F) |
|---|---|---|---|---|---|---|
| PPH | αi (species) | 2 | 46,228 | 23,114 | 8.4 | 0.002 |
| βj(i) (species:ind) | 20 | 89,269 | 4463 | 16.9 | 0.001 | |
| γijk (residuals) | 368 | 97,207 | 264 | |||
| PT | αi (species) | 2 | 81,547 | 40,774 | 144.0 | 0.001 |
| βj(i) (species:ind) | 20 | 8798 | 440 | 7.9 | 0.001 | |
| γijk (residuals) | 368 | 20,525 | 56 | |||
| Nmax | αi (species) | 2 | 78,133 | 39,066 | 129.6 | 0.001 |
| βj(i) (species:ind) | 20 | 9342 | 467 | 7.6 | 0.001 | |
| γijk (residuals) | 368 | 22,523 | 61 | |||
| Nmin | αi (species) | 2 | 84,678 | 42,339 | 155.6 | 0.001 |
| βj(i) (species:ind) | 20 | 8458 | 423 | 8.0 | 0.001 | |
| γijk (residuals) | 368 | 19,549 | 53 | |||
| PP | αi (species) | 2 | 36,622 | 18,311 | 17.3 | 0.001 |
| βj(i) (species:ind) | 20 | 32,934 | 1647 | 8.1 | 0.001 | |
| γijk (residuals) | 368 | 74,518 | 202 | |||
| PS | αi (species) | 2 | 5365 | 2682 | 2.3 | 0.119 |
| βj(i) (species:ind) | 20 | 35,762 | 1788 | 8.3 | 0.001 | |
| γijk (residuals) | 368 | 78,966 | 215 | |||
| DS | αi (species) | 2 | 948 | 473.8 | 1.5 | 0.242 |
| βj(i) (species:ind) | 20 | 9757 | 487.8 | 7.9 | 0.001 | |
| γijk (residuals) | 368 | 22,753 | 61.8 |
| Trait | Description | Microscope Light Exposure |
|---|---|---|
| PPH | Percentage of ordered suberized cells (phellem cells) | UV |
| PT | Mean phellem cell number | UV |
| Nmax | Maximum number of phellem cells | UV |
| Nmin | Minimum number of phellem cells | UV |
| PHT | 1: Rhytidome with non-functional phloem 2: Rhytidome with massive phellem | Both |
| PP | Percentage of parenchymatic cells | Both |
| PS | Percentage of schlerenchymatic cells | VL |
| SG | 0: lack of schlerenchymatic cells 1: isolated schlerenchymatic cells 2: grouped schlerenchymatic cells | VL |
| DS | Percentage of disordered suberized tissue | UV |
2.3. Concluding Remarks
3. Materials and Methods
3.1. Sampling and Laboratory Procedures
3.2. Quantitative Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amaral Franco, J. Quercus L. In Flora Ibérica; Castroviejo, S., Laínz, M., López, G., Montserrat, P., Muñoz, F., Paiva, J., Villar, L., Eds.; Real Jardín Botánico: Madrid, Spain, 1990; Volume 2, pp. 15–36. [Google Scholar]
- McVay, J.D.; Hipp, A.L.; Manos, P.S. A genetic legacy of introgression confounds phylogeny and biogeography in oaks. Proc. Biol. Sci. 2017, 284, 20170300. [Google Scholar] [CrossRef] [PubMed]
- Denk, T.; Grimm, G.W.; Manos, P.S.; Deng, M.; Hipp, A.L. An Updated Infrageneric Classification of the Oaks: Review of Previous Taxonomic Schemes and Synthesis of Evolutionary Patterns. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Tree Physiol 7; Gil-Pelegrín, E., Peguero-Pina, J., Sancho-Knapik, D., Eds.; Springer Int. Publishing: Cham, Switzerland, 2017; pp. 13–38. [Google Scholar] [CrossRef]
- Trockenbrodt, M. Qualitative Structural Changes during bark development in Quercus robur, Ulmus glabra, Populus tremula and Betula pendula. IAWA J. 1991, 12, 5–22. [Google Scholar] [CrossRef]
- Gričar, J.; Jagodic, Š.; Prislan, P. Structure and subsequent seasonal changes in the bark of sessile oak (Quercus petraea). Trees 2015, 29, 747–757. [Google Scholar] [CrossRef]
- Quilho, T.; Sousa, V.; Tavares, F.; Pereira, H. Bark anatomy and cell size variation in Quercus faginea. Turk. J. Bot. 2013, 37, 561–570. [Google Scholar] [CrossRef]
- Sen, A.; Quilho, T.; Pereira, H. Bark anatomy of Quercus cerris L. var. cerris from Turkey. Turk. J. Bot. 2011, 35, 45–55. [Google Scholar] [CrossRef]
- Sousa, V.; Ferreira, J.P.A.; Miranda, I.; Quilhó, T.; Pereira, H. Quercus rotundifolia Bark as a Source of Polar Extracts: Structural and Chemical Characterization. Forests 2021, 12, 1160. [Google Scholar] [CrossRef]
- Graca, J.; Pereira, H. The periderm development in Quercus suber. IAWA J. 2004, 25, 325–335. [Google Scholar] [CrossRef]
- Pereira, H. The extraction of cork. In Cork. Biology Production and Uses; Pereira, H., Ed.; Elsevier Science: Oxford, UK, 2007; pp. 127–144. [Google Scholar] [CrossRef]
- Pausas, J.G.; Pereira, J.S.; Aronson, J. The Tree. In Cork Oak Woodlands on the Edge. Ecology, Adaptive Management and Restoration; Aronson, J., Pereira, J.S., Pausas, J.G., Eds.; Island Press: Washington, DC, USA, 2009; pp. 11–23. [Google Scholar]
- Leite, C.; Pereira, H. Cork-containing barks—A review. Front. Mater. 2017, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- López de Heredia, U.; Vázquez, F.M.; Soto, Á. The Role of Hybridization on the Adaptive Potential of Mediterranean Sclerophyllous Oaks: The Case of the Quercus ilex x Q. suber Complex. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Tree Physiol 7; Gil-Pelegrín, E., Peguero-Pina, J., Sancho-Knapik, D., Eds.; Springer: Cham, Switzerland, 2017; pp. 239–260. [Google Scholar] [CrossRef]
- Colmeiro, M.; Boutelou, E. Examen de las Encinas y Demás Arboles de la Peninsula Iberica ue Producen Bellotas, con la Designación de los que se Llaman Mestos; Imprenta Geofrin: Seville, Spain, 1854. [Google Scholar]
- Laguna, M. Un mesto italiano y varios mestos españoles. Montes 1881, 114, 477–486. [Google Scholar]
- Borzi, A. L’llixi-Suergiu (Quercus morisii-Borzi), nuova Querce della Sardegna. Nuov. Gior. Bot. Ital. 1881, 13, 3–10. [Google Scholar]
- Natividade, J.V. Estudo Histologico das Peridermes do Hibrido Quercus ilex x Suber; P Cout Publ Dir G Serv Flor III (1); Direcçao Geral Serviços Florestais: Lisbon, Portugal, 1936. [Google Scholar]
- Camus, A. Les Chênes: Monographie du Genre Quercus [et Lithocarpus]; Editions Paul Lechevalier: Paris, France, 1936. [Google Scholar]
- Vázquez, F.M.; Sánchez-Gullón, E.; Pinto-Gomes, C.; Pineda, M.A.; García, D.; Márquez, F.; Guerra, M.J.; Blanco, J.; Vilaviçosa, C. Three New Oak Hybrids from Southwest Iberia (Spain and Portugal). J. Int. Oak. Soc. 2015, 26, 43–55. [Google Scholar]
- López de Heredia, U.; Duro-García, M.J.; Soto, Á. Leaf morphology of progenies in Q. suber, Q. ilex, and their hybrids using multivariate and geometric morphometric analysis. iForest 2018, 11, 90–98. [Google Scholar] [CrossRef]
- Varela, M.C.; Bras, R.; Barros, I.R.; Oliveira, P.; Meierrose, C. Opportunity for hybridization between two oak species in mixed stands as monitored by the timing and intensity of pollen production. For. Ecol. Manag. 2008, 256, 1546–1551. [Google Scholar] [CrossRef]
- Llensa de Gelcén, S. Sistemática, fitogeografía y utilidad forestal del hibrido x Quercus morisii Borzi. In Anales de la Escuela de Peritos Agrícolas y Superior de Agricultura y de los Servicios Técnicos de Agricultura; OA.mg: Barcelona, Spain, 1943; Volume 3, pp. 315–328. [Google Scholar]
- Soto, Á.; Rodríguez-Martínez, D.; López De Heredia, U. SimHyb: A simulation software for the study of the evolution of hybridizing populations. Application to Quercus ilex and Q. suber suggests hybridization could be underestimated. iForest 2018, 11, 99–103. [Google Scholar] [CrossRef]
- López de Heredia, U.; Mora-Márquez, F.; Goicoechea, P.G.; Guillardín-Calvo, L.; Simeone, M.C.; Soto, Á. ddRAD Sequencing-Based Identification of Genomic Boundaries and Permeability in Quercus ilex and Q. suber Hybrids. Front. Plant Sci. 2020, 11, 564414. [Google Scholar] [CrossRef] [PubMed]
- Mullick, D.B. A new tissue essential to necrophylactic periderm formation in the bark of four conifers. Can. J. Bot. 1975, 53, 2443–2457. [Google Scholar] [CrossRef]
- Rittinger, P.A.; Biggs, A.R.; Peirson, D.R. Histochemistry of lignin and suberin deposition in boundary layers formed after wounding in various plant species and organs. Can. J. Bot. 1987, 65, 1886–1892. [Google Scholar] [CrossRef]
- Wahlström, K.T.; Johansson, M. Structural responses in bark to mechanical wounding and Armillaria ostoyae infection in seedlings of Pinus sylvestris. Eur. J. For. Pathol. 1992, 22, 65–76. [Google Scholar] [CrossRef]
- Nêmec, B. Botanickà Michroteknika; House of the Academia of Science of Czechoslovakia: Prague, Czechoslovakia, 1962. [Google Scholar]
- Biggs, A.R. Suberized boundary zones and the chronology of wound response in tree bark. Phytopathology 1985, 75, 1191–1195. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.nih.gov/ij/index.html (accessed on 21 April 2022).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genomics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Burgos, G.; Díez-Morales, E.; López de Heredia, U.; Soto, Á. Qualitative and Quantitative Anatomical Analysis of the Constitutive Bark of Q. ilex x Q. suber Hybrids. Plants 2022, 11, 2475. https://doi.org/10.3390/plants11192475
de Burgos G, Díez-Morales E, López de Heredia U, Soto Á. Qualitative and Quantitative Anatomical Analysis of the Constitutive Bark of Q. ilex x Q. suber Hybrids. Plants. 2022; 11(19):2475. https://doi.org/10.3390/plants11192475
Chicago/Turabian Stylede Burgos, Gonzalo, Eduardo Díez-Morales, Unai López de Heredia, and Álvaro Soto. 2022. "Qualitative and Quantitative Anatomical Analysis of the Constitutive Bark of Q. ilex x Q. suber Hybrids" Plants 11, no. 19: 2475. https://doi.org/10.3390/plants11192475






