Efficacy of Sterculia diversifolia Leaf Extracts: Volatile Compounds, Antioxidant and Anti-Inflammatory Activity, and Green Synthesis of Potential Antibacterial Silver Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Compounds
2.2. Synthesis of Silver Nanoparticles
2.3. Biological Assays
2.3.1. Total Phenolics and Flavonoids
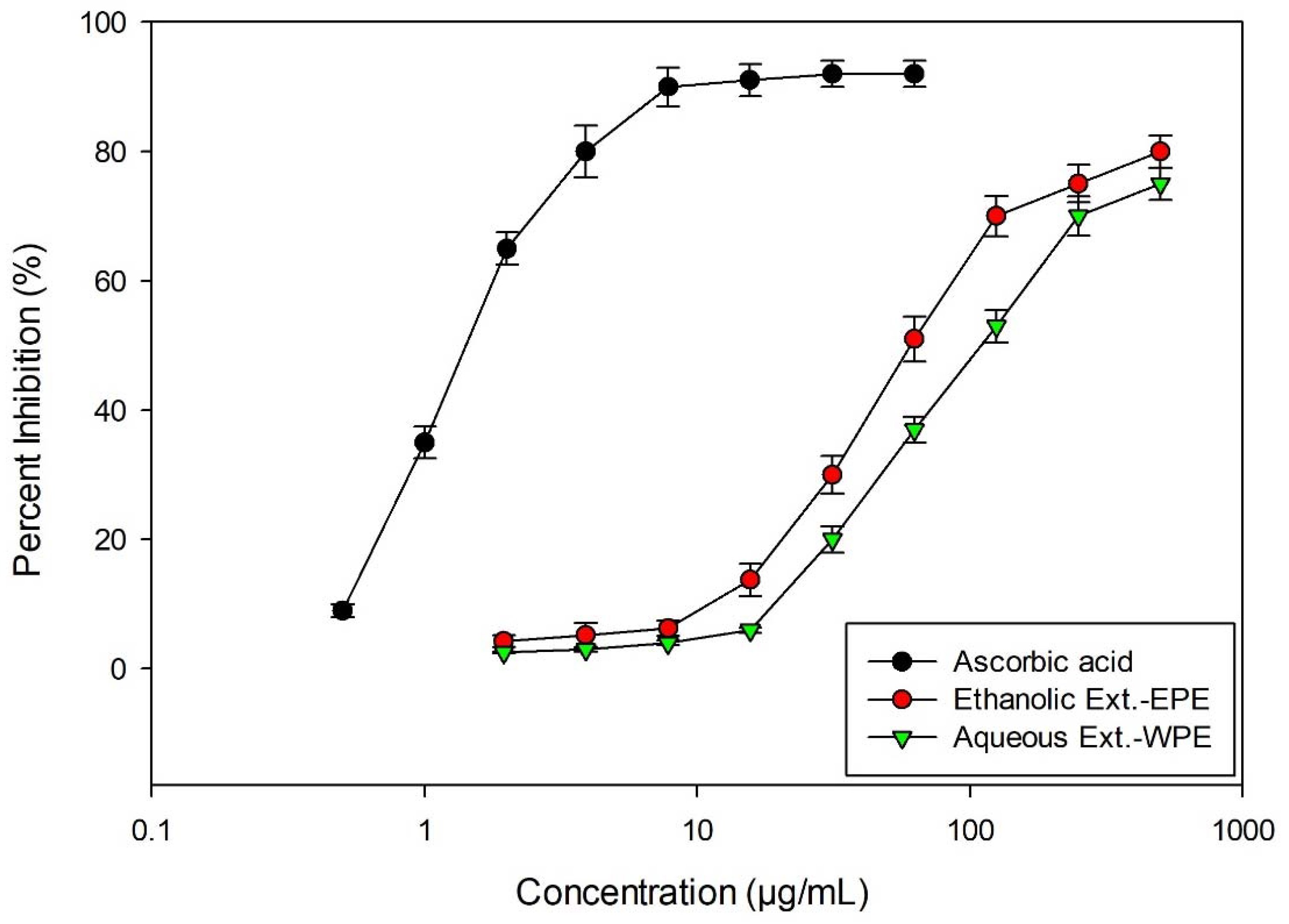
2.3.2. Antioxidant Activity
2.3.3. Inhibition of Protein Denaturation
2.3.4. Antimicrobial
2.3.5. Photosynthetic Pigments and Proline Content
3. Materials and Methods
3.1. Sample Preparation
3.2. Preparation of Ethanol and Water Extract
3.3. Synthesis of Silver Nanoparticles
3.4. AgNPs Characterization: Determination of Particle Size, Shape, and Charge
3.5. GC-MS Analysis of Ethanolic Extract
3.6. Total Flavonoid Content
3.7. Total Phenolic Content
3.8. DPPH Scavenging Activity
3.9. Inhibition of Protein Denaturation
3.10. Antimicrobial Activity
3.11. Determination of Leaf Proline and Photosynthetic Pigments Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Batool, R.; Khan, M.R.; Zai, J.A.; Ali, S.; Maryam, S.; Naz, I.; Bibi, S. Brachychiton populneus (Schott & Endl.) R.Br. ameliorate carbon tetrachloride induced oxidative stress through regulation of endoplasmic reticulum stress markers and inflammatory mediators in Sprague-Dawley male rats. Biomed. Pharmacother. 2018, 107, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- El-Sherei, M.M.; Ragheb, A.Y.; Kassem, M.E.S.; Marzouk, M.M.; Mosharrafa, S.A.; Saleh, N.A.M. Phytochemistry, biological activities and economical uses of the genus Sterculia and the related genera: A review. Asian Pac. J. Trop. Dis. 2016, 6, 492–501. [Google Scholar] [CrossRef]
- Ragheb, A.Y.; Kassem, M.E.; El-Sherei, M.M.; Marzouk, M.M.; Mosharrafa, S.A.; Saleh, N.A. Morphological, phytochemical and anti-hyperglycemic evaluation of Brachychiton populneus. Rev. Bras. Farmacogn. 2019, 29, 559–569. [Google Scholar] [CrossRef]
- Global Plants Database. Available online: https://plants.jstor.org/ (accessed on 22 August 2022).
- Thabet, A.A.; Youssef, F.S.; El-Shazly, M.; Singab, A.N.B. Sterculia and Brachychiton: A comprehensive overview on their ethnopharmacology, biological activities, phytochemistry and the role of their gummy exudates in drug delivery. J. Pharm. Pharmacol. 2018, 70, 450–474. [Google Scholar] [CrossRef]
- Al-Qaraleh, S.Y.; Al-Zereini, W.A.; Oran, S.A. Phyto-Decoration of Selenium Nanoparticles Using Moringa peregrina (Forssk.) Fiori Aqueous Extract: Chemical Characterization and Bioactivity Evaluation. Biointerface Res. Appl. Chem. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of nanotechnology in plant growth and crop protection: A review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Park, T.J.; Lee, K.G.; Lee, S.Y. Advances in microbial biosynthesis of metal nanoparticles. Appl. Microbiol. Biotechnol. 2016, 100, 521–534. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) J.F. Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, N.; Dimopoulou, A.; Georgopoulou, A.; Gallios, N.; Papadopoulos, D.; Tsipas, D.; Theologidis, I.; Michailidis, N.; Chatzinikolaidou, M. The effect of silver nanoparticles size, produced using plant extract from Arbutus unedo, on their antibacterial efficacy. Nanomaterials 2017, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.D.; Shetty, K.; Cecchini, A.L.; da Silva Miglioranza, L.H. Phenolic compounds and total antioxidant activity determination in rosemary and oregano extracts and its use in cheese spread. Semin. Agrar. 2012, 33, 655–666. [Google Scholar] [CrossRef]
- El-Gammal, R.E. Antioxidative Activity of Nanoparticles of Rosemary. Int. J. ChemTech Res. 2016, 9, 844–854. [Google Scholar]
- Abou Zeid, A.H.; Farag, M.A.; Hamed, M.A.A.; Kandil, Z.A.A.; El-Akad, R.H.; El-Rafie, H.M. Flavonoid chemical composition and antidiabetic potential of Brachychiton acerifolius leaves extract. Asian Pac. J. Trop. Biomed. 2017, 7, 389–396. [Google Scholar] [CrossRef]
- Batool, R.; Khan, M.R.; Sajid, M.; Ali, S.; Zahra, Z. Estimation of phytochemical constituents and in vitro antioxidant potencies of Brachychiton populneus (Schott & Endl.) R. Br. BMC Chem. 2019, 13, 32. [Google Scholar]
- Rabbi, F.; Zada, A.; Adhikari, A.; Jabeen, A.; Nisar, A.; Ullah, I. Sterculia diversifolia bears anti-cancer and immunomodulatory activities. Bangladesh J. Pharmacol. 2017, 12, 51–55. [Google Scholar] [CrossRef]
- Naveed, M.; Batool, H.; Javed, A.; Makhdoom, S.I.; Aziz, T.; Mohamed, A.A.; Sameeh, M.Y.; Alruways, M.W.; Dablool, A.S.; Almalki, A.A.; et al. Characterization and evaluation of the antioxidant, antidiabetic, anti-inflammatory, and cytotoxic activities of silver nanoparticles synthesized using Brachychiton populneus leaf extract. Processes 2022, 10, 1521. [Google Scholar] [CrossRef]
- Astuti, S.D.; Widyobroto, B.P.; Agus, A.; Yusiati, L.M. Identification of galactogogues in Gliricidia maculata. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yogyakarta, Indonesia, 23–25 November 2019; IOP Publishing: Bristol, UK, 2019; Volume 387, p. 012119. [Google Scholar] [CrossRef]
- Chinonye, I.; Lynda, O.; Fidelis, O.; Maureen, C. Characterization and Antimicrobial Properties of Volatile Component of The Ethanol Leaf Extract of Momordica Charantia. Int. Res. J. Nat. Sci. 2019, 7, 14–27. [Google Scholar]
- Collakova, E.; Della Penna, D. Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 2001, 127, 1113–1124. [Google Scholar] [CrossRef]
- Shimada, H.; Ohno, R.; Shibata, M.; Ikegami, I.; Onai, K.; Ohto, M.A.; Takamiya, K.I. Inactivation and deficiency of core proteins of photosystems I and II caused by genetical phylloquinone and plastoquinone deficiency but retained lamellar structure in a T-DNA mutant of Arabidopsis. Plant J. 2005, 41, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhou, S.; Shi, K.; Zhang, C.; Shao, H. Chemical profile and phytotoxic action of Onopordum acanthium essential oil. Sci. Rep. 2020, 10, 13568. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.; Munne-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formisano, C.; Rigano, D.; Senatore, F.; Bruno, M.; Rosselli, S.; Raimondo, F.M.; Spadaro, V. Chemical composition of the essential oil of Centaurea sicana and C. giardinae growing wild in Sicily. Nat. Prod. Commun. 2008, 3, 919–922. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant Sources, Extraction Methods, and Uses of Squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef]
- Kim, D.K.; Lim, J.P.; Kim, J.W.; Park, H.W.; Eun, J.S. Antitumor and antiinflammatory constituents from Celtis sinensis. Arch. Pharm. Res. 2005, 28, 39–43. [Google Scholar] [CrossRef]
- Bhui, D.K.; Misra, A. Synthesis of worm like silver nanoparticles in methyl cellulose polymeric matrix and its catalytic activity. Carbohydr. Polym. 2012, 89, 830–835. [Google Scholar] [CrossRef]
- Proudfoot, J.M.; Croft, K.D.; Puddey, I.B.; Beilin, L.J. The role of copper reduction by α-tocopherol in low-density lipoprotein oxidation. Free Radic. Biol. Med. 1997, 23, 720–728. [Google Scholar] [CrossRef]
- Roopan, S.M.; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crops Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Skiba, M.I.; Vorobyova, V.I.; Pivovarov, A.; Makarshenko, N.P. Green synthesis of silver nanoparticles in the presence of polysaccharide: Optimization and characterization. J. Nanomater. 2020, 2020, 3051308. [Google Scholar] [CrossRef]
- Ju Park, E.; Won Lee, S.; Bang, I.C.; Park, H.W. Optimal synthesis and characterization of Ag nanofluids by electrical explosion of wires in liquids. Nanoscale Res. Lett. 2011, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Assis, M.D.; Robeldo, T.; Foggi, C.C.; Kubo, A.M.; Mínguez-Vega, G.; Condoncillo, E.; Beltran-Mir, H.; Torres-Mendieta, R.; Andrés, J.; Oliva, M.; et al. Ag Nanoparticles/α-Ag2WO4 Composite Formed by Electron Beam and Femtosecond Irradiation as Potent Antifungal and Antitumor Agents. Sci. Rep. 2019, 9, 9927. [Google Scholar] [CrossRef] [PubMed]
- Rjeibi, I.; Ben Saad, A.; Ncib, S.; Souid, S.; Allagui, M.S.; Hfaiedh, N. Brachychiton populneus as a novel source of bioactive ingredients with therapeutic effects: Antioxidant, enzyme inhibitory, anti-inflammatory properties and LC–ESI-MS profile. Inflammopharmacology 2020, 28, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Abou Zeid, A.H.; Hamed, M.A.; Kandeel, Z.; El-Rafie, H.M.; El-Akad, R.H. Metabolomic fingerprint classification of Brachychiton acerifolius organs via UPLC-qTOF-PDA-MS analysis and chemometrics. Nat. Prod. Res. 2015, 29, 116–124. [Google Scholar] [CrossRef]
- Irawaty, W.; Ayucitra, A. Assessment on antioxidant and in vitro antidiabetes activities of different fractions of Citrus hystrix peel. Int. Food Res. J. 2018, 25, 2467–2477. [Google Scholar]
- Merrill, R.L.; Dionne, R.A. Medications for Management of Chronic, Non-Odontogenic Pain. In Pharmacology and Therapeutics for Dentistry, 7th ed.; Dowd, F.J., Mariotti, A., Eds.; Mosby: Maryland Heights, MO, USA, 2017; pp. 564–574. [Google Scholar]
- Lewis, J.H.; Stine, J.G. Nonsteroidal antiinflammatory drugs and leukotriene receptor antagonists. In Drug-Induced Liver Disease; Academic Press: Cambridge, MA, USA, 2013; pp. 369–401. [Google Scholar]
- Padilha, M.M.; Vilela, F.C.; Rocha, C.Q.; Dias, M.J.; Soncini, R.; dos Santos, M.H.; Alves-da-Silva, G.; Giusti-Paiva, A. Antiinflammatory properties of Morus nigra leaves. Phyther. Res. 2010, 24, 1496–1500. [Google Scholar] [CrossRef]
- Alahmad, A.; Al-Zereini, W.A.; Hijazin, T.J.; Al-Madanat, O.Y.; Alghoraibi, I.; Al-Qaralleh, O.; Al-Qaraleh, S.; Feldhoff, A.; Walter, J.-G.; Scheper, T. Green Synthesis of Silver Nanoparticles Using Hypericum perforatum L. Aqueous Extract with the Evaluation of Its Antibacterial Activity against Clinical and Food Pathogens. Pharmaceutics 2022, 14, 1104. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Delgado-Beleño, Y.; Martinez-Nuñez, C.E.; Cortez-Valadez, M.; Flores-López, N.S.; Flores-Acosta, M. Optical properties of silver, silver sulfide and silver selenide nanoparticles and antibacterial applications. Mater. Res. Bull. 2018, 99, 385–392. [Google Scholar] [CrossRef]
- Alkhsabah, I.A.; Alsharafa, K.Y.; Kalaji, H.M. Effects of abiotic factors on internal homeostasis of Mentha spicata leaves. Appl. Ecol. Environ. Res. 2018, 16, 2537–2564. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Oelze, M.L.; Vogel, M.O.; Alsharafa, K.; Kahmann, U.; Viehhauser, A.; Maurino, V.G.; Dietz, K.J. Efficient acclimation of the chloroplast antioxidant defence of Arabidopsis thaliana leaves in response to a 10-or 100-fold light increment and the possible involvement of retrograde signals. J. Exp. Bot. 2012, 63, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Alsharafa, K.Y. Mineral deficiencies influence on tomato leaves: Pigments, hydrogen peroxide and total phenolic compounds contents. Plant Omics. 2017, 10, 78–87. [Google Scholar] [CrossRef]
- Al-Sammarraie, O.N.; Alsharafa, K.Y.; Al-limoun, M.O.; Khleifat, K.M.; Al-Sarayreh, S.A.; Al-Shuneigat, J.M.; Kalaji, H.M. Effect of various abiotic stressors on some biochemical indices of Lepidium sativum plants. Sci Rep. 2020, 10, 21131. [Google Scholar] [CrossRef] [PubMed]
- Dellero, Y.; Clouet, V.; Marnet, N.; Pellizzaro, A.; Dechaumet, S.; Niogret, M.F.; Bouchereau, A. Leaf status and environmental signals jointly regulate proline metabolism in winter oilseed rape. J. Exp. Bot. 2020, 71, 2098–2111. [Google Scholar] [CrossRef] [PubMed]
- Khoma, Y.A.; Nesterenko, O.G.; Kutsokon, N.K.; Khudolieieva, L.V.; Shevchenko, V.V.; Rashydov, N.M. Proline content in the leaves of poplar and willow under water deficit. Regul. Mech. Biosyst. 2021, 12, 519–522. [Google Scholar] [CrossRef]
- Naik, R.R.; Shakya, A.K.; Ferri, B.; Oriquat, G.A.; Pistelli, L.; Numan, N.A. Volatile composition and biological activity of Jordanian commercial samples of R. coriaria L. fruits. Molecules 2021, 26, 5691. [Google Scholar] [CrossRef]
- Al-Jaber, H.I.; Shakya, A.K.; Elagbar, Z.A. HPLC profiling of selected phenolic acids and flavonoids in Salvia eigii, Salvia hierosolymitana and Salvia viridis growing wild in Jordan and their in vitro antioxidant activity. PeerJ 2020, 8, e9769. [Google Scholar] [CrossRef]
- Kar, B.; Kumar, R.S.; Karmakar, I.; Dola, N.; Bala, A.; Mazumder, U.K.; Hadar, P.K. Antioxidant and in vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac. J. Trop. Biomed. 2012, 2, 5–7. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dere, Ş.; Güneş, T.; Sivaci, R. Spectrophotometric Determination of Chlorophyll-A, B and Total Carotenoid Contents of Some Algae Species Using Different Solvents. Tr. J. Botany 1998, 22, 13–18. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]

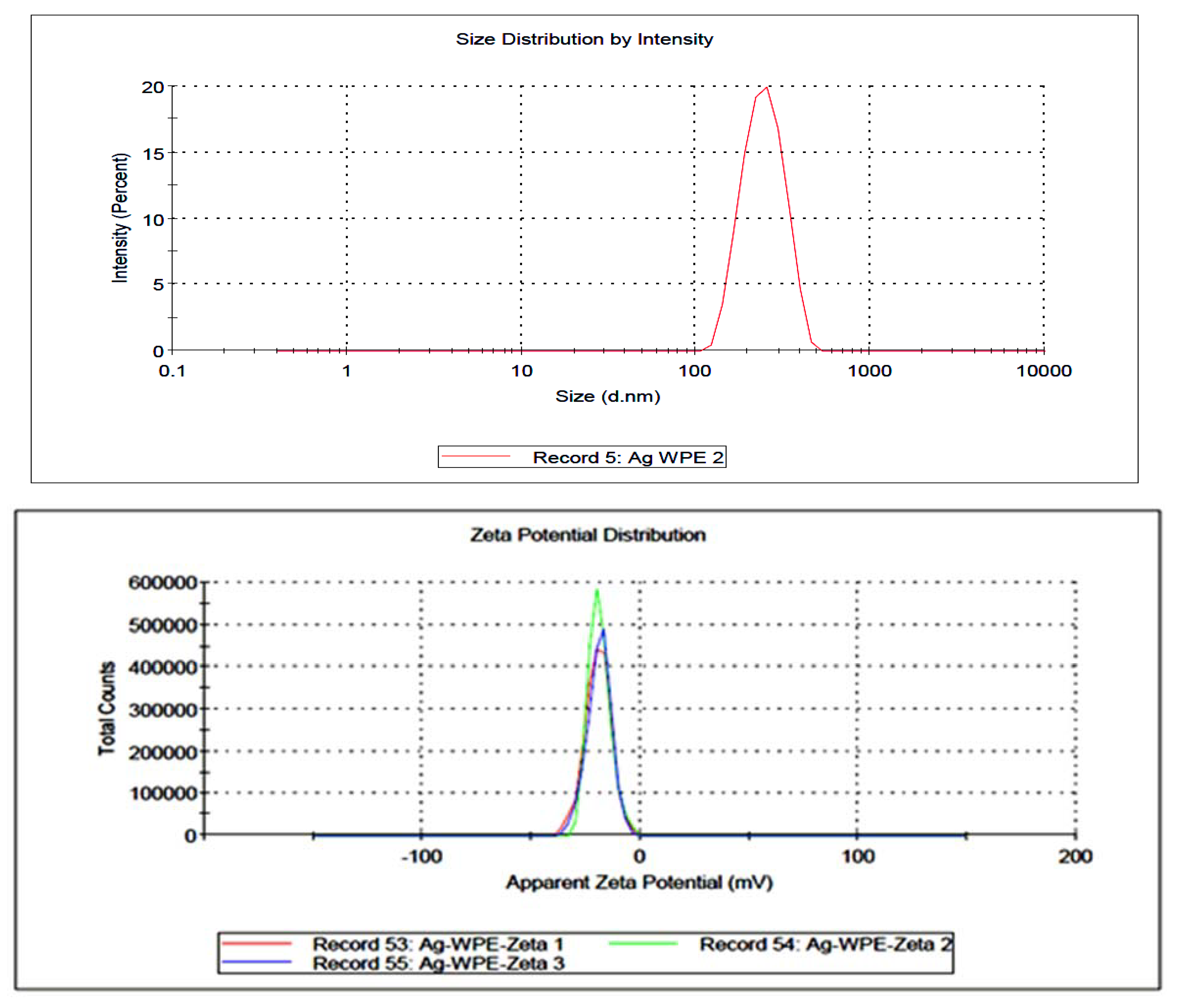
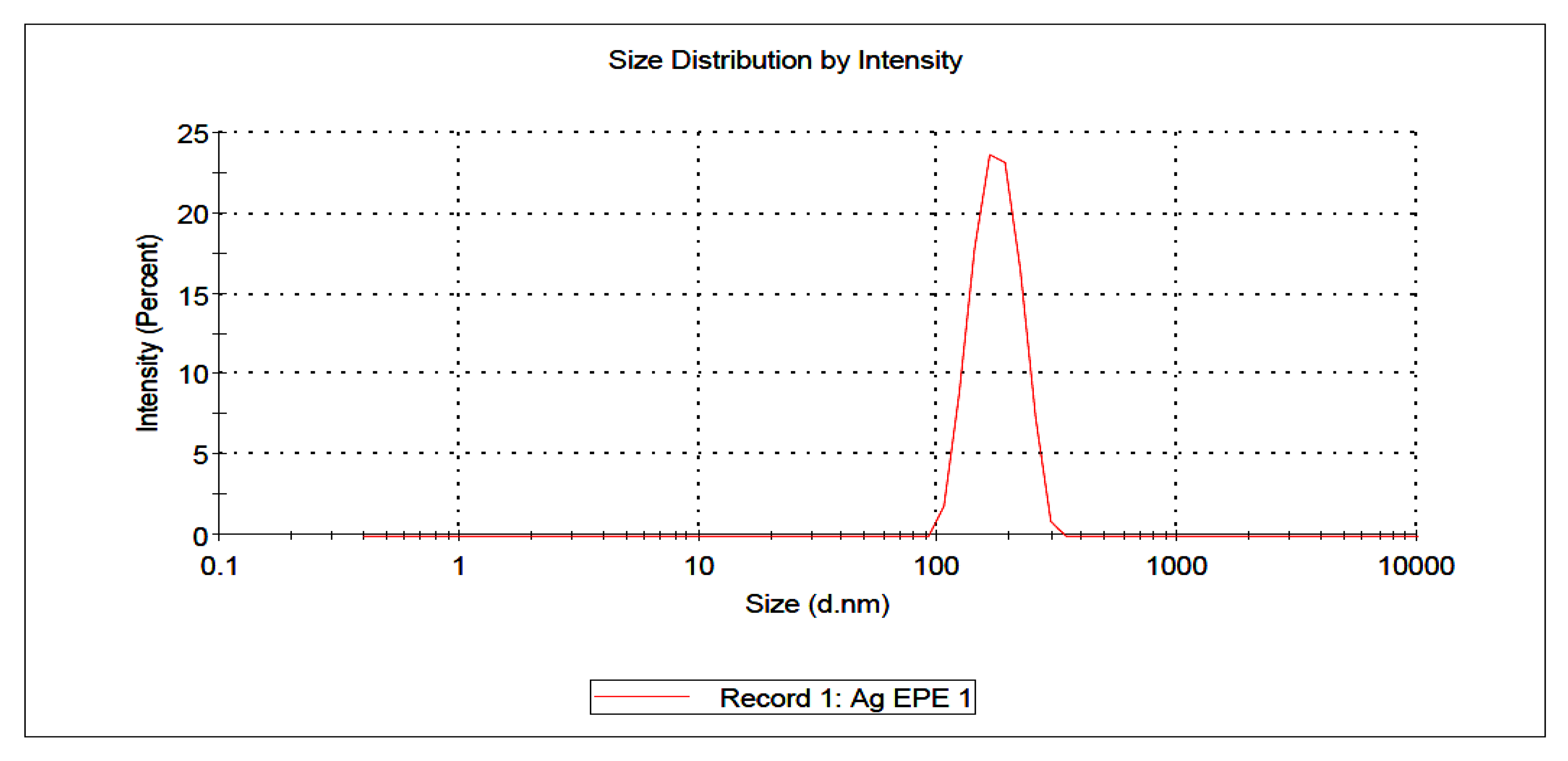
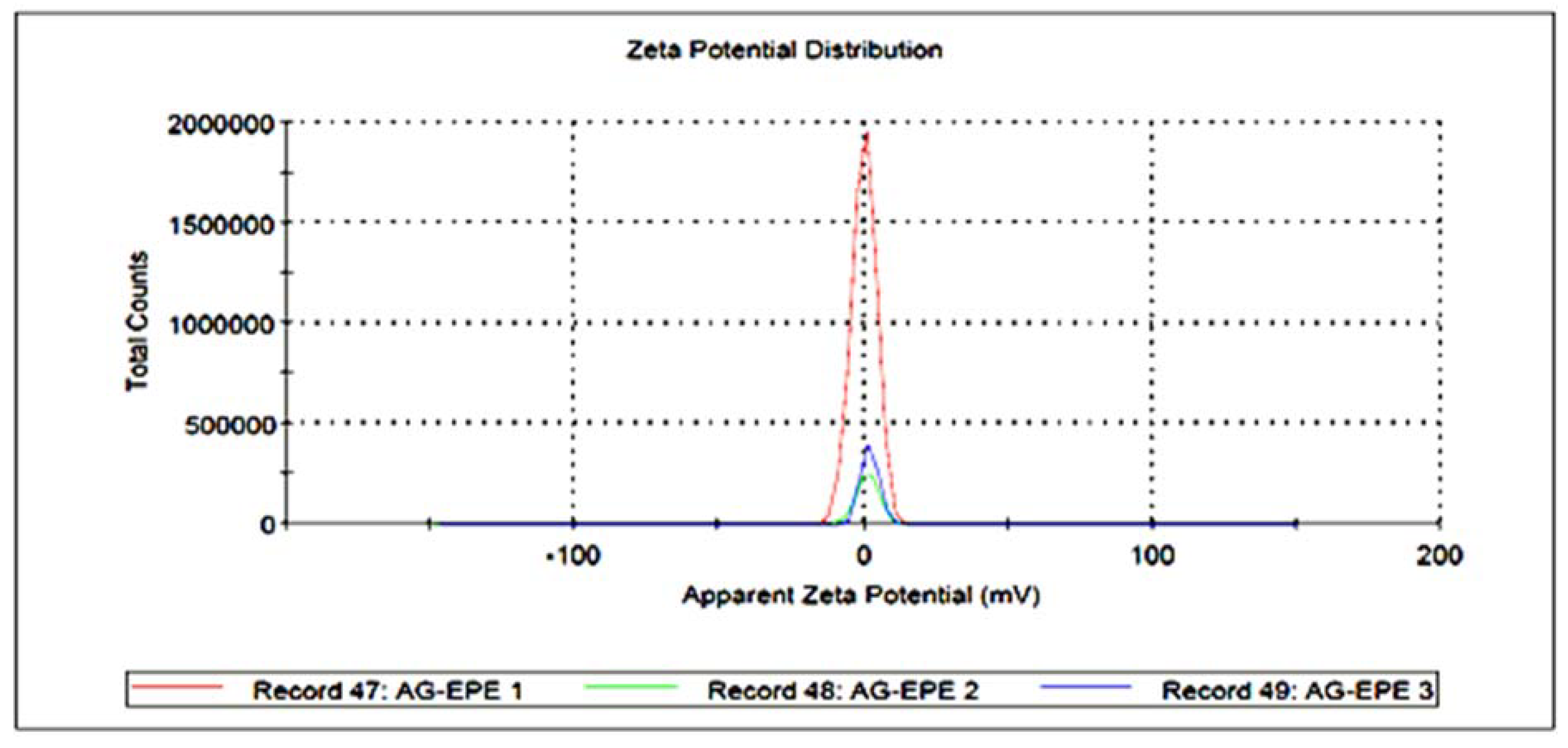

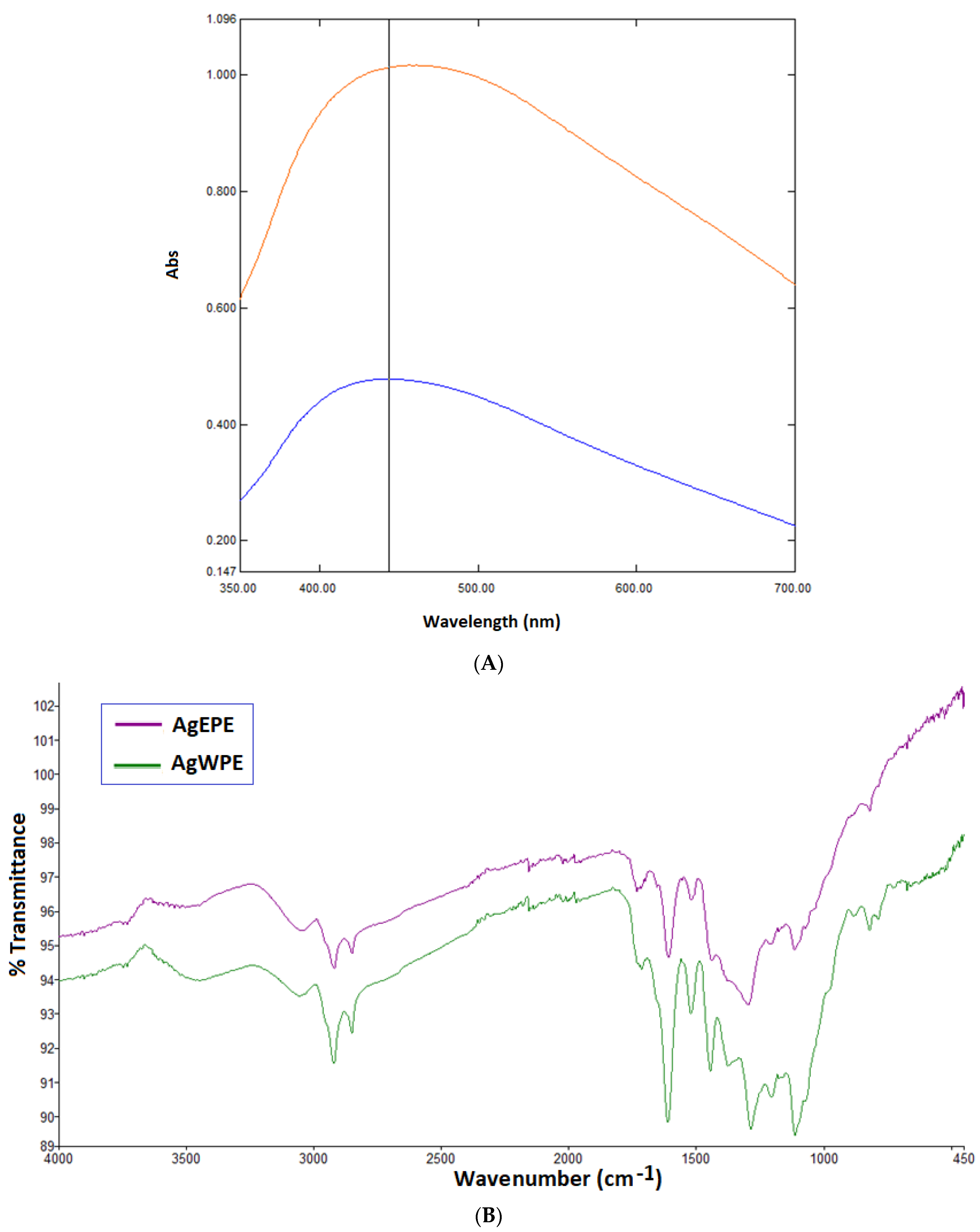
 ) and WPE (
) and WPE ( ); (B) FT-IR spectroscopic analysis of Synthesized AgNPs using EPE (
); (B) FT-IR spectroscopic analysis of Synthesized AgNPs using EPE ( ) and WPE (
) and WPE ( ).
).
 ) and WPE (
) and WPE ( ); (B) FT-IR spectroscopic analysis of Synthesized AgNPs using EPE (
); (B) FT-IR spectroscopic analysis of Synthesized AgNPs using EPE ( ) and WPE (
) and WPE ( ).
).

| Peak | Ret. Time (min.) | Area% | Name | Ret. Index |
|---|---|---|---|---|
| 1 | 23.407 | 24.88 | 1-Octadecyne | 1856 |
| 2 | 24.13 | 1.96 | Unknown | 1878 |
| 3 | 27.442 | 3.54 | Phytol | 2104 |
| 4 | 32.428 | 2.89 | Pentacosane | 2492 |
| 5 | 32.799 | 4.19 | Unknown | 2524 |
| 6 | 34.762 | 4.41 | Heptacosane | 2690 |
| 7 | 36.31 | 7.19 | Squalene | 2804 |
| 8 | 42.546 | 5.24 | dl-α-Tocopherol | 3112 |
| 9 | 49.981 | 6.23 | Germanicol | 3327 |
| 10 | 51.569 | 30.97 | (3β)-Lup-20(29)-en-3-ol acetate | 3362 |
| Compound | Structure |
|---|---|
| Phytol | 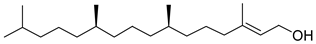 |
| Squalene | 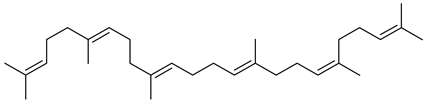 |
| Germanicol | 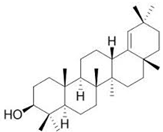 |
| 1-octadecyne | 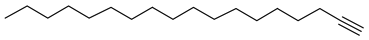 |
| Heptacosane |  |
| 3β-Lup-20(29)-en-3-ol acetate |  |
| dl-α-tocopherol | 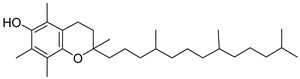 |
| Sample | Particle Size (nm) (Mean ± SD) | Zeta Potential (mV) (Mean ± SE) |
|---|---|---|
| AgWPE | 247.1 ± 3.56 | −19.0 ± 3.20 |
| AgEPE | 304.4 ± 9.61 | 2.12 ± 1.84 |
| Sample | Total Phenolics (mg GAE/g Extract) | Total Flavonoids (mg QE/g Extract) |
|---|---|---|
| WPE | 5.33 ± 0.01 | 64.88 ± 1.26 |
| EPE | 7.10 ± 0.03 | 29.71 ± 0.87 |
| Sample | IC50 (µg/mL) |
|---|---|
| DPPH Radical Activity * | |
| WPE | 119.0 ± 1.25 |
| EPE | 65.4 ± 1.02 |
| Ascorbic Acid (50% EtOH) | 1.25 ± 0.05 |
| Sample * | % Protein Inhibition IC50 (µg/mL) (Mean ± SD) |
|---|---|
| WPE | 95.5 ± 7.4 |
| EPE | 115.1 ± 8.8 |
| Diclofenac Potassium | 51.5 ± 5.2 |
| Inhibition Zone (mm) | ||
|---|---|---|
| Sample Name | E. coli | S. aureus |
| WPE | 8 | 8 |
| EPE | 9 | 8 |
| AgWPE | 18 | 18 |
| AgEPE | 16 | 15 |
| Moxifloxacin (25 µg/mL) | 30 | 52 |
| Measurement * | Content |
|---|---|
| Chlorophyll a (mg g−1 FW) | 0.805495 ± 0.012062 |
| Chlorophyll b (mg g−1 FW) | 0.31829 ± 0.012019 |
| Total chlorophyll (mg g−1 FW) | 1.143893 ± 0.017276 |
| Carotenoids (mg g−1 FW) | 0.298165 ± 0.006863 |
| Chlorophyll a:b | 2.534345 ± 0.102977 |
| Chlorophyll: carotenoids | 3.83719 ± 0.037265 |
| Proline (mg g−1 FW) | 10.02667 ± 0.169967 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ramamneh, E.A.-D.M.; Ghrair, A.M.; Shakya, A.K.; Alsharafa, K.Y.; Al-Ismail, K.; Al-Qaraleh, S.Y.; Mojski, J.; Naik, R.R. Efficacy of Sterculia diversifolia Leaf Extracts: Volatile Compounds, Antioxidant and Anti-Inflammatory Activity, and Green Synthesis of Potential Antibacterial Silver Nanoparticles. Plants 2022, 11, 2492. https://doi.org/10.3390/plants11192492
Al-Ramamneh EA-DM, Ghrair AM, Shakya AK, Alsharafa KY, Al-Ismail K, Al-Qaraleh SY, Mojski J, Naik RR. Efficacy of Sterculia diversifolia Leaf Extracts: Volatile Compounds, Antioxidant and Anti-Inflammatory Activity, and Green Synthesis of Potential Antibacterial Silver Nanoparticles. Plants. 2022; 11(19):2492. https://doi.org/10.3390/plants11192492
Chicago/Turabian StyleAl-Ramamneh, Ezz Al-Dein M., Ayoup M. Ghrair, Ashok K. Shakya, Khalid Y. Alsharafa, Khalid Al-Ismail, Samer Y. Al-Qaraleh, Jacek Mojski, and Rajashri R. Naik. 2022. "Efficacy of Sterculia diversifolia Leaf Extracts: Volatile Compounds, Antioxidant and Anti-Inflammatory Activity, and Green Synthesis of Potential Antibacterial Silver Nanoparticles" Plants 11, no. 19: 2492. https://doi.org/10.3390/plants11192492
APA StyleAl-Ramamneh, E. A.-D. M., Ghrair, A. M., Shakya, A. K., Alsharafa, K. Y., Al-Ismail, K., Al-Qaraleh, S. Y., Mojski, J., & Naik, R. R. (2022). Efficacy of Sterculia diversifolia Leaf Extracts: Volatile Compounds, Antioxidant and Anti-Inflammatory Activity, and Green Synthesis of Potential Antibacterial Silver Nanoparticles. Plants, 11(19), 2492. https://doi.org/10.3390/plants11192492












