A Comprehensive Analysis of Physiologic and Hormone Basis for the Difference in Room-Temperature Storability between ‘Shixia’ and ‘Luosanmu’ Longan Fruits
Abstract
:1. Introduction
2. Results and Discussion
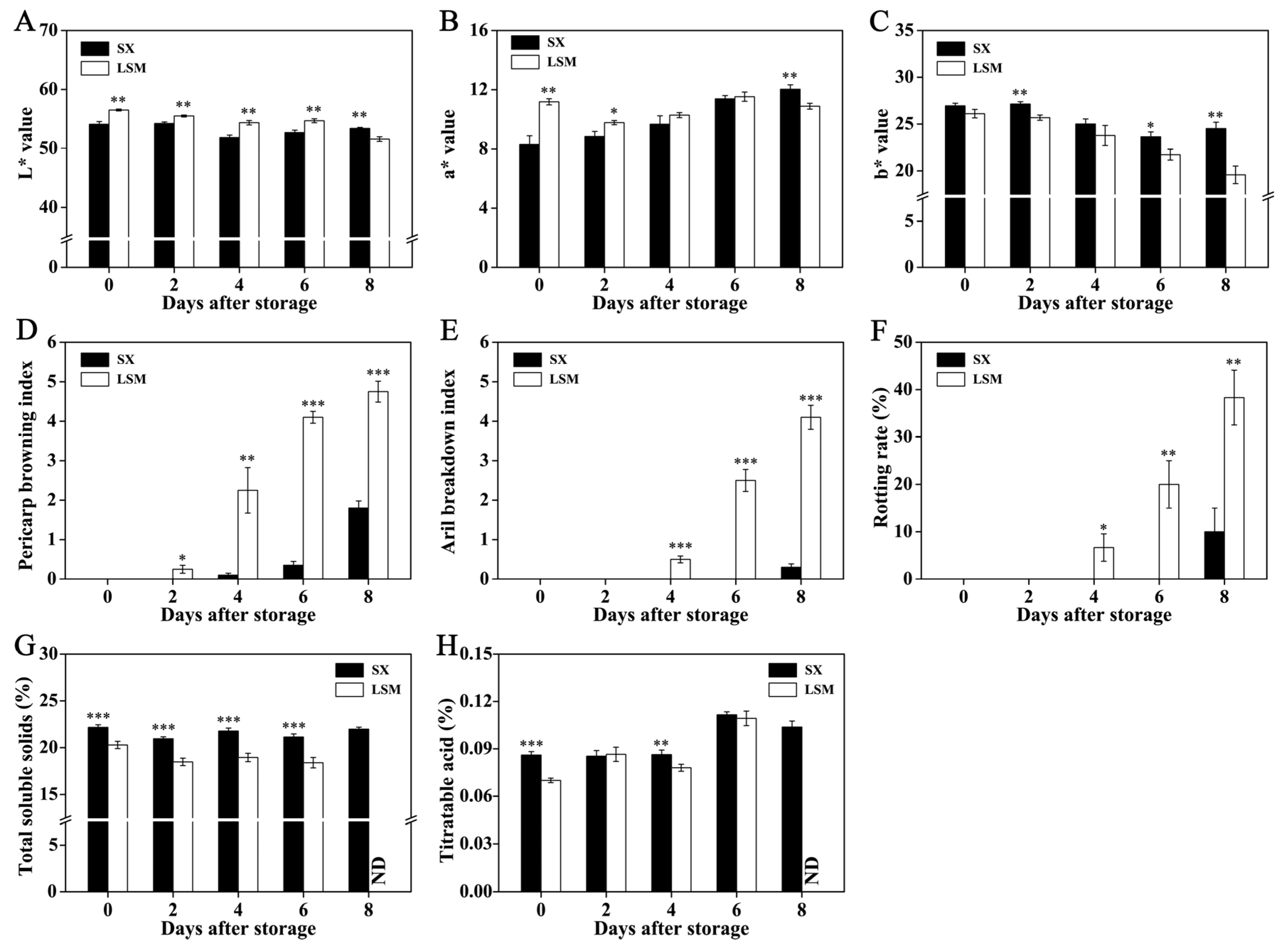
2.1. Difference of Quality Deterioration between ‘Shixia’ and ‘Luosanmu’ Longan during the Room-Temperature Storage
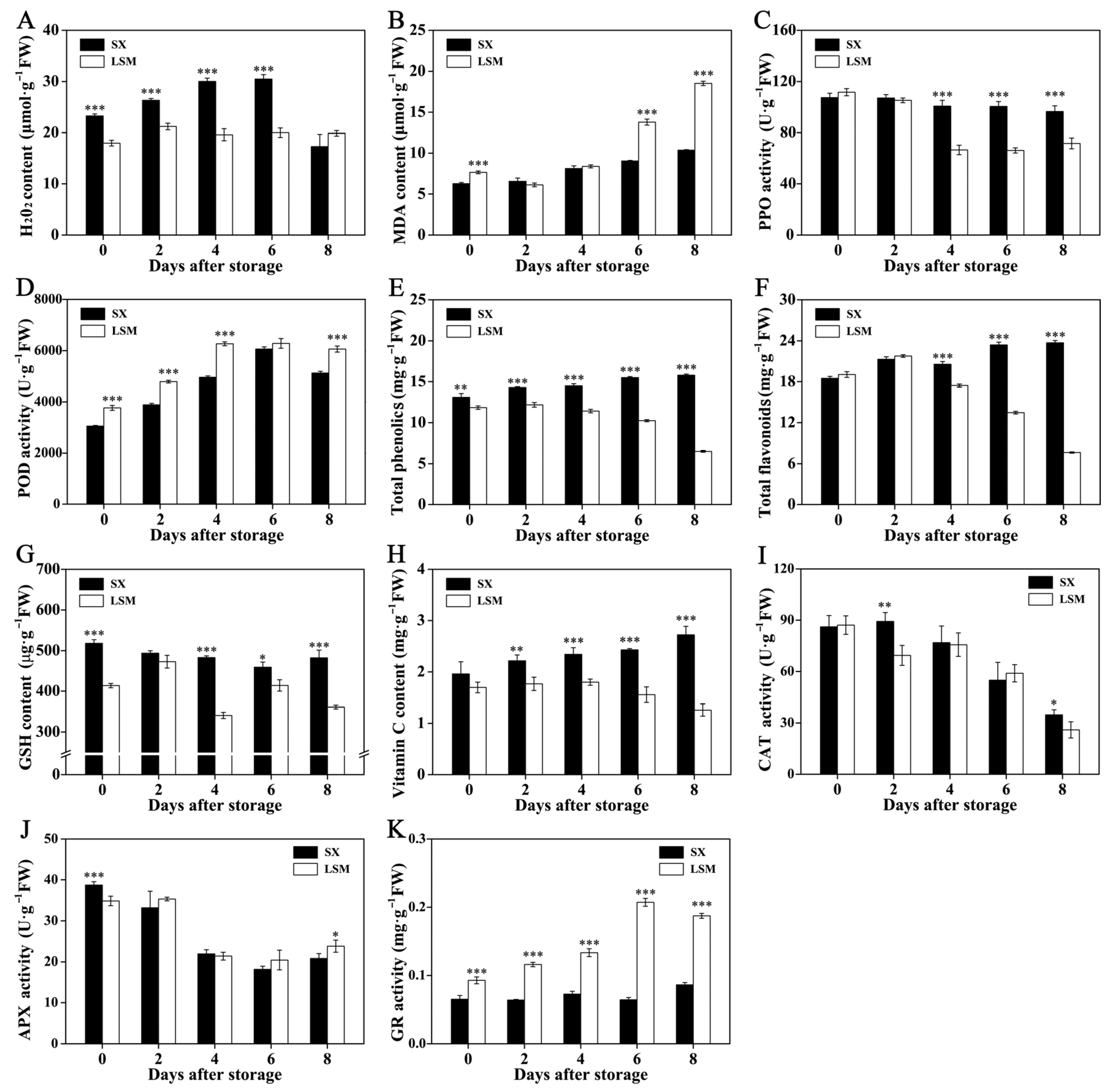
2.2. The Difference of Non-Enzymatic and Enzymatic Radical-Scavenging Capacity in Pericarp between ‘Shixia’ and ‘Luosanmu’ Longan during the Room-Temperature Storage
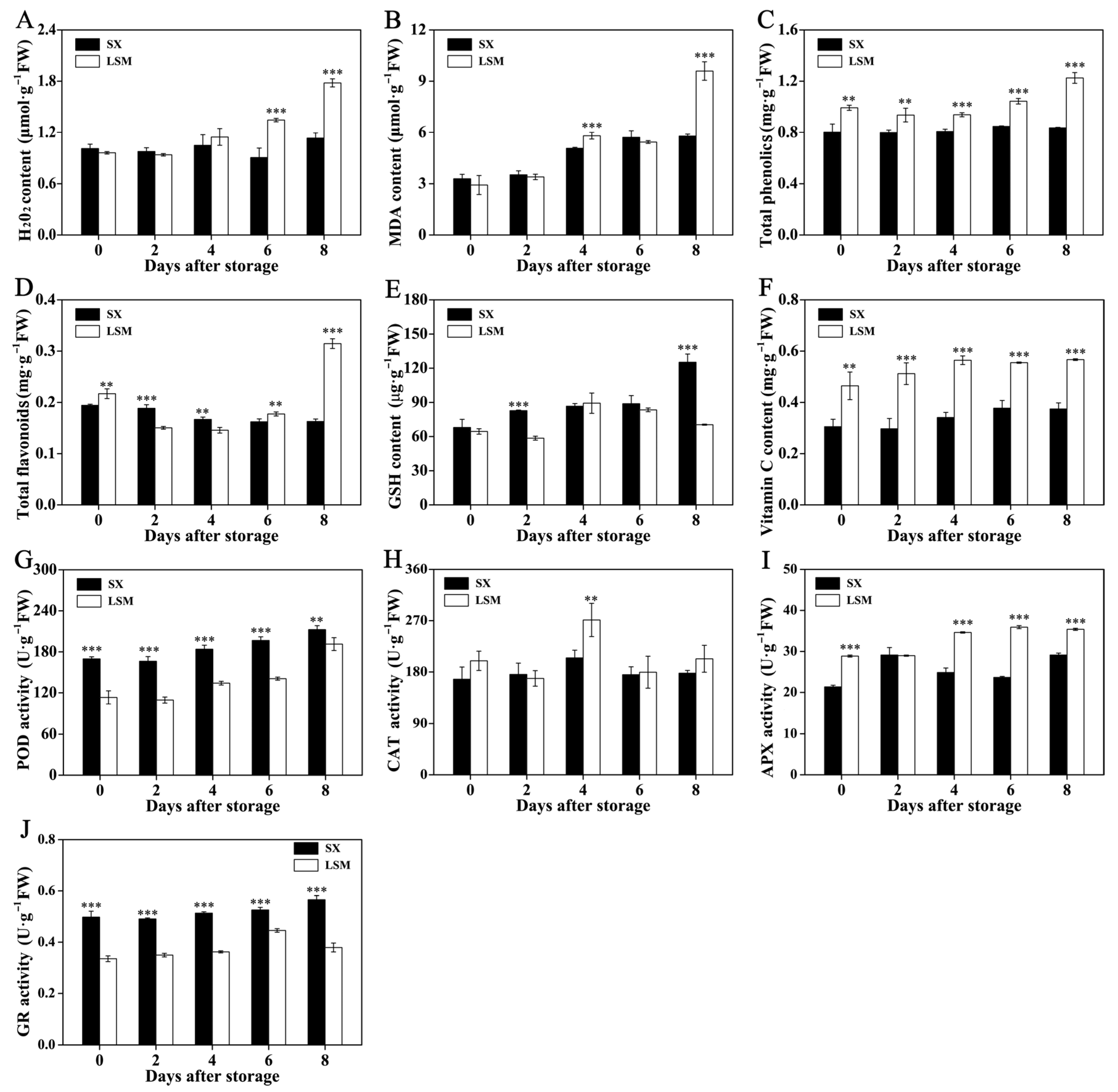
2.3. The Difference of Non-Enzymatic and Enzymatic Radical-Scavenging Capacity in Aril between ‘Shixia’ and ‘Luosanmu’ Longan during the Room-Temperature Storage
2.4. The Difference of Pectin and Cellulose Components and Activities of Hydrolase Enzymes in Aril between ‘Shixia’ and ‘Luosanmu’ Longan during the Room-Temperature Storage
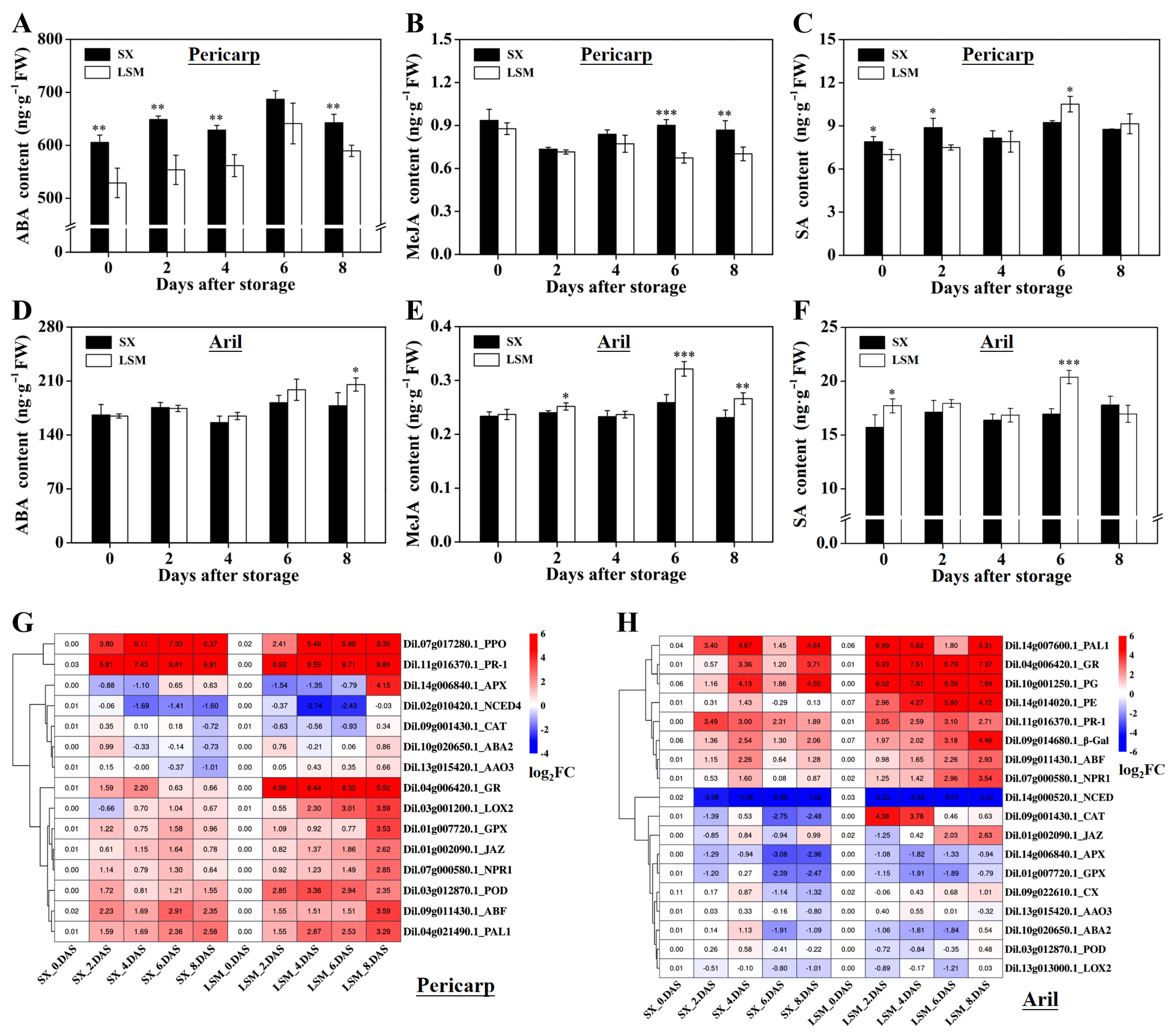
2.5. Difference of ABA, MeJA, SA Content and the Expression of Genes Related to Radical- Scavenging and Hormones between ‘Shixia’ and ‘Luosanmu’ Fruit during the Room-Temperature Storage
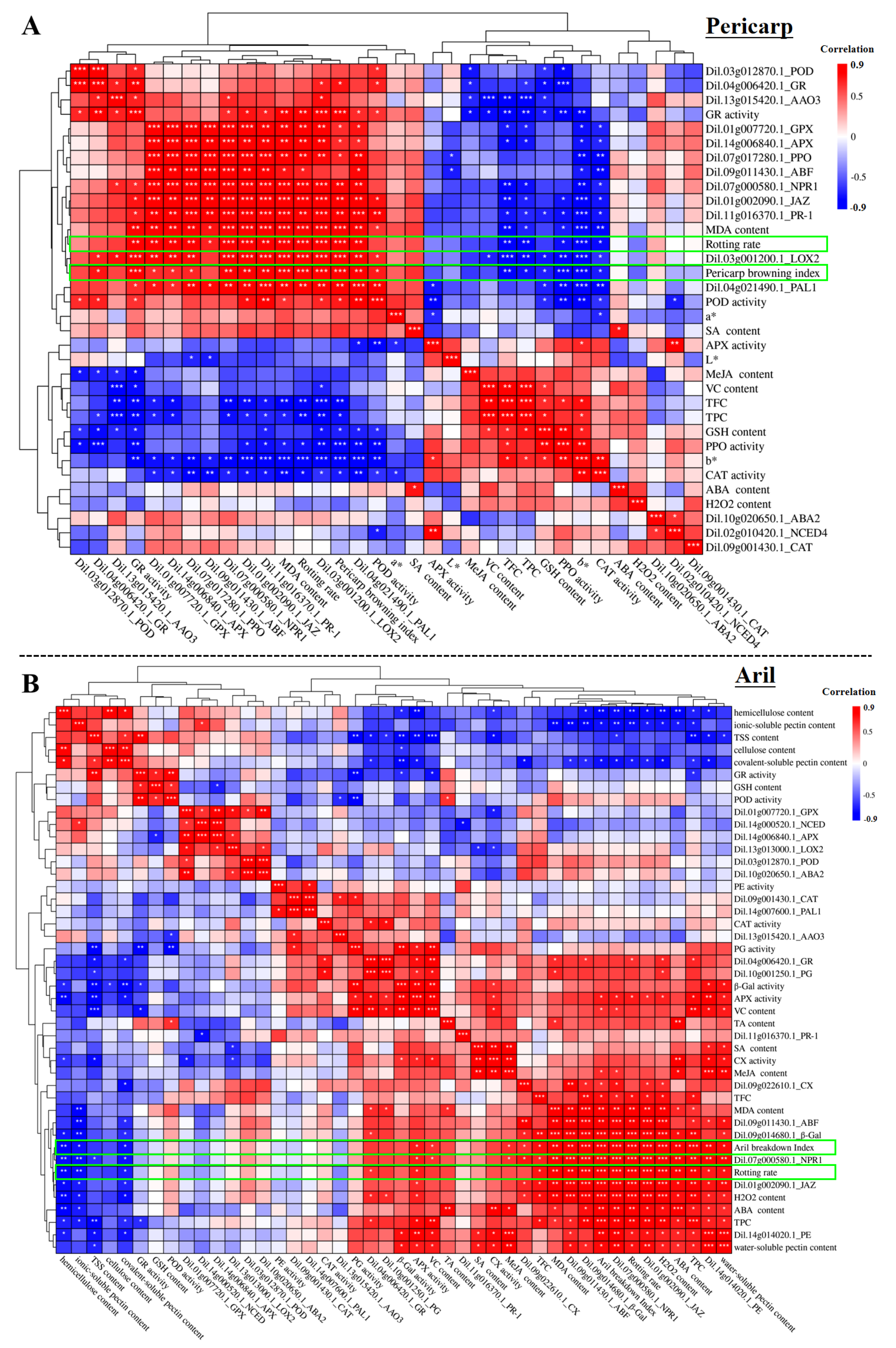
2.6. The Correlation of Physiological Parameters, Enzyme Activities and Gene Expression with the Longan Deterioration
3. Materials and Methods
3.1. Preparation, Treatment and Storage of Longan Fruit
3.2. Determination of Chromatic Value
3.3. Determination of Pericarp Browning Index, Aril Breakdown Index and Rotting Rate
3.4. Sampling and Determination of Total Soluble Solid (TSS), Titratable Acid (TA) and Vitamin C (VC)
3.5. Assay of Total Phenolics, Total Flavonoid, and Reduced Glutathione (GSH)
3.6. Determination of PPO Activity, Malondialdehyde (MDA) Content and H2O2 Content
3.7. Measurement of Peroxidase, Catalase, Ascorbate Peroxidase and Glutathione Reductase Activities
3.8. Determination of Pectin, Cellulose, and Hemicellulose Contents
3.9. Determination of Pectinesterase, Polygalacturonase, β-Galactosidase and Cellulose Activities
3.10. Determination of Abscisic Acid (ABA), Methyl Jasmonate (MeJA) and Salicylic Acid (SA) Content
3.11. RNA Isolation and qRT-PCR Analysis
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Zhu, Q.N.; Zhou, X.F.; Zhang, X.L.; Dang, Z.X.; Liang, S.X.; Li, G.T.; Zhang, Z.Q.; Fang, F.; Pang, X.Q. Characterization of a pericarp browning related LACCASE 14-4 from longan fruit with a focus on (epi)catechin oxidative polymerization. Postharvest Biol. Technol. 2022, 185, 111802. [Google Scholar] [CrossRef]
- Lai, T.T.; Shuai, L.; Han, D.M.; Lai, Z.Y.; Du, X.X.; Guo, X.M.; Hu, W.S.; Wu, Z.X.; Luo, T. Comparative metabolomics reveals differences in primary and secondary metabolites between “Shixia” and “Chuliang” longan (Dimocarpus longan Lour.) pulp. Food Sci. Nutr. 2021, 9, 5785–5799. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Yin, F.L.; Liao, L.Y.; Liu, Y.F.; Guan, B.Y.; Wang, M.; Lai, T.T.; Wu, Z.X.; Shuai, L. Postharvest melatonin treatment inhibited longan (Dimocarpus longan Lour.) pericarp browning by increasing ROS scavenging ability and protecting cytomembrane integrity. Food Sci. Nutr. 2021, 9, 4963–4973. [Google Scholar] [CrossRef]
- Duan, X.W.; Su, X.G.; You, Y.L.; Qu, H.X.; Li, Y.B.; Jiang, Y.M. Effect of nitric oxide on pericarp browning of harvested longan fruit in relation to phenolic metabolism. Food Chem. 2007, 104, 571–576. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lin, H.T.; Zhang, S.; Chen, Y.H.; Chen, M.Y.; Lin, Y.X. The role of active oxygen metabolism in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2014, 96, 42–48. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lin, H.T.; Lin, Y.X.; Zhang, S.; Chen, Y.H.; Jiang, X.J. The roles of metabolism of membrane lipids and phenolics in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2016, 111, 53–61. [Google Scholar] [CrossRef]
- Lin, Y.X.; Lin, Y.F.; Chen, Y.H.; Wang, H.; Shi, J.; Lin, H.T. Hydrogen peroxide induced changes in energy status and respiration metabolism of harvested longan fruit in relation to pericarp browning. J. Agric. Food Chem. 2016, 64, 4627–4632. [Google Scholar] [CrossRef]
- Lin, H.T.; Chen, L.; Lin, Y.F.; Jiang, Y.M. Fruit weight loss and pericarp water loss of harvested longan fruit in relation to pericarp browning. Acta Hortic. 2010, 863, 587–592. [Google Scholar] [CrossRef]
- Chen, Y.H.; Sun, J.Z.; Lin, H.T.; Lin, M.S.; Lin, Y.F.; Wang, H.; Hung, Y.-C. Salicylic acid treatment suppresses Phomopsis longanae Chi-induced disease development of postharvest longan fruit by modulating membrane lipid metabolism. Postharvest Biol. Technol. 2020, 164, 111168. [Google Scholar] [CrossRef]
- Han, D.M.; Luo, T.; Zhang, L.; Wu, J.Q.; Wu, H.T.; Wu, Z.X.; Li, J.G.; Wang, J.; Pan, X.W. Optimized precooling combined with SO2-released paper treatment improves the storability of longan (Dimocarpus longan Lour.) fruits stored at room temperature. Food Sci. Nutr. 2020, 8, 2827–2838. [Google Scholar] [CrossRef]
- Luo, T.; Niu, J.J.; Guo, X.M.; Wu, H.T.; Han, D.M.; Shuai, L.; Wu, Z.X. Preharvest zinc sulfate spray improves the storability of longan (Dimocarpus longan Lour.) fruits by protecting the cell wall components and antioxidants of pericarp. J. Sci. Food Agric. 2019, 99, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Apai, W. Effects of fruit dipping in hydrochloric acid then rinsing in water on fruit decay and browning of longan fruit. Crop Prot. 2010, 29, 1184–1189. [Google Scholar] [CrossRef]
- Lin, Y.F.; Hu, Y.H.; Lin, H.T.; Liu, X.; Chen, Y.H.; Zhang, S.; Chen, Q.X. Inhibitory effects of propyl gallate on tyrosinase and its application in controlling pericarp browning of harvested longan fruits. J. Agric. Food Chem. 2013, 61, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.M.; Sun, J.; Li, C.B.; Sheng, J.F.; Zheng, F.J.; Liao, F.; He, X.M.; Liu, G.M.; Lin, D.N.; et al. Effects of 2-butanol on quality and physiological characteristics of longan fruit stored at ambient temperature. Postharvest Biol. Technol. 2015, 101, 96–102. [Google Scholar] [CrossRef]
- Wang, H.; Zhi, W.; Qu, H.X.; Lin, H.T.; Jiang, Y.M. Application of α-aminoisobutyric acid and β-aminoisobutyric acid inhibits pericarp browning of harvested longan fruit. Chem. Cent. J. 2015, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Khunpon, B.; Uthaibutra, J.; Faiyue, B.; Saengnil, K. Reduction of enzymatic browning of harvested ‘Daw’ longan exocarp by sodium chlorite. Scienceasia 2011, 37, 234–239. [Google Scholar] [CrossRef]
- Saengnil, K.; Chumyam, A.; Faiyue, B.; Uthaibutra, J. Use of chlorine dioxide fumigation to alleviate enzymatic browning of harvested ‘Daw’ longan pericarp during storage under ambient conditions. Postharvest Biol. Technol. 2014, 91, 49–56. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Li, Y.B. Effects of chitosan coating on postharvest life and quality of longan fruit. Food Chem. 2001, 73, 139–143. [Google Scholar] [CrossRef]
- Khan, M.R.; Sripethdee, C.; Chinsirikul, W.; Sane, A.; Chonhenchob, V. Effects of film permeability on reducing pericarp browning, preventing postharvest decay and extending shelf life of modified atmosphere-retail packaged longan fruits. Int. J. Food Sci. Technol. 2016, 51, 1925–1931. [Google Scholar] [CrossRef]
- Tian, S.P.; Xu, Y.; Jiang, A.L.; Gong, Q.Q. Physiological and quality responses of longan fruit to high O2 or high CO2 atmospheres in storage. Postharvest Biol. Technol. 2002, 24, 335–340. [Google Scholar] [CrossRef]
- Su, X.G.; Jiang, Y.M.; Duan, X.W.; Liu, H.; Li, Y.B.; Lin, W.B.; Zheng, Y.H. Effects of pure oxygen on the rate of skin browning and energy status in longan fruit. Food Technol. Biotechnol. 2005, 43, 359–365. [Google Scholar] [CrossRef]
- Whangchai, K.; Saengnil, K.; Uthaibutra, J. Effect of ozone in combination with some organic acids on the control of postharvest decay and pericarp browning of longan fruit. Crop Prot. 2006, 25, 821–825. [Google Scholar] [CrossRef]
- Zhang, J.N.; Weng, H.L.; Lin, Z.Q.; Lin, Y.F.; Chen, Y.H.; Lin, H.T. Comparison of fruit storability and quality changes between harvested ‘Fuyan’ and ‘Dongbi’ longan fruits. Chin. J. Trop. Crops 2013, 34, 989–994. [Google Scholar]
- Lin, L.J.; Lin, Y.X.; Lin, H.T.; Lin, M.S.; Ritenour, M.A.; Chen, Y.H.; Wang, H.; Hung, Y.C.; Lin, Y.F. Comparison between ‘Fuyan’ and ‘Dongbi’ longans in aril breakdown and respiration metabolism. Postharvest Biol. Technol. 2019, 153, 176–182. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Chen, X.D.; Luo, T.; Wu, Z.X.; Han, D.M. Comparison of postharvest quality and storability of different longan varieties. Mod. Food Sci. Technol. 2020, 36, 157–164. [Google Scholar] [CrossRef]
- Suiubon, S.; Supapvanich, S.; Promyou, S. Postharvest quality maintenance of longan fruit by ultra violet-C incorporated with salicylic acid application. Emir. J. Food Agr. 2017, 29, 179–187. [Google Scholar] [CrossRef]
- Promyoua, S.; Supapvanich, S. Combinative effect of salicylic acid immersion and UV-C illumination on chilling injury-related factors of longan (Dimocarpus longan Lour.) fruit. Int. J. Fruit Sci. 2020, 20, 133–148. [Google Scholar] [CrossRef]
- Qu, S.S.; Li, M.M.; Wang, G.; Zhu, S.J. Application of ABA and GA3 alleviated browning of litchi (Litchi chinensis Sonn.) via different strategies. Postharvest Biol. Technol. 2021, 181, 111672. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Liu, J.L.; Huber, D.J.; Qu, H.X.; Yun, Z.; Li, T.T.; Jiang, Y.M. Transcriptome, degradome and physiological analysis provide new insights into the mechanism of inhibition of litchi fruit senescence by melatonin into the mechanism of inhibition of litchi fruit senescence by melatonin. Plant Sci. 2021, 308, 110926. [Google Scholar] [CrossRef]
- Deshi, V.; Homa, F.; Tokala, V.Y.; Mir, H.; Aftab, M.A.; Siddiqui, M.W. Regulation of pericarp browning in cold-stored litchi fruit using methyl jasmonate. J. King Saud Univ. Sci. 2021, 33, 101445. [Google Scholar] [CrossRef]
- Rojas-Murcia, N.; Hématy, K.; Lee, Y.; Emonet, A.; Ursache, R.; Fujita, S.; Bellis, D.D.; Geldner, N. High-order mutants reveal an essential requirement for peroxidases but not laccases in Casparian strip lignification. Proc. Natl. Acad. Sci. USA 2020, 117, 29166–29177. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Lin, H.T.; Wang, H.; Lin, M.S.; Chen, Y.H.; Fan, Z.Q.; Hung, Y.-C.; Lin, Y.F. Effects of hydrogen peroxide treatment on pulp breakdown, softening, and cell wall polysaccharide metabolism in fresh longan fruit. Carbohydr. Polym. 2020, 242, 116427. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.D.; Chang, J.W.; Ma, Q.L.; Chen, L.L.; Liu, S.Z.; Jin, S.; Han, J.W.; Xu, R.W.; Zhu, A.D.; Guo, J.; et al. Network analysis of postharvest senescence process in Citrus fruits revealed by transcriptomic and metabolomic profiling. Plant Physiol. 2015, 168, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.J.; Wang, H.-W.; Sun, D.-W. Phytohormones in postharvest storage of fruit and vegetables: Mechanisms and applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–15. [Google Scholar] [CrossRef]
- Zhu, M.T.; Liu, Z.F.; Zeng, Y.X.; Yu, J. Nordihydroguaiaretic acid reduces postharvest berry abscission in grapes. Postharvest Biol. Technol. 2022, 183, 111748. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, F.; Ji, S.J.; Dai, H.Y.; Zhou, X.; Wei, B.D.; Cheng, S.C.; Wang, A.D. Abscisic acid accelerates postharvest blueberry fruit softening by promoting cell wall metabolism. Sci. Hortic. 2021, 288, 110325. [Google Scholar] [CrossRef]
- Siebeneichler, T.J.; Crizel, R.L.; Camozatto, G.H.; Paim, B.T.; Messias, R.D.; Rombaldi, C.V.; Galli, V. The postharvest ripening of strawberry fruits induced by abscisic acid and sucrose differs from their in vivo ripening. Food Chem. 2020, 317, 126407. [Google Scholar] [CrossRef]
- Liu, Z.C.; Liang, X. Study on the determination of vitamin C in drinks by ultraviolet spectrophotometry. China Foreign Med. Treat. 2010, 29, 16–18. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Huber, D.J.; Hu, M.J.; Jiang, G.X.; Gao, Z.Y.; Xu, X.B.; Jiang, Y.M.; Zhang, Z.K. Delay of postharvest browning in litchi fruit by melatonin via the enhancing of antioxidative processes and oxidation repair. J. Agric. Food Chem. 2018, 66, 7475–7484. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, S.; Lin, H.T.; Lu, W.J.; Wang, H.; Chen, Y.H.; Lin, Y.F.; Fan, Z.Q. The role of cell wall polysaccharides disassembly in Lasiodiplodia theobromae-induced disease occurrence and softening of fresh longan fruit. Food Chem. 2021, 351, 129294. [Google Scholar] [CrossRef]
- He, Z.L.; Zhan, Y.G.; Zeng, F.S.; Zhao, X.T.; Wang, X. Drought physiology and gene expression characteristics of Fraxinus interspecific hybrids. Plant Growth Regul. 2016, 78, 179–193. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, L.; Lai, T.; Han, D.; Lin, X.; Xu, J.; Zhu, D.; Guo, X.; Lin, Y.; Pan, F.; Wang, Y.; et al. A Comprehensive Analysis of Physiologic and Hormone Basis for the Difference in Room-Temperature Storability between ‘Shixia’ and ‘Luosanmu’ Longan Fruits. Plants 2022, 11, 2503. https://doi.org/10.3390/plants11192503
Long L, Lai T, Han D, Lin X, Xu J, Zhu D, Guo X, Lin Y, Pan F, Wang Y, et al. A Comprehensive Analysis of Physiologic and Hormone Basis for the Difference in Room-Temperature Storability between ‘Shixia’ and ‘Luosanmu’ Longan Fruits. Plants. 2022; 11(19):2503. https://doi.org/10.3390/plants11192503
Chicago/Turabian StyleLong, Libing, Tingting Lai, Dongmei Han, Xiaolan Lin, Jianhang Xu, Difa Zhu, Xiaomeng Guo, Yuqiong Lin, Fengyi Pan, Yihang Wang, and et al. 2022. "A Comprehensive Analysis of Physiologic and Hormone Basis for the Difference in Room-Temperature Storability between ‘Shixia’ and ‘Luosanmu’ Longan Fruits" Plants 11, no. 19: 2503. https://doi.org/10.3390/plants11192503




