Evaluation of Trigonella foenum-graecum L. Plant Food Safety after Lead Exposure: Phytochemical Processes
Abstract
:1. Introduction
2. Results
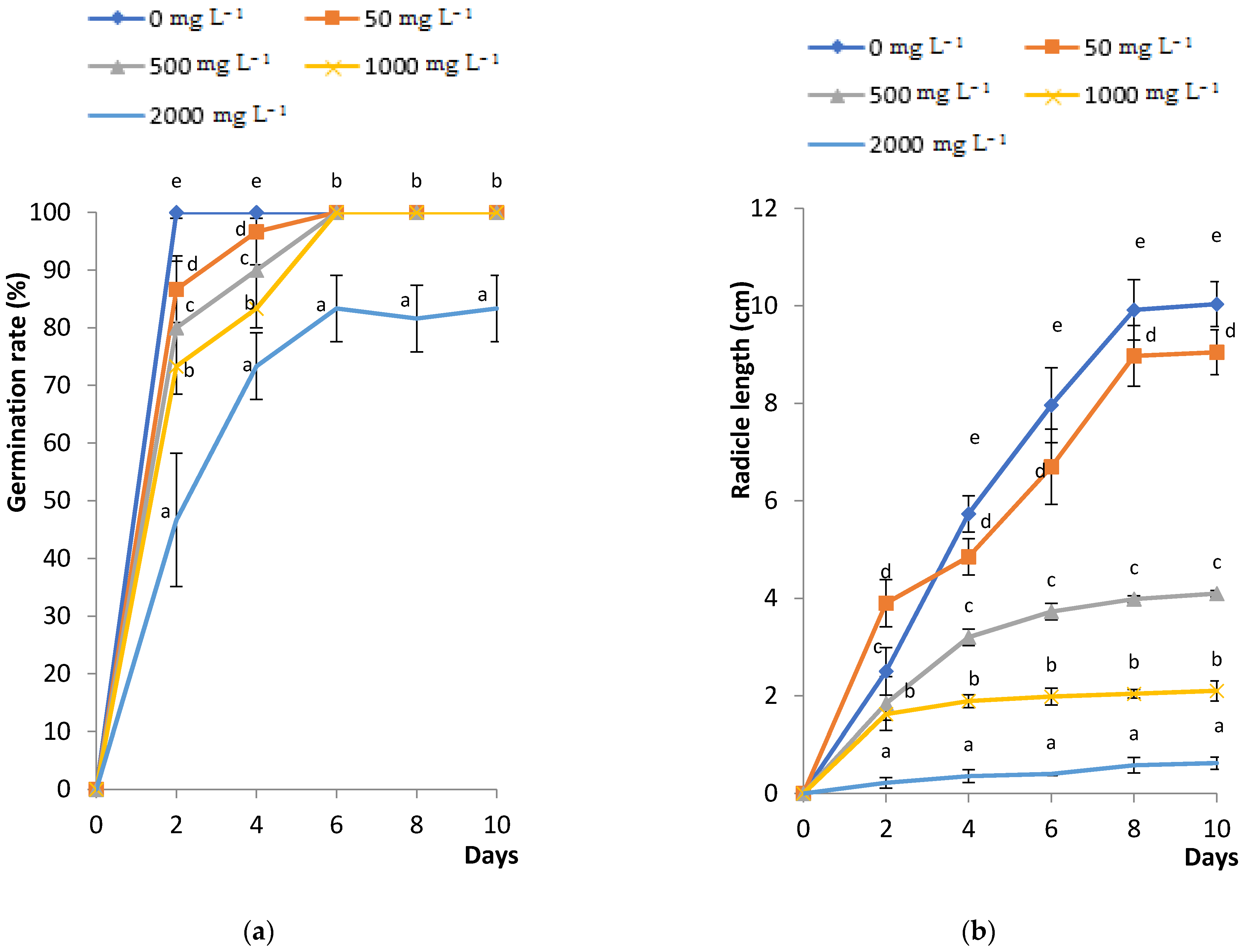
2.1. Effects of PbCl2 Stress on Fenugreek Seed Germination, Radicle Length and Amylase Activity
2.2. Determination of Pb Contents in Fenugreek Tissues
2.3. Chlorophyll Content
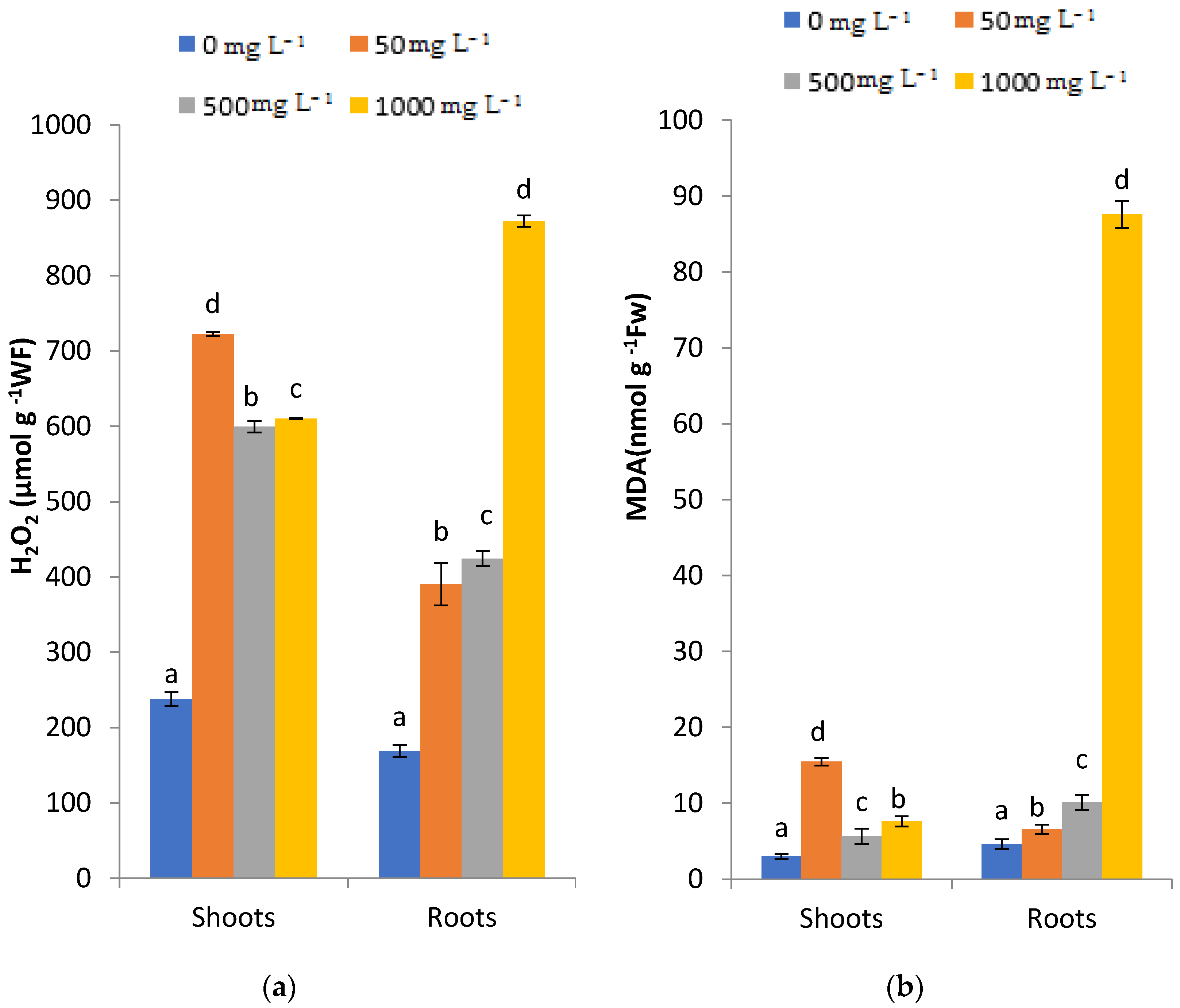
2.4. Effects of PbCl2 Stress on hydrogen peroxide (H2O2) and lipid peroxide (MalonDiAldehyde, MDA) Levels
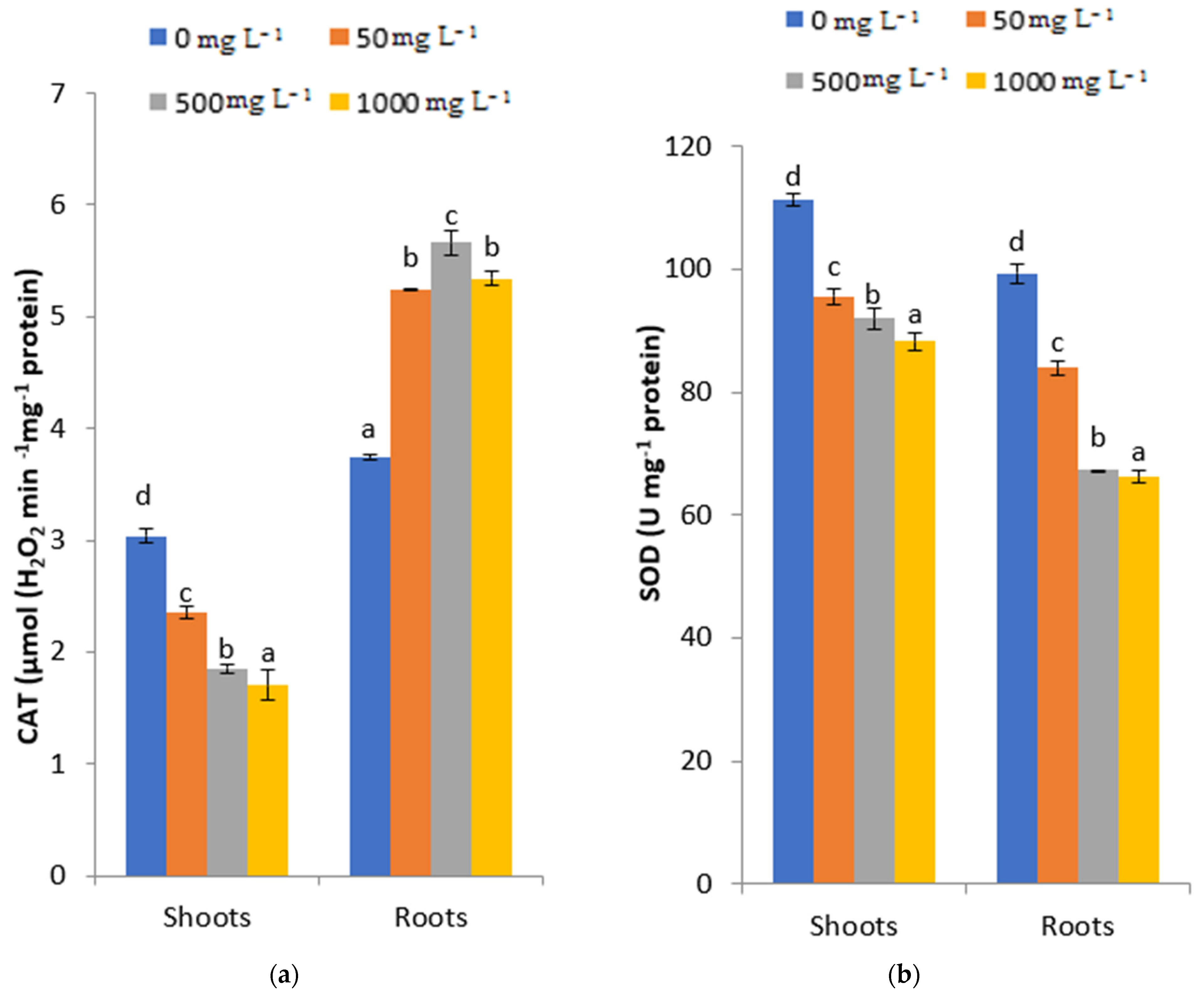
2.5. Effects of PbCl2 Stress on Catalase (CAT) and Superoxide Dismutase (SOD)
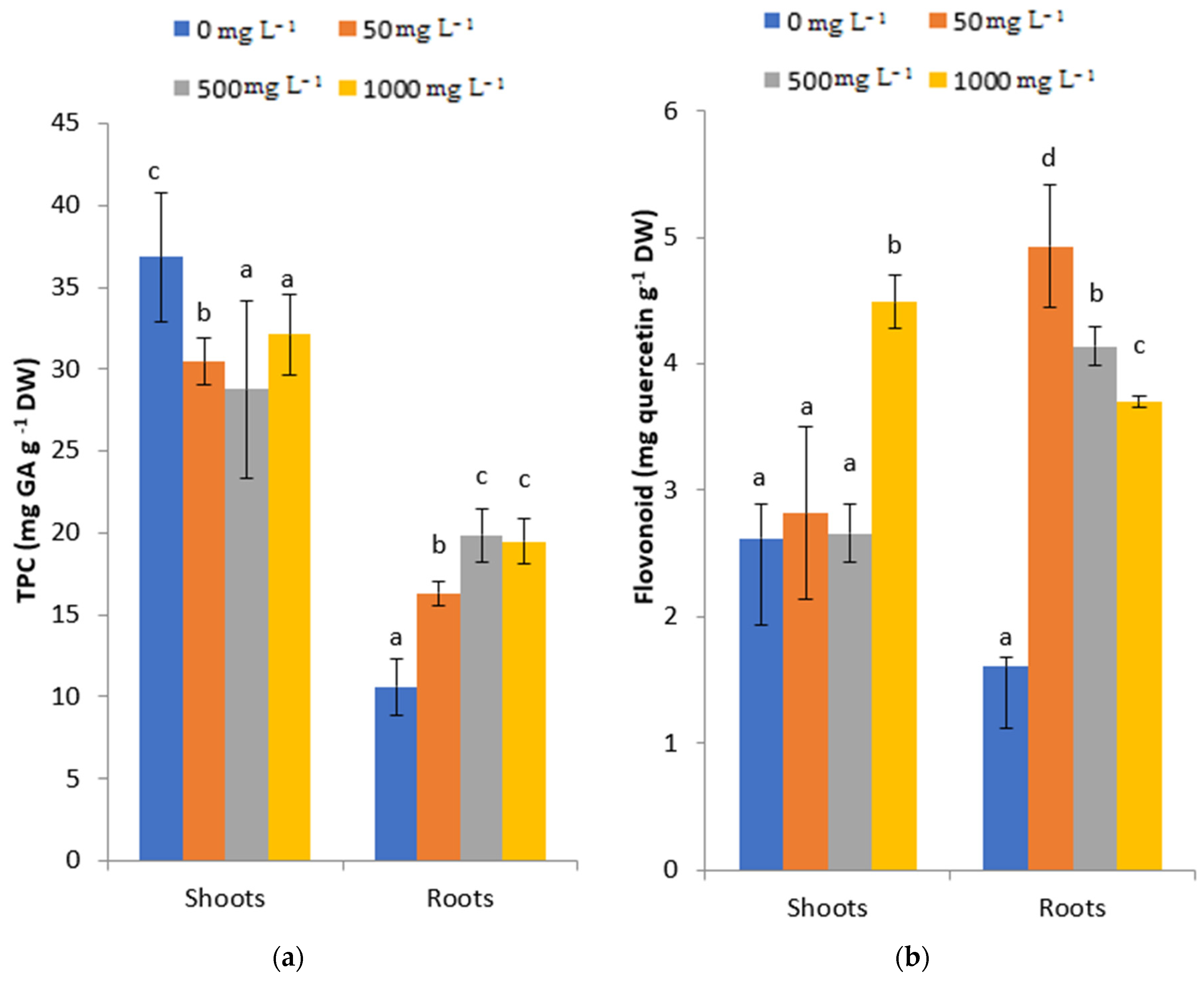
2.6. Effects of Lead Stress on Total Phenol and Flavonoid Contents
2.7. Quantification and Identification of Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Seed Germination, Radicle Length Measurements and α-Amylase Activity Assay
4.2. Plant Growth Conditions
4.3. Pb content, Translocation Factor and Bioaccumulation Factor Determination
4.4. Leaf Chlorophyll Content
4.5. H2O2 and MDA Determination
4.6. CAT and SOD Determination
4.7. Determination of the Total Phenolic and Flavonoid Content
4.8. Determination of Phenols by HPLC
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouni, A.; Ghemari, C.; Ben Said, A.; Pruvot, C.; Douay, F.; Nasri-Ammar, K. Monitoring of heavy metal contamination in soils and terrestrial isopods sampled from the industrialized areas of Sfax (Southeastern Tunisia). Environ. Earth Sci. 2019, 78, 440. [Google Scholar] [CrossRef]
- Chabbi, I.; Baati, H.; Dammak, R.; Bahloul, M.; Azri, C. Toxic metal pollution and ecological risk assessment in superficial soils of “rural-agricultural and coastal-urban” of monastir region, eastern Tunisia. Hum. Ecol. Risk Assess. 2021, 27, 575–594. [Google Scholar] [CrossRef]
- Sebei, A.; Chaabani, A.; Abdelmalek-Babbou, C.; Helali, M.A.; Dhahri, F.; Chaabani, F. Evaluation of pollution by heavy metals of an abandoned Pb-Zn mine in northern Tunisia using sequential fractionation and geostatistical mapping. Environ. Sci. Pollut. Res. 2020, 27, 43942–43957. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Pourrut, B.; Abbas, G.; Shahid, N.; Pinelli, E. Role of metal speciation in lead induced oxidative stress to Vicia faba roots. J. Soil Sediments 2015, 62, 448–454. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food Chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, S.A.; Tiwari, S.; Thul, S.; Sarangi, B.K. Evaluation of lead and chromium tolerance and accumulation level in Gomphrena celosoides: A novel metal accumulator from lead acid battery waste contaminated site in Nigeria. Int. J. Phytoremediat. 2019, 21, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.E.; Al-Jabri, M.; El-Ghareeb, D.K.; Al-Maroai, Y.A. Impact of aluminum and zinc oxides on morphological characters, germination, metals accumulation and DNA in fenugreek (Trigonella foenum-graecum). J. Saudi Soc. Agric. Sci. 2020, 19, 510–520. [Google Scholar] [CrossRef]
- Dheri, G.S.; Brar, M.S.; Malhi, S.S. Comparative phytoremediation of chromium-contaminated soils by fenugreek, spinach, and raya. Commun. Soil Sci. Plant Anal. 2007, 38, 1655–1672. [Google Scholar] [CrossRef]
- Zemzmi, J.; Ródenas, L.; Blas, E.; Abdouli, H.; Najar, T.; Pascual, J.J. Preliminary evaluation of fenugreek (Trigonella foenum-graecum) seed gum as a potential prebiotic for growing rabbits in tunisia: Effects on in vivo faecal digestibility and in vitro fermentation. World Rabbit Sci. 2020, 28, 113–122. [Google Scholar] [CrossRef]
- Xalxo, R.; Keshavkant, S. Growth and antioxidant responses of Trigonella foenum-graecum L. seedlings to lead and simulated acid rain exposure. Biologia 2020, 75, 1115–1126. [Google Scholar] [CrossRef]
- Zayneb, C.; Bassem, K.; Zeineb, K.; Grubb, C.D.; Noureddine, D.; Hafedh, M.; Amine, E. Physiological responses of fenugreek seedlings and plants treated with cadmium. Environ. Sci. Pollut. Res. 2015, 22, 10679–10689. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L. Tolerance and accumulation of lead by fenugreek. J. Agric. Ecol. 2016, 1, 22–34. [Google Scholar] [CrossRef]
- Wannes, W.A.; Tounsi, M.S. Can medicinal plants contribute to the cure of Tunisian COVID-19 patients? J. Med. Plants Stud. 2020, 8, 218–226. [Google Scholar] [CrossRef]
- Ashraf, U.; Mahmood, M.H.-U.-R.; Hussain, S.; Abbas, F.; Anjum, S.A.; Tang, X. Lead (Pb) distribution and accumulation in different plant parts and its associations with grain Pb contents in fragrant rice. Chemosphere 2020, 248, 126003. [Google Scholar] [CrossRef]
- Alaraidh, I.A.; Alsahli, A.A.; Abdel Razik, E.S. Alteration of antioxidant gene expression in response to heavy metal stress in Trigonella foenum-graecum L. S. Afr. J. Bot. 2018, 115, 90–93. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing adverse effects of Pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Salinitro, M.; Hoogerwerf, S.; Casolari, S.; Zappi, A.; Melucci, D.; Tassoni, A. Production of antioxidant molecules in Polygonum aviculare (L.) and Senecio vulgaris (L.) under metal stress: A possible tool in the evaluation of plant metal tolerance. Int. J. Mol. Sci. 2020, 21, 7317. [Google Scholar] [CrossRef]
- Lamhamdi, M.; Bakrim, A.; Aarab, A.; Lafont, R.; Sayah, F. Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. C. R.-Biol. 2011, 334, 118–126. [Google Scholar] [CrossRef]
- Farooqi, Z.R.; Iqbal, M.Z.; Kabir, M.; Shafiq, M. Toxic effects of lead and cadmium on germination and seedling growth of Albizia Lebbeck, L. Pak. J. Bot. 2009, 41, 27–33. [Google Scholar]
- Elleuch, A.; Chaâbene, Z.; Grubb, D.C.; Drira, N.; Mejdoub, H.; Khemakhem, B. Morphological and biochemical behavior of fenugreek (Trigonella foenum-graecum) under copper stress. Ecotoxicol. Environ. Saf. 2013, 98, 46–53. [Google Scholar] [CrossRef]
- Islam, E.; Liu, D.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q.; Tian, S.; Li, J. Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2008, 154, 914–926. [Google Scholar] [CrossRef]
- Mihoub, A.; Chaoui, A.; El Ferjani, E. Changements biochimiques induits par le cadmium et le cuivre au cours de la germination des graines de petit pois (Pisum sativum L.). C. R.-Biol. 2005, 328, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ramesar, N.S.; Tavarez, M.; Ebbs, S.D.; Sankaran, R.P. Transport and partitioning of lead in indian mustard (Brassica juncea) and wheat (Triticum aestivum). Bioremediat. J. 2014, 18, 345–355. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S. A review on phytoremediation of heavy metals and utilization of its byproducts. J. Appl. Ecol. Environ. Res. 2005, 3, 153–179. [Google Scholar]
- Adejumo, S.A.; Oniosun, B.; Akpoilih, O.A.; Adeseko, A.; Arowo, D.O. Anatomical changes, osmolytes accumulation and distribution in the native plants growing on Pb-contaminated sites. Environ. Geochem. Health. 2021, 43, 1537–1549. [Google Scholar] [CrossRef]
- Fatemi, H.; Esmaiel Pour, B.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Aarti, P.D.; Tanaka, R.; Tanaka, A. Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol. Plant. 2006, 128, 186–197. [Google Scholar] [CrossRef]
- Cenkci, S.; Ciǧerci, I.H.; Yildiz, M.; Özay, C.; Bozdaǧ, A.; Terzi, H. Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environ. Exp. Bot. 2010, 67, 467–473. [Google Scholar] [CrossRef]
- Biteur, N.; Aoues, A.; Kharoubi, O.; Slimani, M. Oxidative stress induction by lead in leaves of radish (Raphanus sativus) seedlings. Not Sci. Biol. 2011, 3, 93–99. [Google Scholar] [CrossRef]
- Zaid, A. Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol. Mol. Biol. Plants 2019, 26, 25–39. [Google Scholar] [CrossRef]
- Ali, M.; Nas, F.S. The effect of lead on plants in terms of growing and biochemical parameters: A review. MOJ Ecol. Environ. Sci. 2018, 3, 265–268. [Google Scholar] [CrossRef]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C. EDTA-enhanced phytoremediation of heavy metals: A review. Soil Sediment Contam. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- Zanganeh, R.; Jamei, R.; Rahmani, F. Response of maize plant to sodium hydrosulfide pretreatment under lead stress conditions at early stages of growth. Cereal Res. Commun. 2020, 49, 267–276. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Pourrut, B.; Silvestre, J.; Laplanche, C.; Pinelli, E. Influence of EDTA and citric acid on lead-induced oxidative stress to Vicia faba roots. J. Soils Sediments 2014, 14, 835–843. [Google Scholar] [CrossRef]
- Kikui, S.; Sasaki, T.; Maekawa, M.; Miyao, A.; Hirochika, H.; Matsumoto, H.; Yamamoto, Y. Physiological and genetic analyses of aluminium tolerance in rice, focusing on root growth during germination. J. Inorg. Biochem. 2005, 99, 1837–1844. [Google Scholar] [CrossRef]
- Gopal, R.; Rizvi, A.H. Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 2008, 70, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Mnafgui, W.; Rizzo, V.; Muratore, G.; Hajlaoui, H.; de Oliveira Schinoff, B.; Mnafgui, K.; Elleuch, A. Trigonella foenum-graecum L. Morphophysiological and Phytochemical Processes Controlling Iron Uptake and Translocation. Crop Pasture Sci. 2021. Available online: https://www.publish.csiro.au/CP/justaccepted/CP21419 (accessed on 22 November 2021).
- Gill, S.S.; Tuteja, N. Plant physiology and biochemistry reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Al Khazan, M.M. Priming with moringa (Moringa oleifera lam.) leaf extract boosts the growth and physio-biochemical attributes of lead-stressed fenugreek (Trigonella foenum-graecum L.) seedlings. Appl. Ecol. Environ. Res. 2020, 18, 6949–6967. [Google Scholar] [CrossRef]
- Sevindik, M.; Rasul, A.; Hussain, G.; Anwar, H.; Zahoor, M.K.; Sarfraz, I.; Kamran, K.S.; Akgul, H.; Akata, I.; Selamoglu, Z. Determination of anti-oxidative, anti-microbial activity and heavy metal contents of Leucoagaricus leucothites. Pak. J. Pharm. Sci. 2018, 31, 2163–2168. [Google Scholar]
- Kisa, D.; Kayır, Ö.; Sağlam, N.; Şahin, S.; Öztürk, L.; Elmastaş, M. Changes of phenolic compounds in tomato associated with the heavy metal stress. J. Nat. Appl. Sci. JONAS 2019, 2, 35–43. [Google Scholar]
- Vidal, C.; Ruiz, A.; Ortiz, J.; Larama, G.; Perez, R.; Santander, C.; Ademar, P.; Ferreira, A.; Cornejo, P. Immobilization of Copper in Imperata Cylindrica, a plant with potential use for bioremediation of Cu contaminated environments. Plants 2020, 9, 1397. [Google Scholar] [CrossRef]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Allaro, H.B.; Kassa, B.; Hundie, B. A time series analysis of structural break time in the macroeconomic variables in Ethiopia. Afr. J. Agric. Res. 2011, 6, 392–400. [Google Scholar]
- Sagisaka, S. The occurrence of peroxide in a perennial plant, Populus gelrica. Plant Physiol. 1976, 57, 308–309. [Google Scholar] [CrossRef]
- Duan, X.; Liu, T.; Zhang, D.; Su, X.; Lin, H.; Jiang, Y. Effect of pure oxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage. Food Res. Int. 2011, 44, 1905–1911. [Google Scholar] [CrossRef]
- Aebi, H. Oxygen radicals in biological systems. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Salehi, A.; Fallah, S.; Kaul, H.P.; Zitterl-Eglseer, K. Antioxidant capacity and polyphenols in buckwheat seeds from fenugreek/buckwheat intercrops as influenced by fertilization. J. Cereal Sci. 2018, 84, 142–150. [Google Scholar] [CrossRef]
- Hanafy, R.S.; Akladious, S.A. Physiological and molecular studies on the effect of gamma radiation in fenugreek (Trigonella foenum-graecum L.) plants. J. Genet. Eng. Biotechnol. 2018, 16, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Akrimi, R.; Hajlaoui, H.; Rizzo, V.; Muratore, G.; Mhamdi, M. Agronomical traits, phenolic compounds and antioxidant activity in raw and cooked potato tubers growing under saline conditions. J. Sci. Food Agric. 2020, 100, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, V.; Clifford, M.N.; Brown, J.E.; Siracusa, L.; Muratore, G. Effects of processing on the polyphenol and phenolic acid content and antioxidant capacity of semi-dried cherry tomatoes (Lycopersicon esculentum M.). J. Sci. Food Agric. 2016, 96, 2040–2046. [Google Scholar] [CrossRef] [PubMed]


| PbCl2 Concentration (mg L−1) | Content of Pb in Shoots (mg g−1 DW) | Content of Pb in Roots (mg g−1 DW) | Content of Pb in Seeds (mg g−1 DW) | Translocation Factor (TF) | Bioaccumulation Factor (BAF) |
|---|---|---|---|---|---|
| 0 | 0a | 0.0009 ± 0.002 a | 0 | 0 | 0 |
| 50 | 0.0198 ± 0.003 b | 0.0275 ± 0.008 c | 0 | 0.72 | 0.44 |
| 500 | 0.0281 ± 0.006 c | 0.255 ± 0.005 b | 0 | 0.11 | 1.32 |
| 1000 | 0.031 ± 0.005 c | 0.769 ± 0.031 d | 0.0005 ± 0.0001 | 0.04 | 1.55 |
| PbCl2 Concentration (mg L−1) | Gallic Acid (mg kg−1) | Syringic Acid (mg kg−1) | Chlorogenic Acid (mg kg−1) | Quercetin (mg kg−1) |
|---|---|---|---|---|
| Shoots | ||||
| 0 | 0.105 ±0.014 c | 0.040 ± 0.01 c | n.d. | n.d. |
| 50 | 0.104 ± 0.009 c | 0.027 ± 0.039 b | n.d. | 0.004 ± 9 × 10−5 b |
| 500 | 0.095 ± 0.008 b | 0.026 ± 0.034 b | 0.005 ± 0.002 b | 0.002 ± 6 × 10−7 a |
| 1000 | 0.078 ± 0.001 a | 0.022 ± 0.001 a | 0.003 ± 0.0012 a | n.d. |
| Roots | ||||
| 0 | 0.105 ± 10−4 a | 0.107 ± 0.004 a | 0.002 a | 0.0034 ± 8 × 10−8 b |
| 50 | 0.198 ± 2 × 10−4 d | n.d. | n.d. | 0.0066 ± 3 × 10−4 d |
| 500 | 0.132 ± 5 × 10−3 c | n.d. | n.d. | 0.0044 ± 1 × 10−3 c |
| 1000 | 0.103 ± 9 × 10−3 b | n.d. | n.d. | 0.0031 ± 14 × 10−5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mnafgui, W.; Rizzo, V.; Muratore, G.; Hajlaoui, H.; Elleuch, A. Evaluation of Trigonella foenum-graecum L. Plant Food Safety after Lead Exposure: Phytochemical Processes. Plants 2022, 11, 2526. https://doi.org/10.3390/plants11192526
Mnafgui W, Rizzo V, Muratore G, Hajlaoui H, Elleuch A. Evaluation of Trigonella foenum-graecum L. Plant Food Safety after Lead Exposure: Phytochemical Processes. Plants. 2022; 11(19):2526. https://doi.org/10.3390/plants11192526
Chicago/Turabian StyleMnafgui, Wiem, Valeria Rizzo, Giuseppe Muratore, Hicham Hajlaoui, and Amine Elleuch. 2022. "Evaluation of Trigonella foenum-graecum L. Plant Food Safety after Lead Exposure: Phytochemical Processes" Plants 11, no. 19: 2526. https://doi.org/10.3390/plants11192526
APA StyleMnafgui, W., Rizzo, V., Muratore, G., Hajlaoui, H., & Elleuch, A. (2022). Evaluation of Trigonella foenum-graecum L. Plant Food Safety after Lead Exposure: Phytochemical Processes. Plants, 11(19), 2526. https://doi.org/10.3390/plants11192526






