Effect of Relative Humidity on the Population Dynamics of the Predator Amblyseius swirskii and Its Prey Carpoglyphus lactis in the Context of Slow-Release Sachets for Use in Biological Control in Greenhouses
Abstract
:1. Introduction
2. Results
2.1. Trial 1: Dynamic Changes in the Populations of A. swirskii and C. lactis Inside the Sachets According to RH
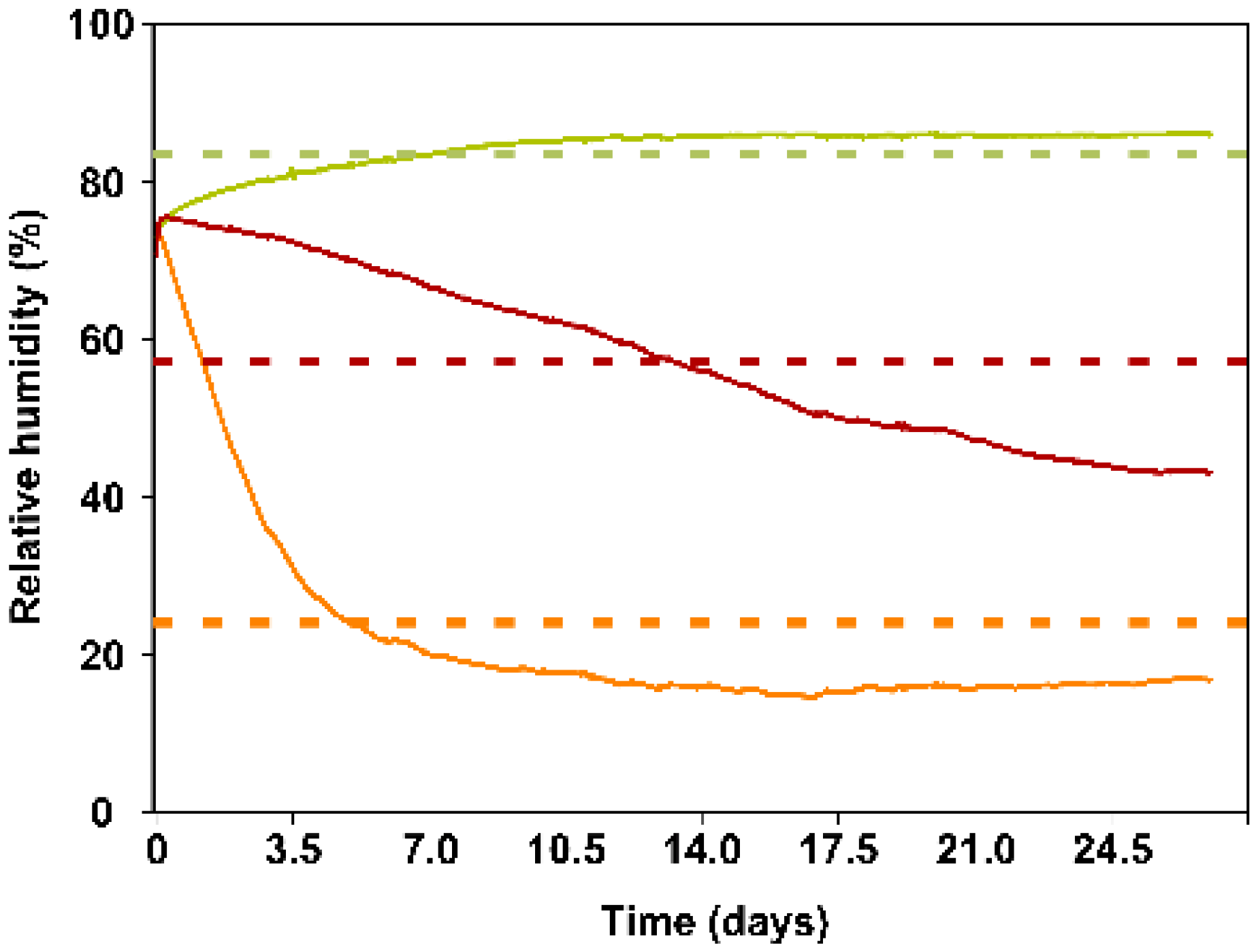
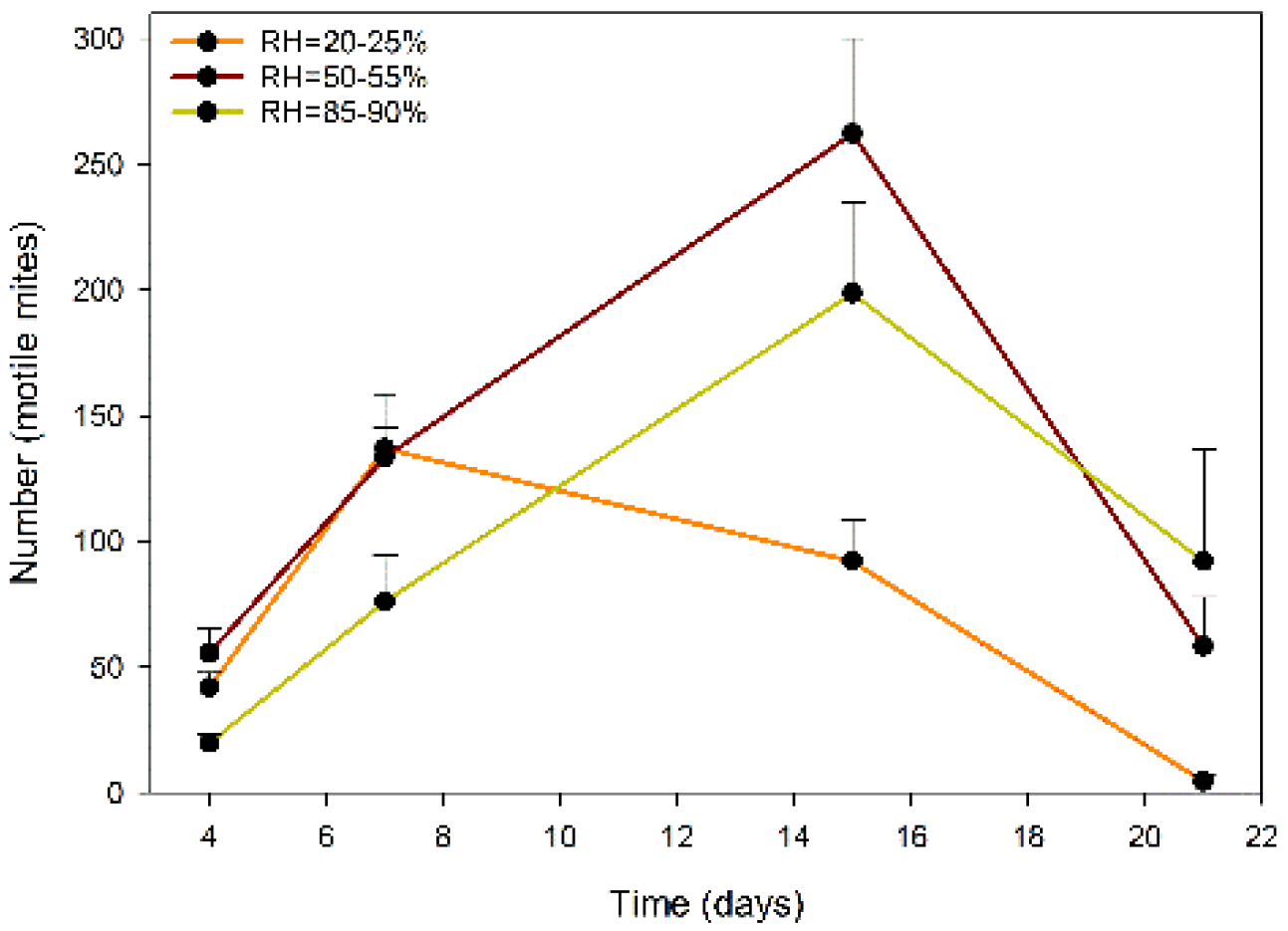
2.2. Trial 2: Evaluation of the Release of A. swirskii from the Sachets, According to RH
2.3. Fit of the Mathematical Model to the Number of Predatory Mites Released from the Sachets: Rate and Period of Mite Release, According to the RH Regime
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Trials Conditions
4.3. Trial 1: Evaluation of the Populations of A. swirskii and C. lactis Inside the Sachets
4.4. Evaluation of the Release of A. swirskii from the Sachets
4.5. Statistical Analysis
4.6. Mathematical Model Biological Material
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghazy, N.A.; Osakabe, M.; Waleed, M.; Schausberger, P.; Gotoh, T.; Amano, H. Phytoseiid mites under environmental stress. Biol. Control 2016, 96, 120–134. [Google Scholar] [CrossRef]
- San, P.P.; Tuda, M.; Takagi, M. Impact of relative humidity and water availability on the life history of the predatory mite Amblyseius swirskii. Bio. Control 2021, 66, 497–510. [Google Scholar] [CrossRef]
- Goleva, I.; Zebitz, C.P.W. Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp. Appl. Acarol. 2013, 61, 259–283. [Google Scholar] [CrossRef] [PubMed]
- Nemati, A.; Riahi, E. Does feeding on pollen grains affect the performance of Amblyseius swirskii (Acari: Phytoseiidae) during subsequent generations? Bull. Entomol. Res. 2019, 110, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.J.; Knapp, M.; Van Houten, Y.M.; Hoogerbrugge, H.; Belda, J.E. Amblyseius swirskii: What made this predatory mite such a successful biocontrol agent? Exp. Appl. Acarol. 2015, 65, 419–433. [Google Scholar] [CrossRef]
- Midthassel, A.; Baxter, I.H.; Stepman, W.; Boullenger, A. The effect of relative humidity and temperature on predator release from an Amblyseius swirskii (Acari: Phytoseiidae) breeding sachet. IOBC-WPRS Bull. 2014, 102, 151–155. [Google Scholar]
- Vila, E.; Cabello, T. Biosystems engineering applied to greenhouse pest control. In Biosystems Engineering: Biofactories for Food Production in the XXI Century; Torres, I., Guevara, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 99–128. [Google Scholar] [CrossRef]
- Bolckmans, K.J.F.; Van Houten, Y.M. Mite Composition, Use Thereof, Method for Rearing the Phytoseiid Predatory Mite Amblyseius swirskii, Rearing System for Rearing Said Phytoseiid Mite and Methods for Biological Pest Control on a Crop. WO Patent WO/2006/057552, 1 June 2006. [Google Scholar]
- Bolckmans, K.J.F.; Van Houten, Y.M.; Van Baal, A.E.; Stam, A.T. Phytoseiid Predatory Mite Releasing System and Method for Production. World Patent WO/2013/043,050, 28 March 2013. [Google Scholar]
- Shimoda, T.; Kagawa, Y.; Yoshizawa, H.; Nakano, A.; Matsuhira, K.; Yanagita, H.; Shimomoto, M.; Adachi-Hagimori, T.; Mori, K.; Hinomoto, N.; et al. Moisturized sheltered sachets are potentially useful for the efficient release of selected predators in a wide range of humidity environments. Bio. Control 2019, 64, 65–67. [Google Scholar] [CrossRef]
- Sampson, C. The commercial development of an Amblyseius cucumeris controlled release method for the control of Frankliniella occidentalis in protected crops. The 1998 Brighton conference—Pests and Diseases. In Proceedings of the Brighton Crop Protection Conference, Brighton, UK, 16–19 November 1998; British Crop Protection Council: Farnham, UK, 1998; Volume 5B-4, pp. 409–416. [Google Scholar]
- Jacobson, R.J.; Croft, P.; Fenlon, J. Suppressing establishment of Frankliniella occidentalis (Thysanoptera: Thripidae) in cucumber crops by prophylactic release of Amblyseius cucumeris (Acarina: Phytoseiidae). Biocontrol Sci. Technol. 2001, 11, 27–34. [Google Scholar] [CrossRef]
- Midthassel, A.; Leather, S.R.; Wright, D.J.; Baxter, I.H. The functional and numerical response of Typhlodromips swirskii (Acari: Phytoseiidae) to the factitious prey Suidasia medanensis (Acari: Suidasidae) in the context of a breeding sachet. Biocontrol Sci. Technol. 2014, 24, 361–374. [Google Scholar] [CrossRef]
- Asgari, F.; Sarraf, H.R.; Kavousi, A.; Enkegaard, A.; Chi, H. Demography and mass rearing of Amblyseius swirskii (Acari: Phytoseiidae) fed on two species of stored-product mites and their mixture. J. Econ. Entomol. 2020, 113, 2604–2612. [Google Scholar] [CrossRef]
- Collins, D.A. A review on the factors affecting mite growth in stored grain commodities. Exp. Appl. Acarol. 2012, 56, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.; Gigot, C.; Tixier, M.S.; Van Houten, Y.M.; Kreiter, S. Egg hatching response to a range of air humidities for six species of predatory mites. Entomol. Exp. Appl. 2010, 135, 237–244. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Hale, A.; Klapwijk, J.N. Guidelines for quality control of commercially produced natural enemies. In Quality Control and Production of Biological Control Agents. Theory and Testing Procedures; Van Lenteren, J.C., Ed.; CAB International: Wallingford, UK, 2003; pp. 265–303. [Google Scholar] [CrossRef]
- Taylor, T.C.H. Report of the entomologist. In Report of the Department of Agriculture; Authority of the Government of the Uganda: Kawande, Uganda, 1940; pp. 9–24. [Google Scholar]
- van Emden, H.F. Conservation biological control: From theory to practice. In Proceedings of the 1st International Symposium on Biological Control of Arthropods, Honolulu, HI, USA, 14–18 January 2002; Publication FHTET-03-05 199-208. USDA Forest Service: Washington, DC, USA, 2002; pp. 199–208. [Google Scholar]
- Vazquez, L.L. Climate change and biological control of pests in agriculture. In Natural Enemies of Insect Pests in Neotropical Agroecosystems; Souza, B., Vazquez, R., Marucci, C., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 63–69. [Google Scholar] [CrossRef]
- De Courcy Williams, M.E.; Kravar-Garde, L.; Fenlon, J.S.; Sunderland, K.D. Phytoseiid mites in protected crops: The effect of humidity and food availability on egg hatch and adult life span of Iphiseius degenerans, Neoseiulus cucumeris, N. californicus and Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2004, 32, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Midthassel, A. Interactions between the Predatory Mite Amblyseius swirskii and Its Factitious Prey Suidasia medenensis with Implications for Field Release. Ph.D. Thesis, Imperial College, London, UK, 2015; p. 278. Available online: https://spiral.imperial.ac.uk/bitstream/10044/1/26986/1/Midthassel-A-2015-PhD-Thesis.pdf (accessed on 15 August 2022).
- Shimoda, T.; Kagawa, Y.; Mori, K.; Hinomoto, N.; Hiraoka, T.; Nakajima, T. A novel method for protecting slow-release sachets of predatory mites against environmental stresses and increasing predator release to crops. Bio. Control 2017, 62, 495–503. [Google Scholar] [CrossRef]
- Bakker, F.M.; Klein, M.E.; Mesa, N.C.; Braun, A.R. Saturation deficit tolerance spectra of phytophagous mites and their phytoseiid predators on cassava. Exp. Appl. Acarol. 1993, 17, 97–113. [Google Scholar] [CrossRef]
- Walzer, A.; Castagnoli, M.; Simoni, S.; Liguori, M.; Palevsky, E.; Schausberger, P. Intraspecific variation in humidity susceptibility of the predatory mite Neoseiulus californicus: Survival, development, and reproduction. Biol. Control 2007, 41, 42–52. [Google Scholar] [CrossRef]
- Walzer, A.; Castagnoli, M.; Simoni, S.; Liguori, M.; Palevsky, E.; Schausberger, P. Identification of a drought-adapted Neoseiulus californicus strain: Egg hatchability, juvenile survival and oviposition at low humidities. In Trends in Acarology; Sabelis, M., Bruin, J., Eds.; Springer: Dordrecht, The Netherland, 2010; pp. 279–283. [Google Scholar] [CrossRef]
- Rasmy, A.H.; Abou-El-Ella, G.M.; Hussein, H.E. Cannibalism and interspecific predation of the phytoseiid mite, Amblyseius swirskii. J. Pest Sci. 2004, 77, 23–25. [Google Scholar] [CrossRef]
- Okamoto, M. Studies on the environmental factors for the life cycle of Carpoglyphus lactis. 1. The effects of relative humidities on individual rearing. Med. Entomol. Zool. 1984, 35, 269–275. [Google Scholar] [CrossRef]
- Schausberger, P. The influence of relative humidity on egg hatch in Euseius finlandicus, Typhlodromus pyri and Kampimodromus aberrans (Acari: Phytoseiidae). J. Appl. Entomol. 1998, 122, 497–500. [Google Scholar] [CrossRef]
- Shipp, J.L.; Van Houten, Y.M. Influence of temperature and vapor pressure deficit on survival of the predatory mite Amblyseius cucumeris (Acari: Phytoseiidae). Environ. Entomol. 1997, 26, 106–113. [Google Scholar] [CrossRef]
- Gomez-Moya, C.A.; Gondim, M.G.; De Moraes, G.J.; De Morais, E.G. Effect of relative humidity on the biology of the predatory mite Amblyseius largoensis (Acari: Phytoseiidae). Int. J. Acarol. 2018, 44, 400–411. [Google Scholar] [CrossRef]
- Jafari, S.; Sarraf, H.; Kavousi, A. Temperature-dependent life table of the predatory mite, Amblyseius swirskii (Mesostigmata: Phytoseidae) fed on stored product mite Carpoglyphus lactis (Astigmata: Carpoglyphidae). J. Entomol. Soc. Iran 2016, 36, 163–179. [Google Scholar]
- Nomikou, M.; Janssen, A.; Sabelis, M.W. Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp. Appl. Acarol. 2003, 31, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Midthassel, A.; Leather, S.R.; Baxter, I.H. Life table parameters and capture success ratio studies of Typhlodromips swirskii (Acari: Phytoseiidae) to the factitious prey Suidasia medanensis (Acari: Suidasidae). Exp. Appl. Acarol. 2013, 61, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Chant, D.A. The influence of prey density, relative humidity, and starvation on the predacious behavior of Phytoseiulus persimilis (Acarina: Phytoseiidae). Can. J. Zool. 1966, 44, 483–491. [Google Scholar] [CrossRef]
- Döker, I.; Kazak, G.; Karut, K. Functional response and fecundity of a native Neoseiulus californicus population to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae) at extreme humidity conditions. Syst. Appl. Acarol. 2016, 21, 1463–1472. [Google Scholar] [CrossRef]
- Abou-Awad, B.A.; Afia, S.I.; Al-Azzazy, M.M. Mango powdery mildew Oidium mangiferae and alternative food for the predatory mites Typhlodromus mangiferus and Typhlodromips swirskii (Phytoseiidae) in absence or presence increasing prey density of Oligonichus mangiferus (Tetranychidae) in Egypt. Arch. Phytopathol. PFL 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Buitenhuis, R.; Glemser, E.; Brommit, A. Practical placement improves the performance of slow-release sachets of Neoseiulus cucumeris. Biocontrol Sci. Technol. 2014, 24, 1153–1166. [Google Scholar] [CrossRef]
- Addesso, K.M.; Witcher, A.L.; Fare, D.C. Swirski mite controlled-release sachets as a pest management tool in container tree production. HortTechnology 2018, 28, 391–398. [Google Scholar] [CrossRef]
- Gazquez, J.C.; Lopez, J.C.; Perez, C.; Baeza, E.; Meca, D.E.; Perez, J. Tecnologia de invernaderos y gestion integrada de plagas. In Gestion Integrada de Invernaderos en el Area Mediterranea; Gazquez, J.C., Racero, J.L., Eds.; Cajamar Caja Rural: Almeria, Spain, 2015; pp. 69–98. [Google Scholar]
- Winston, P.W.; Bates, D.H. Saturated solution for the control of humidity in biological research. Ecology 1960, 41, 232–237. [Google Scholar] [CrossRef]
- Arduino-Home. Available online: https://www.arduino.cc (accessed on 25 November 2021).
- Martinez, E.; Fernandez, I. Determinación de la Humedad de un Alimento por un Método Gravimetrico Indirecto por Desecacion; Universitat Politecnica de Valencia: Valencia, Spain, 2012; pp. 1–5. Available online: https://riunet.upv.es/bitstream/handle/10251/16339/Determinación%20de%20humedad.pdf (accessed on 15 August 2022).
- Gallego, J.R.; Solano-Rojas, Y.; Tiseyra, B.; Gamez, M.; Cabello, T. Population dynamics of mites in slow-release sachets used in biological control: A new study methodology. Exp. Appl. Acarol. 2022, 87, 325–335. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows; Version 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
- Dugaw, C.J. Birth-death models. In Encyclopedia of Theoretical Ecology; Hasting, A., Gross, L.J., Eds.; University of California Press: Berkeley, CA, USA, 2012; pp. 101–106. [Google Scholar]
- Jandel Scientific. Table Curve 2D-User’s Manual; Version 5.0; Jandel Scientific: San Rafael, CA, USA, 1994. [Google Scholar]

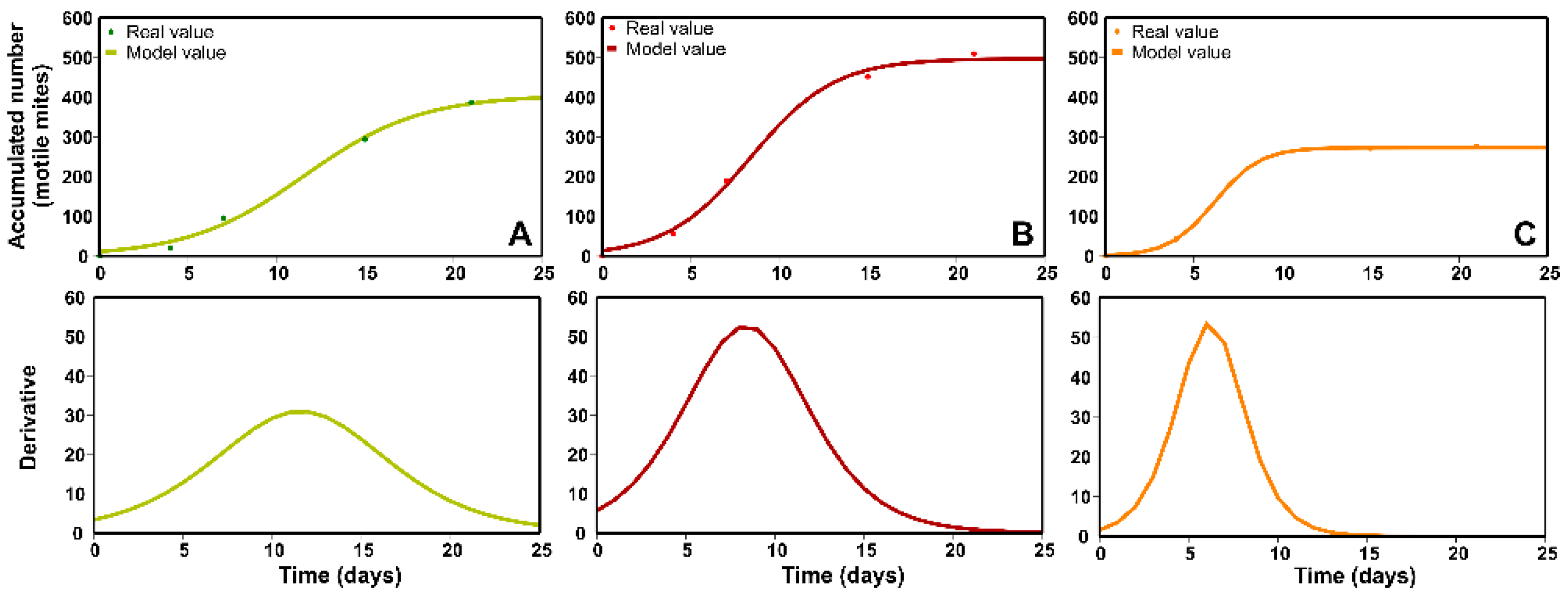
| RH(%) | Fitting Parameters | Statistical Parameters | ||||
|---|---|---|---|---|---|---|
| K | x0 | r | d.f. | R2adj. | p | |
| 22.5 | 273.378 | 2.096 | 0.7857 | 3 | 0.9991 | <0.01 |
| 52.5 | 497.206 | 13.805 | 0.4243 | 3 | 0.9815 | <0.01 |
| 87.5 | 405.467 | 11.250 | 0.3059 | 3 | 0.9769 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solano-Rojas, Y.; Gallego, J.R.; Gamez, M.; Lopez, I.; Castillo, P.; Cabello, T. Effect of Relative Humidity on the Population Dynamics of the Predator Amblyseius swirskii and Its Prey Carpoglyphus lactis in the Context of Slow-Release Sachets for Use in Biological Control in Greenhouses. Plants 2022, 11, 2493. https://doi.org/10.3390/plants11192493
Solano-Rojas Y, Gallego JR, Gamez M, Lopez I, Castillo P, Cabello T. Effect of Relative Humidity on the Population Dynamics of the Predator Amblyseius swirskii and Its Prey Carpoglyphus lactis in the Context of Slow-Release Sachets for Use in Biological Control in Greenhouses. Plants. 2022; 11(19):2493. https://doi.org/10.3390/plants11192493
Chicago/Turabian StyleSolano-Rojas, Yohan, Juan R. Gallego, Manuel Gamez, Inmaculada Lopez, Patricia Castillo, and Tomas Cabello. 2022. "Effect of Relative Humidity on the Population Dynamics of the Predator Amblyseius swirskii and Its Prey Carpoglyphus lactis in the Context of Slow-Release Sachets for Use in Biological Control in Greenhouses" Plants 11, no. 19: 2493. https://doi.org/10.3390/plants11192493







