Silicon Supplementation Alleviates the Salinity Stress in Wheat Plants by Enhancing the Plant Water Status, Photosynthetic Pigments, Proline Content and Antioxidant Enzyme Activities
Abstract
:1. Introduction
2. Results
2.1. Effect of Si on RWC and Electrolyte Leakage of Leaves and Roots under Salinity
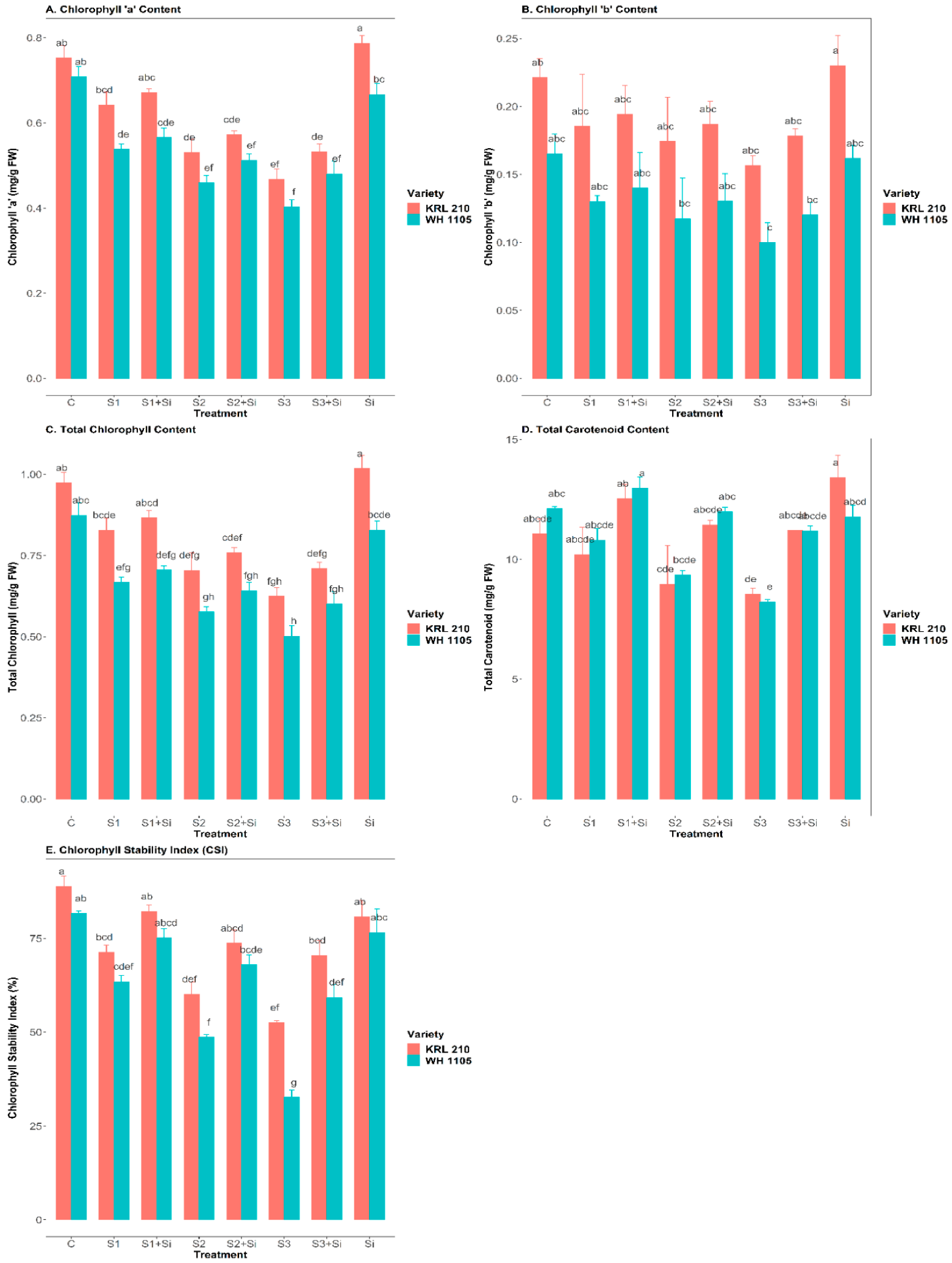
2.2. Effect of Si on Chlorophyll ‘a’ and ‘b’ Content under Salinity
2.3. Effect of Si on Total Chlorophyll, Carotenoid Content, and Chlorophyll Stability Index (CSI) under Salinity
2.4. Effect of Si on Total Proline, Total Phenol Content, and Lipid Peroxidation under Salinity
2.5. Effect of Si on Total Protein and Total Carbohydrate Content under Salinity
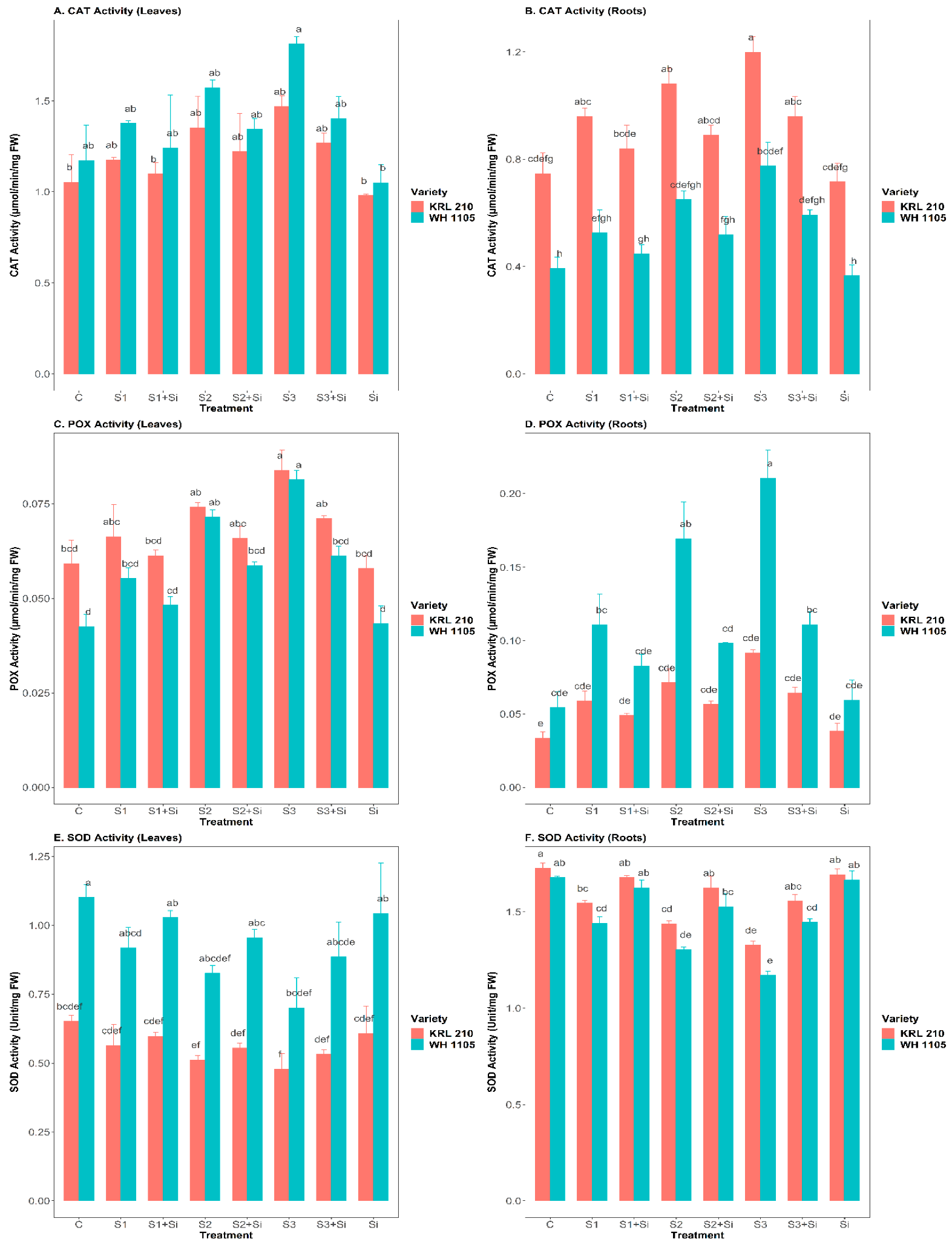
2.6. Effect of Si on the Antioxidant Enzymes Activity (CAT, POX, and SOD) under Salinity
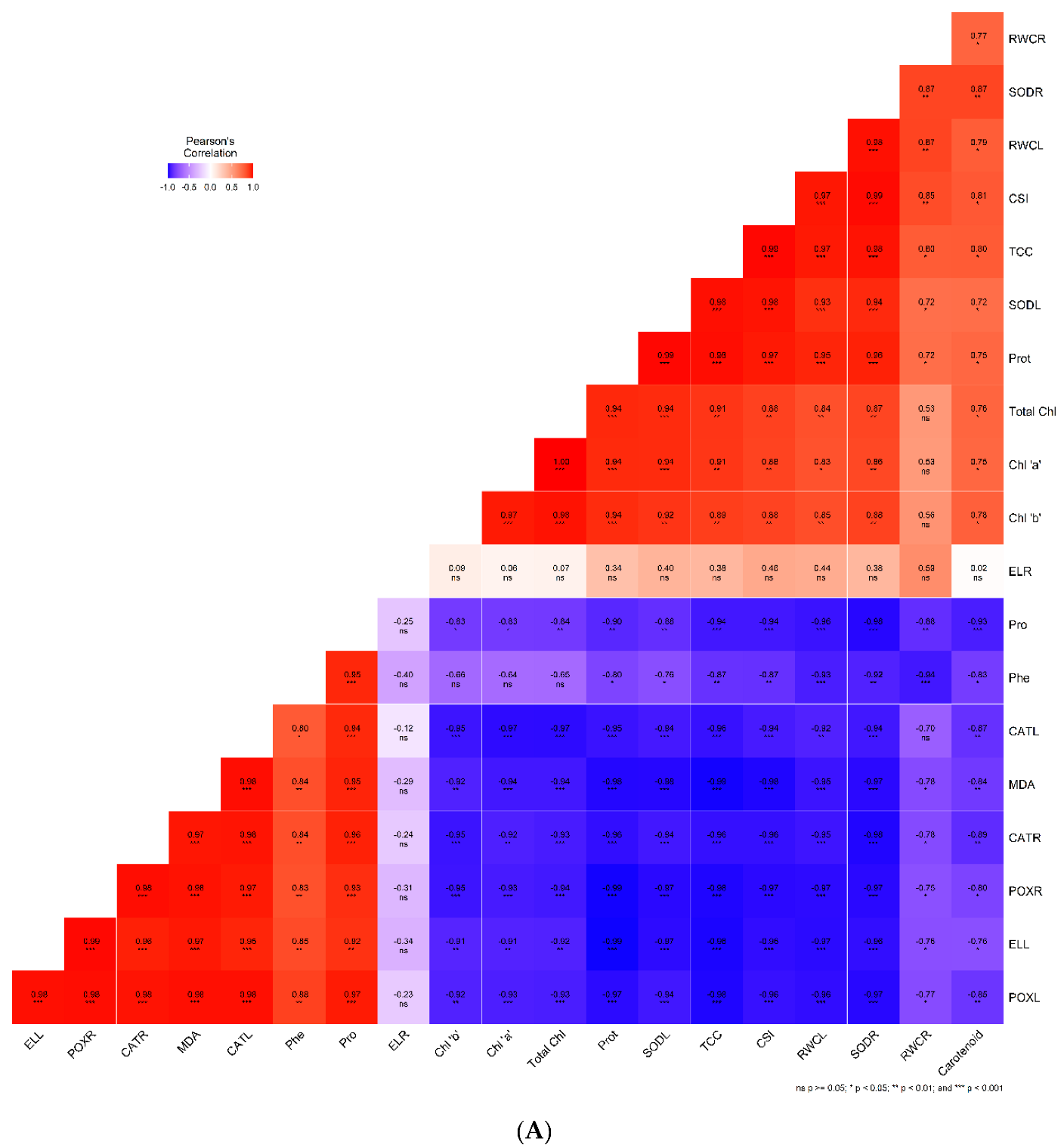
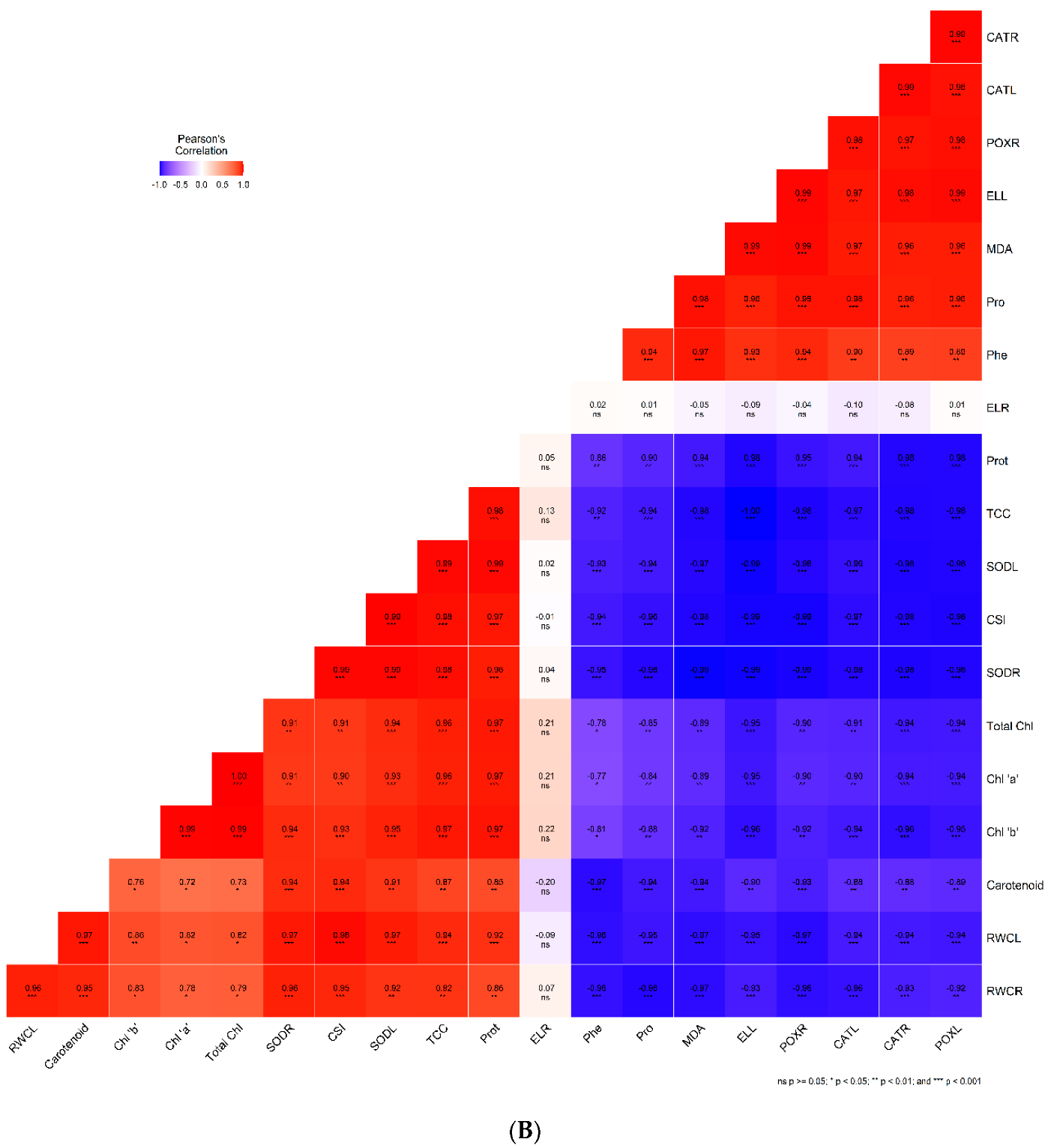
2.7. Correlation Analysis
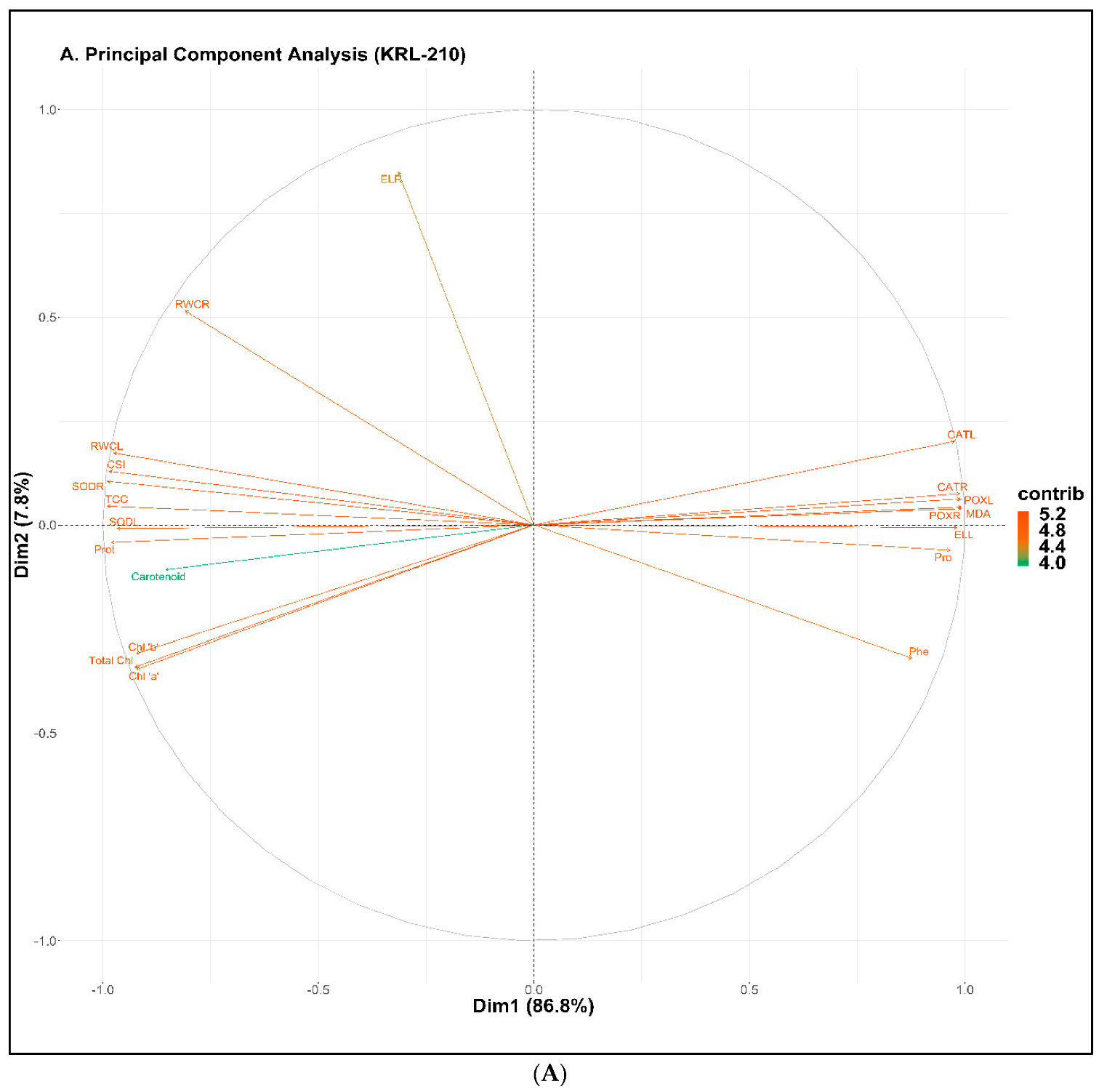
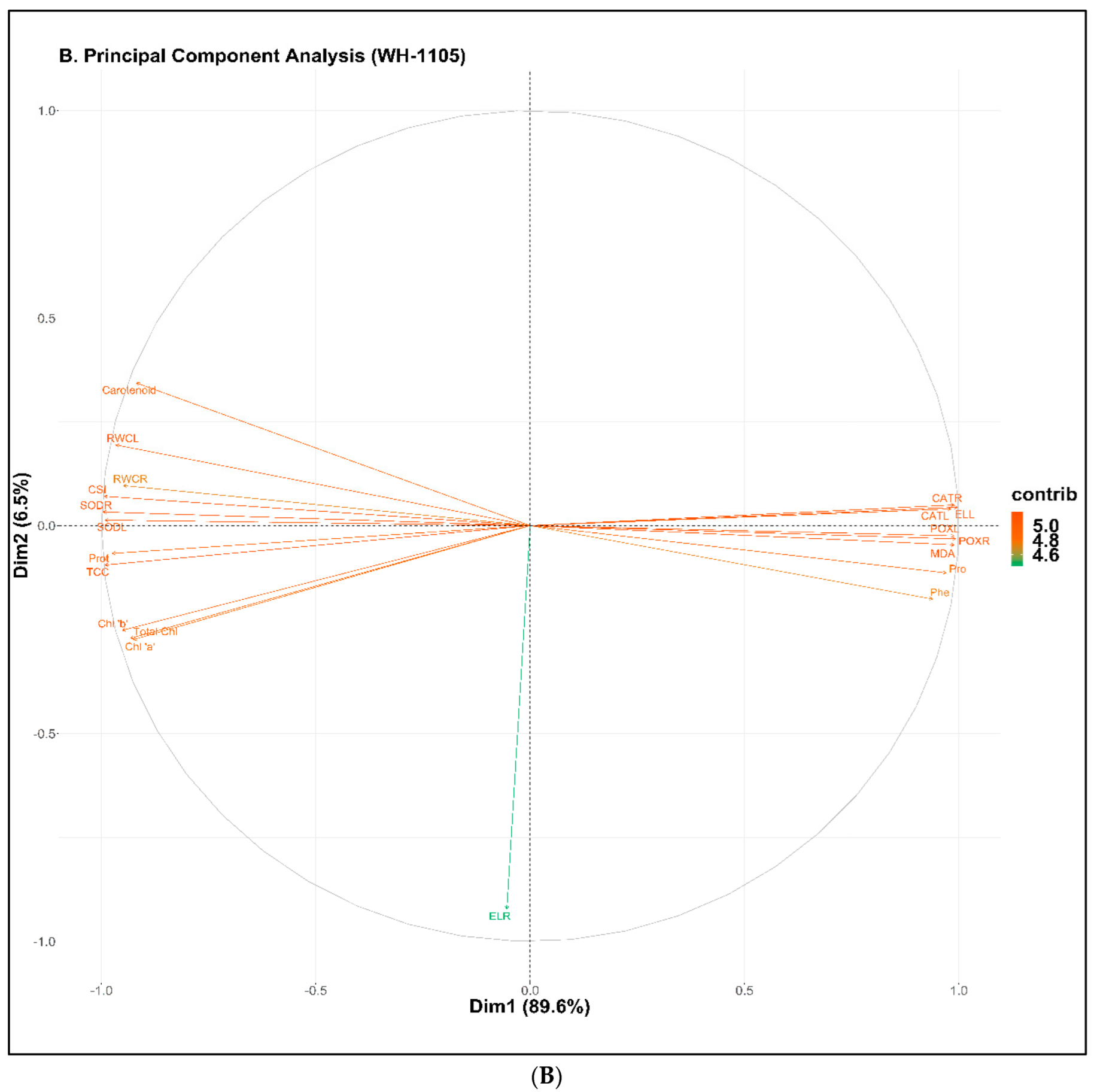
2.8. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Material, Treatments Combinations, and Design
4.2. Relative Water Content (Leaves and Root)
4.3. Electrolyte Leakage (Leaves and Root)
4.4. Photosynthetic Pigments (Chlorophyll a, b and Carotenoid)
4.5. Chlorophyll Stability Index (CSI)
4.6. Lipid Peroxidation or Malondialdehyde (MDA) Content
4.7. Total Protein Content
4.8. Total Proline Content
4.9. Total Carbohydrate Content
4.10. Total Phenol Content
4.11. Antioxidant Enzyme Activity
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Kumar, P.; Yadava, R.K.; Gollen, B.; Kumar, S.; Verma, R.K.; Yadav, S. Nutritional contents and medicinal properties of wheat: A review. Life Sci. Med. Res. 2011, 22, 1–10. [Google Scholar]
- Gul, Z.; Tang, Z.H.; Arif, M.; Ye, Z. An Insight into Abiotic Stress and Influx Tolerance Mechanisms in Plants to Cope in Saline Environments. Biology 2022, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-Doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef]
- Begum, N.; Hasanuzzaman, M.; Li, Y.; Akhtar, K.; Zhang, C.; Zhao, T. Seed germination behavior, growth, physiology and antioxidant metabolism of four contrasting cultivars under combined drought and salinity in soybean. Antioxidants 2022, 11, 498. [Google Scholar] [CrossRef]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity stress in potato: Understanding physiological, biochemical and molecular responses. Life 2021, 11, 545. [Google Scholar] [CrossRef]
- Liu, B.; Soundararajan, P.; Manivannan, A. Mechanisms of silicon-mediated amelioration of salt stress in plants. Plants 2019, 8, 307. [Google Scholar] [CrossRef]
- Gong, D.H.; Wang, G.Z.; Si, W.T.; Zhou, Y.; Liu, Z.; Jia, J. Effects of salt stress on photosynthetic pigments and activity of ribulose-1, 5-bisphosphate carboxylase/oxygenase in Kalidium foliatum. Russ. J. Plant Physiol. 2018, 65, 98–103. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In Plant Abiotic Stress Tolerance; Springer: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar]
- Ferchichi, S.; Hessini, K.; Dell’Aversana, E.; D’Amelia, L.; Woodrow, P.; Ciarmiello, L.F.; Fuggi, A.; Carillo, P. Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct. Plant Biol. 2018, 45, 1096–1109. [Google Scholar] [CrossRef]
- Yongchao, L.; Ruixing, D. Influence of silicon on microdistribution of mineral ions in roots of salt-stressed barley as associated with salt tolerance in plants. Sci. China C: Life Sci. 2002, 45, 298–308. [Google Scholar]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Morton, M.J.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrão, S.; Tester, M. Salt stress under the scalpel–dissecting the genetics of salt tolerance. Plant J. 2019, 97, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Akram, N.A.; Al-Qurainy, F.; Foolad, M.R. Drought tolerance: Roles of organic osmolytes, growth regulators, and mineral nutrients. Adv. Agron. 2011, 111, 249–296. [Google Scholar]
- Vyrides, I.; Stuckey, D.C. Compatible solute addition to biological systems treating waste/wastewater to counteract osmotic and other environmental stresses: A review. Crit. Rev. Biotechnol. 2017, 37, 865–879. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omics. 2012, 5, 60–67. [Google Scholar]
- Mahmud, J.A.; Bhuyan, M.H.M.; Anee, T.I.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In Plant Abiotic Stress Tolerance; Springer: Cham, Switzerland, 2019; pp. 221–257. [Google Scholar]
- Abdelaal, K.A.; Mazrou, Y.S.; Hafez, Y.M. Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Sharma, J.; Verma, S.; Sharma, A. Importance of silicon in combating a variety of stresses in plants: A review. J. Appl. Nat. Sci. 2022, 14, 607–630. [Google Scholar]
- Ali, A.; Basra, S.M.; Iqbal, J.; Hussain, S.; Subhani, M.N.; Sarwar, M.; Haji, A. Silicon mediated biochemical changes in wheat under salinized and non-salinized solution cultures. Afr. J. Biotechnol. 2012, 11, 606–615. [Google Scholar]
- Conceição, S.S.; Oliveira Neto, C.F.D.; Marques, E.C.; Barbosa, A.V.C.; Galvão, J.R.; Oliveira, T.B.D.; Okumura, R.S.; Martins, J.T.D.S.; Costa, T.C.; Gomes-Filho, E. Silicon modulates the activity of antioxidant enzymes and nitrogen compounds in sunflower plants under salt stress. Arch. Agron. Soil Sci. 2019, 65, 1237–1247. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; de Mello Prado, R.; Junior, G.D.S.S.; Felisberto, G. Silicon attenuates sodium toxicity by improving nutritional efficiency in sorghum and sunflower plants. Plant Physiol. Biochem. 2019, 142, 224–233. [Google Scholar] [CrossRef]
- Garg, N.; Bhandari, P. Silicon nutrition and mycorrhizal inoculations improve growth, nutrient status, K+/Na+ ratio and yield of Cicer arietinum L. genotypes under salinity stress. Plant Growth Regul. 2016, 78, 371–387. [Google Scholar] [CrossRef]
- Ali, A.; Mahmood, R.; Jaan, M.; Abbas, M.N. Stimulating the anti-oxidative role and wheat growth improvement through silicon under salt stress. Silicon 2019, 11, 2403–2406. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Çakmak, İ.; Ma, J.F.; Rengel, Z.; Zhao, F. Beneficial elements. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 249–269. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar]
- Gong, Z. Plant abiotic stress: New insights into the factors that activate and modulate plant responses. J. Integr. Plant Biol. 2021, 63, 429. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Hu, Y.; Han, W.; Gong, H. Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiol. Plant. 2015, 37, 1–9. [Google Scholar]
- El-Banna, M.F.; Abdelaal, K.A. Response of strawberry plants grown in the hydroponic system to pretreatment with H2O2 before exposure to salinity stress. J. Plant Prod. 2018, 9, 989–1001. [Google Scholar] [CrossRef]
- Sharma, S.; Villamor, J.G.; Verslues, E. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Al Hinai, M.S.; Ullah, A.; Al-Rajhi, R.S.; Farooq, M. Proline accumulation, ion homeostasis and antioxidant defence system alleviate salt stress and protect carbon assimilation in bread wheat genotypes of Omani origin. Environ. Ex Bot. 2022, 193, 104687. [Google Scholar] [CrossRef]
- Shen, Z.; Pu, X.; Wang, S.; Dong, X.; Cheng, X.; Cheng, M. Silicon improves ion homeostasis and growth of liquorice under salt stress by reducing plant Na+ uptake. Sci. Rep. 2022, 12, 1–13. [Google Scholar]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yin, L.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S.; Tanaka, K. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Ex Bot. 2015, 111, 42–51. [Google Scholar] [CrossRef]
- Alkhatib, R.; Abdo, N.; Mheidat, M. Photosynthetic and ultrastructural properties of eggplant (Solanum melongena) under salinity stress. Horticulturae 2021, 7, 181. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.N.A.; Atteya, A.K.; Al-Hashimi, A.; Abbasi, A.M.; Al-Ashkar, I. Seed priming with silicon as a potential to increase salt stress tolerance in Lathyrus odoratus. Plants 2021, 10, 2140. [Google Scholar] [CrossRef]
- Detmann, K.C.; Araújo, W.L.; Martins, S.C.; Sanglard, L.M.; Reis, J.V.; Detmann, E.; Rodrigues, F.Á.; Nunes-Nesi, A.; Fernie, A.R.; DaMatta, F.M. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; García-Jiménez, A.; Escobar-Sepúlveda, H.F.; Ramírez-Olvera, S.M.; Bello-Bello, J.J.; Gómez-Merino, F.C. Silicon induces hormetic dose-response effects on growth and concentrations of chlorophylls, amino acids and sugars in pepper plants during the early developmental stage. PeerJ 2020, 8, e9224. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiang, X.; Zhang, J.; He, Y.; Zhu, X.; Zhou, X.; Gong, H.; Yin, J.; Liu, Y. Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and salinity: Crosstalk in crop-mediated stress tolerance mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, R.; McCabe, M.F. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Saibi, W.; Feki, K.; Ben Mahmoud, R.; Brini, F. Durum wheat dehydrin (DHN-5) confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta 2015, 242, 1187–1194. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Mushtaq, A.; Khan, Z.; Khan, S.; Rizwan, S.; Jabeen, U.; Bashir, F.; Ismail, T.; Anjum, S.; Masood, A. Effect of silicon on antioxidant enzymes of wheat (Triticum aestivum L.) grown under salt stress. Silicon 2020, 12, 2783–2788. [Google Scholar] [CrossRef]
- Oraee, A.; Tehranifar, A. Relationship between Silicon through Potassium Silicate and Salinity Tolerance in Bellis perennis L. Silicon 2022, 1–15. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Kafi, M.; Nabati, J.; Ahmadi-Lahijani, M.J.; Oskoueian, A. Silicon compounds and potassium sulfate improve salinity tolerance of potato plants through instigating the defense mechanisms, cell membrane stability, and accumulation of osmolytes. Commun. Soil Sci. Plant Anal. 2021, 52, 843–858. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Li, J.; Bao, Q.; Zhang, F.; Tulaxi, G.; Wang, Z. Salt-induced hydrogen peroxide is involved in modulation of antioxidant enzymes in cotton. Crop. J. 2016, 4, 490–498. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Schellenberg, M.; Meyer, S.; Keller, F.; Gehrig, P.; Riedel, K.; Lee, Y.; Eberl, L.; Martinoia, E. Quantitative detection of changes in the leaf-mesophyll tonoplast proteome in dependency of a cadmium exposure of barley (Hordeum vulgare L.) plants. Proteomics 2009, 9, 2668–2677. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. California agriculture experiment station. Circular 1957, 347, 30–37. [Google Scholar]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Ex Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Kaloyereas, S.A. A New Method of Determining Drought Resistance. Plant Physiol. 1958, 33, 232. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Foiln phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Aiyegoro, O.A.; Okoh, A.I. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010, 10, 21. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]


Salt Concentration (mM) | 0 | 40 | 80 | 120 | |
|---|---|---|---|---|---|
 Si Concentration (mM) | |||||
| 0 | 0 + 0 (Control) | 40 + 0 (S1) | 80 + 0 (S2) | 120 + 0 (S3) | |
| 2 | 0 + 2 (Si) | 40 + 2 (S1 + Si) | 80 + 2 (S2 + Si) | 120 + 2 (S3 + Si) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, P.; Kumar, V.; Sharma, J.; Saini, S.; Sharma, P.; Kumar, S.; Sinhmar, Y.; Kumar, D.; Sharma, A. Silicon Supplementation Alleviates the Salinity Stress in Wheat Plants by Enhancing the Plant Water Status, Photosynthetic Pigments, Proline Content and Antioxidant Enzyme Activities. Plants 2022, 11, 2525. https://doi.org/10.3390/plants11192525
Singh P, Kumar V, Sharma J, Saini S, Sharma P, Kumar S, Sinhmar Y, Kumar D, Sharma A. Silicon Supplementation Alleviates the Salinity Stress in Wheat Plants by Enhancing the Plant Water Status, Photosynthetic Pigments, Proline Content and Antioxidant Enzyme Activities. Plants. 2022; 11(19):2525. https://doi.org/10.3390/plants11192525
Chicago/Turabian StyleSingh, Pooja, Vikram Kumar, Jyoti Sharma, Sakshi Saini, Priyanka Sharma, Sandeep Kumar, Yogesh Sinhmar, Dhirendra Kumar, and Asha Sharma. 2022. "Silicon Supplementation Alleviates the Salinity Stress in Wheat Plants by Enhancing the Plant Water Status, Photosynthetic Pigments, Proline Content and Antioxidant Enzyme Activities" Plants 11, no. 19: 2525. https://doi.org/10.3390/plants11192525
APA StyleSingh, P., Kumar, V., Sharma, J., Saini, S., Sharma, P., Kumar, S., Sinhmar, Y., Kumar, D., & Sharma, A. (2022). Silicon Supplementation Alleviates the Salinity Stress in Wheat Plants by Enhancing the Plant Water Status, Photosynthetic Pigments, Proline Content and Antioxidant Enzyme Activities. Plants, 11(19), 2525. https://doi.org/10.3390/plants11192525








