Improving Yield and Yield Stability in Winter Rye by Hybrid Breeding
Abstract
:1. Introduction
2. Rye Has Strong Potential for Adaptation to a Changing Climate
3. Hybrid Breeding, the Cutting-Edge Technology for a Systematic Improvement of Grain Yield in Rye
3.1. Trapping and Managing Genetic Diversity in Rye
3.2. Unlocking Genetic Diversity for Selective Matings on a Large Scale
3.3. Fertility Restoration, Ergot Defense, and Yield Potential—The Challenging Triad
3.3.1. The Genetics of Fertility Restoration in G-type CMS Is Coming of Age
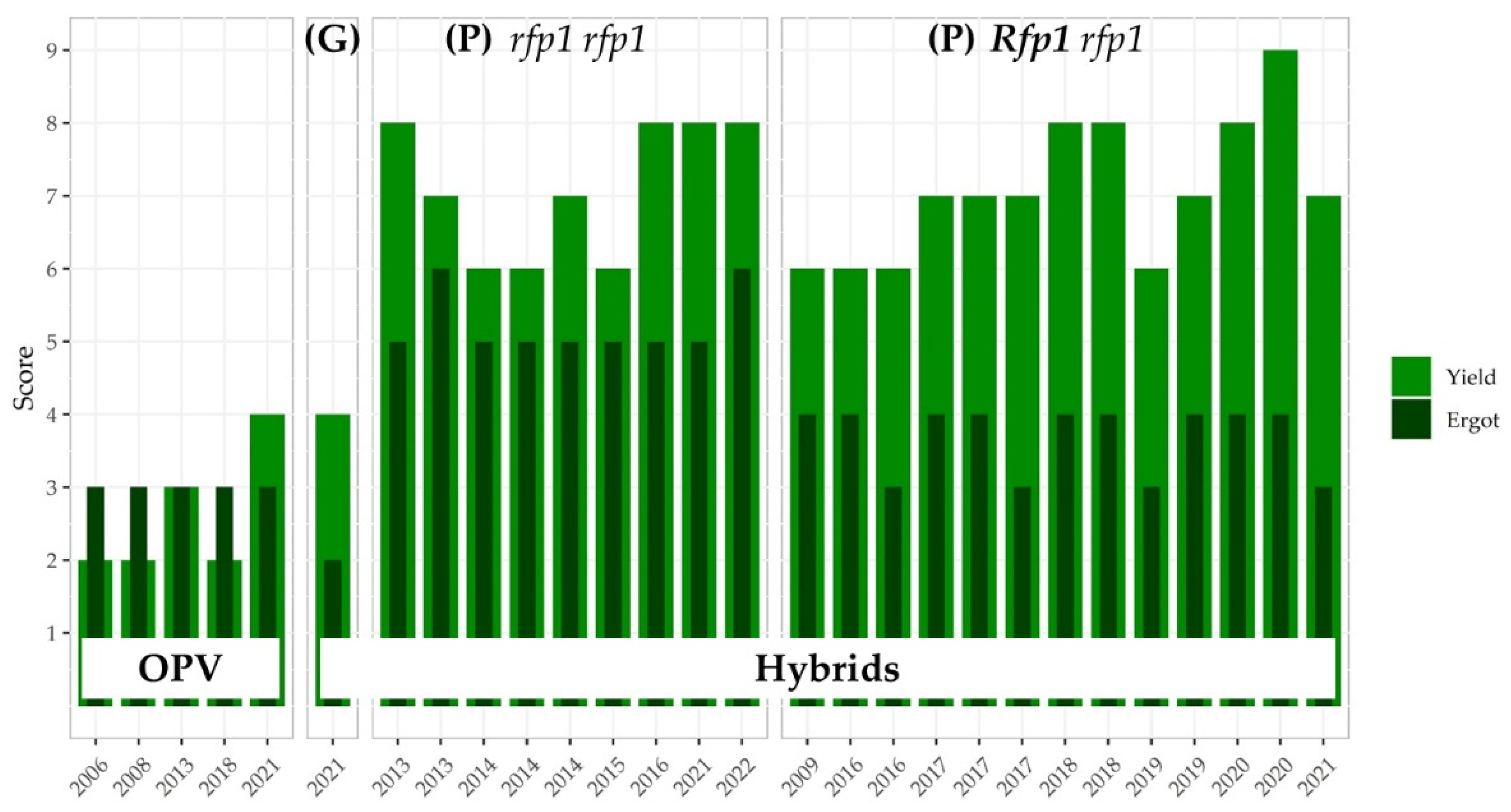
3.3.2. Impact of Major Rfp Genes on Grain Yield in P-type CMS Hybrid Rye
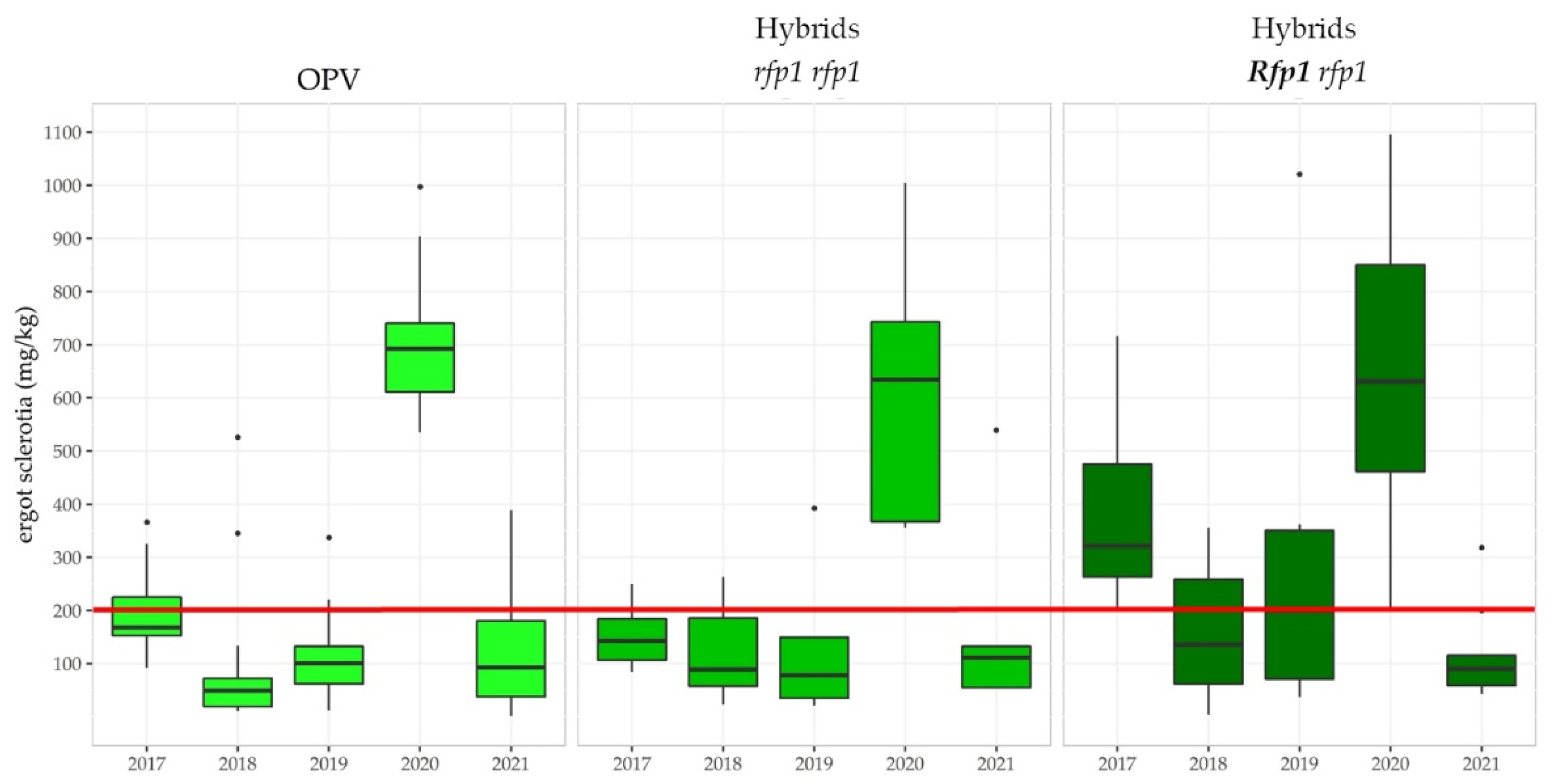
3.3.3. The Ergot Challenge
3.3.4. SMART Breeding to Counterbalance Linkage Drag Effects on Grain Yield
3.3.5. Linkage Drag Constrains the Ergot Defense of Hybrid Rye
3.4. Heterotic Groups, the Enigma of Complementing Components for Yield Improvement
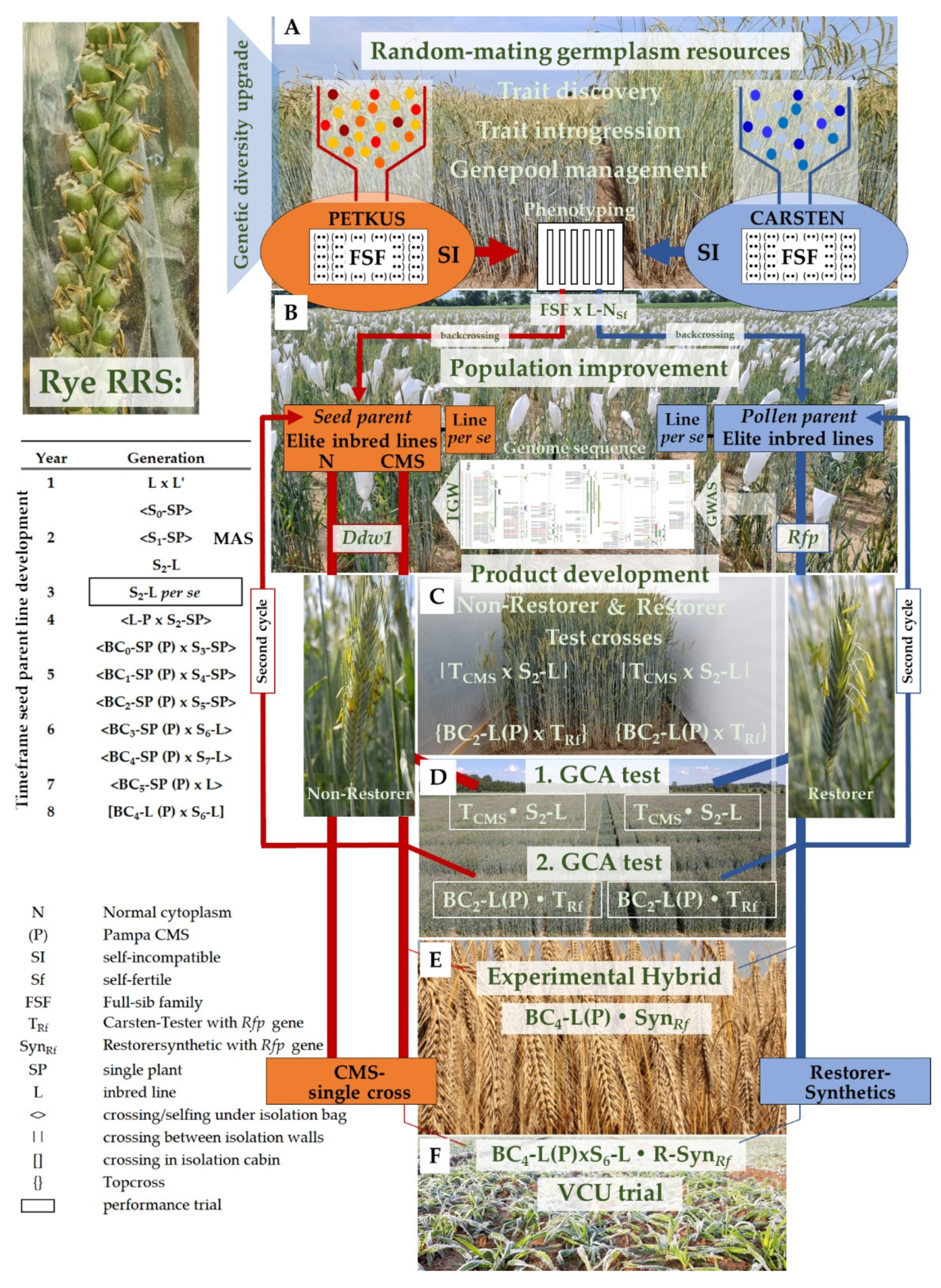
4. A Recurrent Selection Scheme to Enrich Desirable Alleles for Grain Yield
5. Random-Mating Rye Populations, the Genetic Diversity Booster
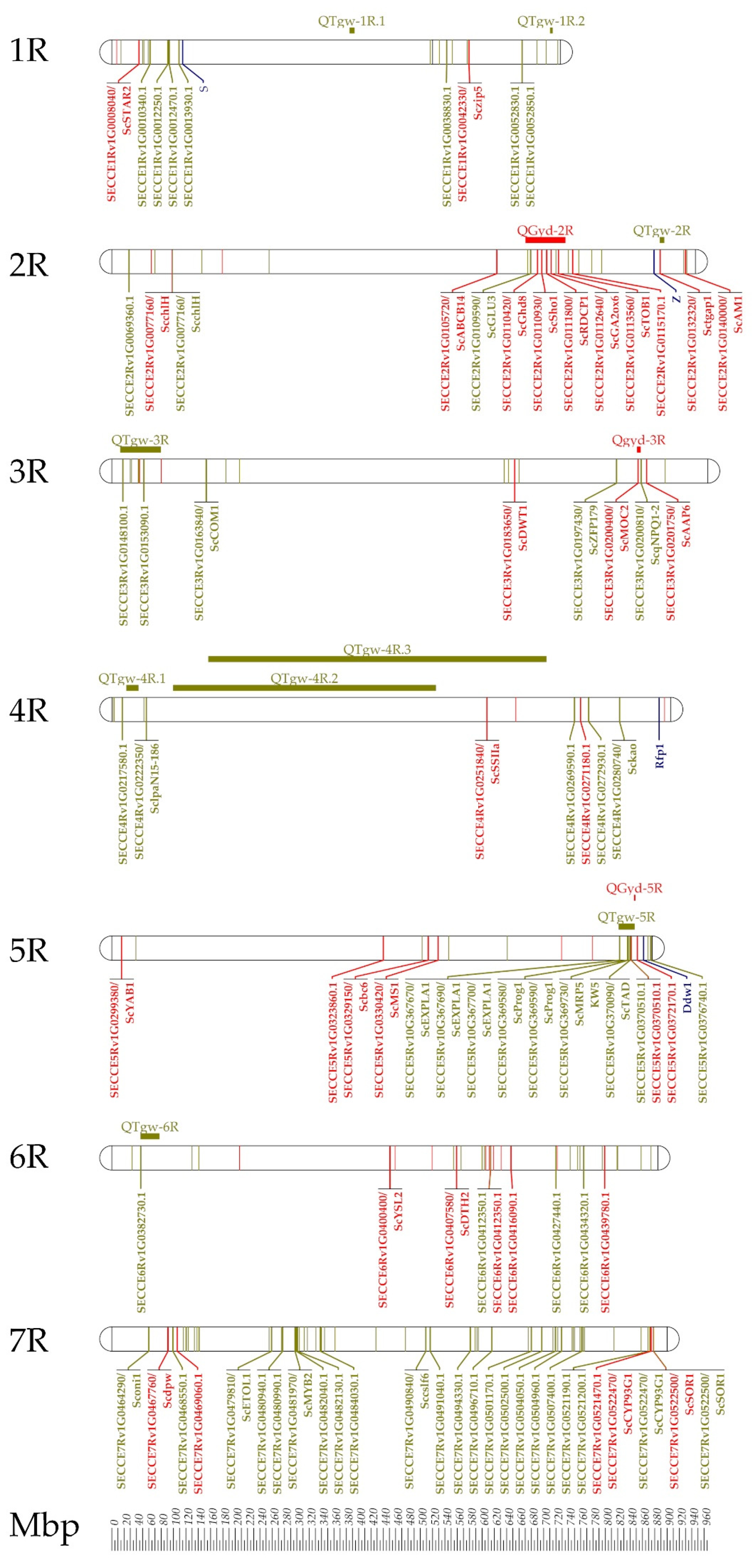
6. Approaching Rye Genes Controlling Grain Yield
7. Novel Ideotypes to Improve the Yield Potential of Rye
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McElroy, J.S. Vavilovian Mimicry: Nikolai Vavilov and His Little-Known Impact on Weed Science. Weed Sci. 2014, 62, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Behre, K.-E. The history of rye cultivation in Europe. Veg. Hist. Archaeobotany 1992, 1, 141–156. [Google Scholar] [CrossRef]
- Küster, H. Am Anfang War das Korn: Eine andere Geschichte der Menschheit, 1st ed.; C.H. Beck: München, Germany, 2012; ISBN 9783406652189. [Google Scholar]
- Mitterauer, M. Why Europe?: The Medieval Origins of Its Special Path; University of Chicago Press: Chicago, IL, USA, 2010; ISBN 9780226532387. [Google Scholar]
- Eurostat. Agricultural Production—Crops. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_crops#Cereals (accessed on 18 August 2022).
- Riedesel, L.; Laidig, F.; Hadasch, S.; Rentel, D.; Hackauf, B.; Piepho, H.-P.; Feike, T. Breeding progress reduces carbon footprints of wheat and rye. J. Clean. Prod. 2022, 377, 134326. [Google Scholar] [CrossRef]
- Geiger, H.H.; Miedaner, T. Rye (Secale cereale L.). In Cereals; Springer: New York, NY, USA, 2009; pp. 157–181. [Google Scholar]
- Pasqui, M.; Di Giuseppe, E. Climate change, future warming, and adaptation in Europe. Anim. Front. 2019, 9, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Drought Observatory. Situation of Combined Drought Indicator in Europe—3rd Ten-Day Period of July 2022. Available online: https://edo.jrc.ec.europa.eu/edov2/php/index.php?id=1000 (accessed on 20 August 2022).
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 317. [Google Scholar] [CrossRef]
- Middleton, C.P.; Senerchia, N.; Stein, N.; Akhunov, E.D.; Keller, B.; Wicker, T.; Kilian, B. Sequencing of chloroplast genomes from wheat, barley, rye and their relatives provides a detailed insight into the evolution of the Triticeae tribe. PLoS ONE 2014, 9, e85761. [Google Scholar] [CrossRef] [Green Version]
- Francis, C.A.; Jensen, E.S.; Lieblein, G.; Breland, T.A. Agroecologist Education for Sustainable Development of Farming and Food Systems. Agron. J. 2017, 109, 23–32. [Google Scholar] [CrossRef]
- Mabhaudhi, T.; Chimonyo, V.G.P.; Hlahla, S.; Massawe, F.; Mayes, S.; Nhamo, L.; Modi, A.T. Prospects of orphan crops in climate change. Planta 2019, 250, 695–708. [Google Scholar] [CrossRef] [Green Version]
- Hadasch, S.; Laidig, F.; Macholdt, J.; Bönecke, E.; Piepho, H.P. Trends in mean performance and stability of winter wheat and winter rye yields in a long-term series of variety trials. Field Crops Res. 2020, 252, 107792. [Google Scholar] [CrossRef]
- Ahrends, H.E.; Siebert, S.; Rezaei, E.E.; Seidel, S.J.; Hüging, H.; Ewert, F.; Döring, T.; Rueda-Ayala, V.; Eugster, W.; Gaiser, T. Nutrient supply affects the yield stability of major European crops—A 50 year study. Environ. Res. Lett. 2021, 16, 14003. [Google Scholar] [CrossRef]
- Buttriss, J.L. Rye: The overlooked cereal. Nutr. Bull. 2006, 31, 3–5. [Google Scholar] [CrossRef]
- ter Steeg, E.M.S.; Struik, P.C.; Visser, R.G.F.; Lindhout, P. Crucial factors for the feasibility of commercial hybrid breeding in food crops. Nat. Plants 2022, 8, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Laidig, F.; Piepho, H.-P.; Rentel, D.; Drobek, T.; Meyer, U.; Huesken, A. Breeding progress, variation, and correlation of grain and quality traits in winter rye hybrid and population varieties and national on-farm progress in Germany over 26 years. Theor. Appl. Genet. 2017, 130, 981–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, H.H. Hybrid breeding in rye. In Cereal Section, Eucarpia Meeting of the Cereal Section on Rye, Svalöv, Sweden, 11–13 June 1985; Persson, G., Gunnarsson, E., Lehmann, L., Jönsson, S., Persson, E., Eds.; European Association for Research on Plant Breeding: Wageningen, The Netherlands, 1986; pp. 237–266. [Google Scholar]
- Bundessortenamt. Beschreibende Sortenliste: Getreide, Mais; Öl- und Faserpflanzen; Leguminosen, Rüben, Zwischen-Früchte; Bundessortenamt: Hannover, Germany, 2022. [Google Scholar]
- de Vries, A.P. Flowering biology of wheat, particularly in view of hybrid seed production—A review. Euphytica 1971, 20, 152–170. [Google Scholar] [CrossRef]
- Lundqvist, A. Self-Incompatibility in Rye. 1. Genetic Control in the Diploid. Hereditas 1956, 42, 293–348. [Google Scholar] [CrossRef]
- Goldberg, E.E.; Kohn, J.R.; Lande, R.; Robertson, K.A.; Smith, S.A.; Igić, B. Species selection maintains self-incompatibility. Science 2010, 330, 493–495. [Google Scholar] [CrossRef] [Green Version]
- Melonek, J.; Korzun, V.; Hackauf, B. Genomics of Self-Incompatibility and Male-Fertility Restoration in Rye. In The Rye Genome; Stein, N., Rabanus-Wallace, M.T., Eds.; Springer: Cham, Switzerland, 2021; pp. 181–212. [Google Scholar]
- Hackauf, B.; Rabanus-Wallace, M.T.; Korzun, V. Bridging the Genotype–Phenotype Gap for Precision Breeding in Rye. In The Rye Genome; Springer: Cham, Switzerland, 2021; pp. 135–180. [Google Scholar]
- Nilsson, H. Populationsanalysen und Erblichkeitsversuche iiber die Selbststerilitat, Selbstfertilitat und Sterilitat bei dem Roggen. Z. Pflanzenzücht. 1916, 4, 1–44. [Google Scholar]
- Ossent, H.P. 10 Jahre Roggenzüchtung in Müncheberg. Der Züchter 1938, 10, 255–261. [Google Scholar] [CrossRef]
- Melz, G.; Kaczmarek, J.; Szigat, G. Genetical analysis of rye (Secale cereale L.). Location of self-fertility genes in different inbred lines. Genet. Pol. 1990, 31, 1–7. [Google Scholar]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Warchoł, M.; Zieliński, K.; Dubas, E. Obtaining of winter rye (Secale cereale L. ssp. cereale) haploid embryos through hybridization with maize (Zea Mays, L.). Cereal Res. Commun. 2018, 46, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Zieliński, K.; Krzewska, M.; Żur, I.; Juzoń, K.; Kopeć, P.; Nowicka, A.; Moravčiková, J.; Skrzypek, E.; Dubas, E. The effect of glutathione and mannitol on androgenesis in anther and isolated microspore cultures of rye (Secale cereale L.). Plant Cell Tiss. Organ Cult. 2020, 140, 577–592. [Google Scholar] [CrossRef] [Green Version]
- Roemer, T. Über die Reichweite des Pollens beim Roggen. Z. Pflanzenzüchtg. 1932, 17, 14–21. [Google Scholar]
- Wang, N.; Gent, J.I.; Dawe, R.K. Haploid induction by a maize cenh3 null mutant. Sci. Adv. 2021, 7, eabe2299. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Liu, C.; Chen, S.; Jin, W. Haploid induction and its application in maize breeding. Mol. Breed. 2021, 41, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Yue, Y.; Zhao, H.; Fei, X.; E., L.; Liu, C.; Chen, S.; Lai, J.; Song, W. Loss-of-function alleles of ZmPLD3 cause haploid induction in maize. Nat. Plants 2021, 7, 1579–1588. [Google Scholar] [CrossRef]
- Liu, C.; Zhong, Y.; Qi, X.; Chen, M.; Liu, Z.; Chen, C.; Tian, X.; Li, J.; Jiao, Y.; Wang, D.; et al. Extension of the in vivo haploid induction system from diploid maize to hexaploid wheat. Plant Biotechnol. J. 2020, 18, 316–318. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Liu, C.; Qi, X.; Jiao, Y.; Wang, D.; Wang, Y.; Liu, Z.; Chen, C.; Chen, B.; Tian, X.; et al. Mutation of ZmDMP enhances haploid induction in maize. Nat. Plants 2019, 5, 575–580. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Y.; Liu, C.; Liu, Y.; Wang, Y.; Liang, D.; Liu, J.; Sahoo, G.; Kelliher, T. OsMATL mutation induces haploid seed formation in indica rice. Nat. Plants 2018, 4, 530–533. [Google Scholar] [CrossRef]
- Gilles, L.M.; Khaled, A.; Laffaire, J.-B.; Chaignon, S.; Gendrot, G.; Laplaige, J.; Bergès, H.; Beydon, G.; Bayle, V.; Barret, P.; et al. Loss of pollen-specific phospholipase NOT LIKE DAD triggers gynogenesis in maize. EMBO J. 2017, 36, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, X.; Meng, D.; Zhong, Y.; Chen, C.; Dong, X.; Xu, X.; Chen, B.; Li, W.; Li, L.; et al. A 4-bp Insertion at ZmPLA1 Encoding a Putative Phospholipase A Generates Haploid Induction in Maize. Mol. Plant. 2017, 10, 520–522. [Google Scholar] [CrossRef] [Green Version]
- Kelliher, T.; Starr, D.; Richbourg, L.; Chintamanani, S.; Delzer, B.; Nuccio, M.L.; Green, J.; Chen, Z.; McCuiston, J.; Wang, W.; et al. MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 2017, 542, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Rabanus-Wallace, M.T.; Hackauf, B.; Mascher, M.; Lux, T.; Wicker, T.; Gundlach, H.; Baez, M.; Houben, A.; Mayer, K.F.X.; Guo, L.; et al. Chromosome-scale genome assembly provides insights into rye biology, evolution and agronomic potential. Nat. Genet. 2021, 53, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, L.; Yang, J.; He, H.; Jin, H.; Li, X.; Ren, T.; Ren, Z.; Li, F.; Han, X.; et al. A high-quality genome assembly highlights rye genomic characteristics and agronomically important genes. Nat. Genet. 2021, 53, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.A.; Beshir, A.; Khokhar, E.S. Doubled haploids in maize: Development, deployment, and challenges. Crop. Sci. 2020, 60, 2815–2840. [Google Scholar] [CrossRef]
- Ahokas, H. Evidence for cytoplasmic male sterility in two Finnish rye cultivars. Hereditas 1980, 92, 373–375. [Google Scholar] [CrossRef]
- Warzecha, R.; Salak-Warzecha, K. A new source of male sterility in rye (Secale cereale L.). Plant Breed. Seed Sci. 2003, 48, 61–65. [Google Scholar]
- Madej, L. Research on male sterility in rye. Hod. Rosl. Aklim. Nasienn. 1975, 19, 421–422. [Google Scholar]
- Kobyljanskij, V.D.; Katerova, A.G. Inheritance of cytoplasmatic and nuclear male sterility in Oshima diploid rye. Genetika 1973, 6, 5–11. [Google Scholar]
- Łapiński, M. Cytoplasmic-genic type of male sterility in Secale montanum Guss. Wheat Inf. Serv. 1972, 35, 25–28. [Google Scholar]
- Geiger, H.H.; Schnell, F.W. Cytoplasmic Male Sterility in Rye (Secale cereale L.) 1. Crop. Sci. 1970, 10, 590–593. [Google Scholar] [CrossRef]
- Adolf, K.; Winkel, A. A new source of sponatnous sterility in winter rye—Preliminary results. In Cereal Section, Proceedings of the Eucarpia Meeting of the Cereal Section on Rye, Svalöv, Sweden, 11–13 June 1985; Persson, G., Gunnarsson, E., Lehmann, L., Jönsson, S., Persson, E., Eds.; European Association for Research on Plant Breeding: Wageningen, The Netherlands, 1986; pp. 293–306. [Google Scholar]
- Melz, G.; Melz, G.; Hartmann, F. Genetics of a male-sterile rye of ‘G-type’ with results of the first F1 hybrids. Plant Breed. Seed Sci. 2003, 47, 47–55. [Google Scholar]
- Dufay, M.; Billard, E. How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Ann. Bot. 2012, 109, 505–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwin, C. The Different Forms of Flowers on Plants of the Same Species; Murray: London, UK, 1877. [Google Scholar]
- Varga, S. Female advantage in gynodioecious plants: A meta-analysis focused on seed quality. Plant Biol. 2021, 23, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Marker, R.M.; Geiger, H.H.; Wilde, P. Influence of ‘Pampa’ cytoplasm on agronomic characters of F2 crosses among inbred lines of winter rye. In Cereal Section, Proceedings of the Eucarpia Meeting of the Cereal Section on Rye, Svalöv, Sweden, 11–13 June 1985; Persson, G., Gunnarsson, E., Lehmann, L., Jönsson, S., Persson, E., Eds.; European Association for Research on Plant Breeding: Wageningen, The Netherlands, 1986; pp. 279–292. [Google Scholar]
- Stojałowski, S.; Kociuba, M.; Stochmal, B.; Kondzioła, M.; Jaciubek, M. Determining the plasmotypic structure of rye populations by SCAR markers. J. Appl. Genet. 2008, 49, 229–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieseberg, L.H.; Blackman, B.K. Speciation genes in plants. Ann. Bot. 2010, 106, 439–455. [Google Scholar] [CrossRef] [Green Version]
- Łapiński, M.; Stojałowski, S. Occurrence and genetic identity of male sterility-inducing cytoplasm in rye (Secale spp.). Plant Breed. Seed Sci. 2003, 48, 7–23. [Google Scholar]
- Vendelbo, N.M.; Sarup, P.; Orabi, J.; Kristensen, P.S.; Jahoor, A. Genetic structure of a germplasm for hybrid breeding in rye (Secale cereale L.). PLoS ONE 2020, 15, e0239541. [Google Scholar] [CrossRef]
- Siekmann, D.; Jansen, G.; Zaar, A.; Kilian, A.; Fromme, F.J.; Hackauf, B. A Genome-Wide Association Study Pinpoints Quantitative Trait Genes for Plant Height, Heading Date, Grain Quality, and Yield in Rye (Secale cereale L.). Front. Plant Sci. 2021, 12, 718081. [Google Scholar] [CrossRef]
- Vendelbo, N.M.; Mahmood, K.; Sarup, P.; Kristensen, P.S.; Orabi, J.; Jahoor, A. Genomic Scan of Male Fertility Restoration Genes in a ‘Gülzow’ Type Hybrid Breeding System of Rye (Secale cereale L.). Int. J. Mol. Sci. 2021, 22, 9277. [Google Scholar] [CrossRef]
- Börner, A.; Korzun, V.; Polley, A.; Malyshev, S.; Melz, G. Genetics and molecular mapping of a male fertility restoration locus (Rfg1) in rye (Secale cereale L.). Theor. Appl. Genet. 1998, 97, 99–102. [Google Scholar] [CrossRef]
- Steinborn, R.; Schwabe, W.; Weihe, A.; Adolf, K.; Melz, G.; Börner, T. A new type of cytoplasmic male sterility in rye (Secale cereale L.): Analysis of mitochondrial DNA. Theor. Appl. Genet. 1993, 85, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Glass, C.; Dreyer, F.; Wilde, P.; Wortmann, H.; Geiger, H.H. Mapping of genes for male-fertility restoration in ‘Pampa’ CMS winter rye (Secale cereale L.). Theor Appl Genet. 2000, 101, 1226–1233. [Google Scholar] [CrossRef]
- Hackauf, B.; Korzun, V.; Wortmann, H.; Wilde, P.; Wehling, P. Development of conserved ortholog set markers linked to the restorer gene Rfp1 in rye. Mol. Breed. 2012, 30, 1507–1518. [Google Scholar] [CrossRef]
- Falke, K.C.; Wilde, P.; Miedaner, T. Rye introgression lines as source of alleles for pollen-fertility restoration in Pampa CMS. Plant Breed. 2009, 128, 528–531. [Google Scholar] [CrossRef]
- Melz, G.; Adolf, K. Genetic analysis of rye (Secale cereale L.) Genetics of male sterility of the G-type. Theor. Appl. Genet. 1991, 82, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Wilde, P.; Korzun, V.; Menzel, J.; Zhou, R.; Stein, N.; Hackauf, B. Restorer Plants. KWS SAAT SE. Patent WO2017109012A1, 29. Juni 2017. U.S. Patent App. 16/064304, 9 May 2019. Available online: https://uspto.report/patent/app/20190136245 (accessed on 10 August 2022).
- Hackauf, B.; Bauer, E.; Korzun, V.; Miedaner, T. Fine mapping of the restorer gene Rfp3 from an Iranian primitive rye (Secale cereale L.). Theor. Appl. Genet. 2017, 130, 1179–1189. [Google Scholar] [CrossRef]
- Stracke, S.; Schilling, A.G.; Förster, J.; Weiss, C.; Glass, C.; Miedaner, T.; Geiger, H.H. Development of PCR-based markers linked to dominant genes for male-fertility restoration in Pampa CMS of rye (Secale cereale L.). Theor. Appl. Genet. 2003, 106, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Herter, C.P.; Goßlau, H.; Wilde, P.; Hackauf, B. Correlated effects of exotic pollen-fertility restorer genes on agronomic and quality traits of hybrid rye. Plant Breed. 2017, 136, 224–229. [Google Scholar] [CrossRef]
- Miedaner, T.; Korzun, V.; Wilde, P. Effective Pollen-Fertility Restoration Is the Basis of Hybrid Rye Production and Ergot Mitigation. Plants 2022, 11, 1115. [Google Scholar] [CrossRef]
- Miedaner, T.; Geiger, H.H. Biology, genetics, and management of ergot (Claviceps spp.) in rye, sorghum, and pearl millet. Toxins 2015, 7, 659–678. [Google Scholar] [CrossRef] [Green Version]
- Wäli, P.P.; Wäli, P.R.; Saikkonen, K.; Tuomi, J. Is the pathogenic ergot fungus a conditional defensive mutualist for its host grass? PLoS ONE 2013, 8, e69249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oeser, B.; Kind, S.; Schurack, S.; Schmutzer, T.; Tudzynski, P.; Hinsch, J. Cross-talk of the biotrophic pathogen Claviceps purpurea and its host Secale cereale. BMC Genom. 2017, 18, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodisch, A.; Oberforster, M.; Raditschnig, A.; Rodemann, B.; Tratwal, A.; Danielewicz, J.; Korbas, M.; Schmiedchen, B.; Eifler, J.; Gordillo, A.; et al. Covariation of Ergot Severity and Alkaloid Content Measured by HPLC and One ELISA Method in Inoculated Winter Rye across Three Isolates and Three European Countries. Toxins 2020, 12, 676. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) 2021/1399 of 24 August 2021 amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Ergot Sclerotia and Ergot Alkaloids in certain Foodstuffs. Off. J. Eur. Union 2021, 301, 1–5. Available online: https://eur-lex.europa.eu/eli/reg/2021/1399/oj (accessed on 19 August 2022).
- Parat, F.; Schwertfirm, G.; Rudolph, U.; Miedaner, T.; Korzun, V.; Bauer, E.; Schön, C.-C.; Tellier, A. Geography and end use drive the diversification of worldwide winter rye populations. Mol. Ecol. 2016, 25, 500–514. [Google Scholar] [CrossRef]
- Preece, C.; Livarda, A.; Christin, P.-A.; Wallace, M.; Martin, G.; Charles, M.; Jones, G.; Rees, M.; Osborne, C.P. How did the domestication of Fertile Crescent grain crops increase their yields? Funct. Ecol. 2017, 31, 387–397. [Google Scholar] [CrossRef]
- Castillo-Bravo, R.; Fort, A.; Cashell, R.; Brychkova, G.; McKeown, P.C.; Spillane, C. Parent-of-Origin Effects on Seed Size Modify Heterosis Responses in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 835219. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, G.; Ren, X.; Du, B.; Cheng, Y.; Wang, Y.; Li, C.; Sun, D. Dissecting the Genetic Basis of Grain Size and Weight in Barley (Hordeum vulgare L.) by QTL and Comparative Genetic Analyses. Front. Plant Sci. 2019, 10, 469. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Łyskowski, A.; Jaremko, Ł.; Jaremko, M. Genetic and Molecular Factors Determining Grain Weight in Rice. Front. Plant Sci. 2021, 12, 605799. [Google Scholar] [CrossRef]
- Xiao, W.; Brown, R.C.; Lemmon, B.E.; Harada, J.J.; Goldberg, R.B.; Fischer, R.L. Regulation of seed size by hypomethylation of maternal and paternal genomes. Plant Physiol. 2006, 142, 1160–1168. [Google Scholar] [CrossRef]
- Bauer, E.; Schmutzer, T.; Barilar, I.; Mascher, M.; Gundlach, H.; Martis, M.M.; Twardziok, S.O.; Hackauf, B.; Gordillo, A.; Wilde, P.; et al. Towards a whole-genome sequence for rye (Secale cereale L.). Plant J. 2017, 89, 853–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, G.P.; D’Occhio, M.J.; Hetzel, D. Smart Breeding: Selection with Markers and Advanced Reproductive Technologies; Association for the Advancement of Animal Breeding and Genetics: Armidale, NSW, Australia, 1997. [Google Scholar]
- Miedaner, T.; Mirdita, V.; Rodemann, B.; Drobeck, T.; Rentel, D. Genetic variation of winter rye cultivars for their ergot (Claviceps purpurea) reaction tested in a field design with minimized interplot interference. Plant Breed. 2010, 129, 58–62. [Google Scholar] [CrossRef]
- Steiner, B.; Buerstmayr, M.; Michel, S.; Schweiger, W.; Lemmens, M.; Buerstmayr, H. Breeding strategies and advances in line selection for Fusarium head blight resistance in wheat. Trop. Plant Pathol. 2017, 42, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Brodführer, S.; Schmehe, B.; Gabriel, D.; Janowski, D.; Herrmann, M.H. Effects of varying levels of cleistogamy on natural smut infection in oats. Crop. Sci. 2022, 62, 704–712. [Google Scholar] [CrossRef]
- Centralny Ośrodek Badania Odmian Roślin Uprawnych. Wyniki porejestrowych Doświadczeń Odmianowych (WPDO) Zboża Ozime: Results of Post-Registration Varietal Trials (WPDO) Winter Cereals. Available online: https://coboru.gov.pl/pdo/pdoPublikacjeCentralne (accessed on 20 August 2022).
- Hackauf, B.; Siekmann, D.; Fromme, F.J. Hybrid breeding in cereals: Lessons from rye. In Tagungsband der 68. Jahrestagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreich, Raumberg-Gumpenstein, Österreich, 20–22 November 2017; Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs, Ed.; HBLFA Raumberg-Gumpenstein: Irdning, Österreich, 2018; pp. 17–18. [Google Scholar]
- Blum, A. Heterosis, stress, and the environment: A possible road map towards the general improvement of crop yield. J. Exp. Bot. 2013, 64, 4829–4837. [Google Scholar] [CrossRef] [Green Version]
- Song, G.-S.; Zhai, H.-L.; Peng, Y.-G.; Zhang, L.; Wei, G.; Chen, X.-Y.; Xiao, Y.-G.; Wang, L.; Chen, Y.-J.; Wu, B.; et al. Comparative transcriptional profiling and preliminary study on heterosis mechanism of super-hybrid rice. Mol. Plant. 2010, 3, 1012–1025. [Google Scholar] [CrossRef]
- Fujimoto, R.; Taylor, J.M.; Shirasawa, S.; Peacock, W.J.; Dennis, E.S. Heterosis of Arabidopsis hybrids between C24 and Col is associated with increased photosynthesis capacity. Proc. Natl. Acad. Sci. USA 2012, 109, 7109–7114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, D.K.; Rohozinski, D.; Song, Q.; Taylor, S.H.; Juenger, T.E.; Harmon, F.G.; Chen, Z.J. Temporal Shift of Circadian-Mediated Gene Expression and Carbon Fixation Contributes to Biomass Heterosis in Maize Hybrids. PLoS Genet. 2016, 12, e1006197. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zhang, Z.; Tan, X.; Jiang, Y.; Gao, J.; Lin, L.; Wang, Z.; Ren, J.; Wang, X.; Qin, L.; et al. Association of the molecular regulation of ear leaf senescence/stress response and photosynthesis/metabolism with heterosis at the reproductive stage in maize. Sci. Rep. 2016, 6, 29843. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Hou, P.; Duan, F.; Niu, L.; Dai, T.; Wang, K.; Zhao, M.; Li, S.; Zhou, W. Improving photosynthesis to increase grain yield potential: An analysis of maize hybrids released in different years in China. Photosynth. Res. 2021, 150, 295–311. [Google Scholar] [CrossRef]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Li, Z.; Liu, G.; Jiang, Y.; Maurer, H.P.; Würschum, T.; Mock, H.-P.; Matros, A.; Ebmeyer, E.; Schachschneider, R.; et al. Genome-based establishment of a high-yielding heterotic pattern for hybrid wheat breeding. Proc. Natl. Acad. Sci. USA 2015, 112, 15624–15629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melchinger, A.E.; Gumber, R.K. Overview of Heterosis and Heterotic Groups in Agronomic Crops. In Concepts and Breeding of Heterosis in Crop. Plants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 29–44. [Google Scholar]
- Hepting, L. Analyse eines 7 × 7 Sortendiallels zur Ermittlung geeigneten Ausgangsmaterials fur die Hybridzuchtung bei Roggen. Z Pflanzenzucht 1978, 80, 188–197. [Google Scholar]
- Meißner, B.; von Brook, R. Der Roggenkönig: Leben und Wirken des Züchters Ferdinand von Lochow; Wallstein-Verl.: Göttingen, Germany, 2014. [Google Scholar]
- Anonymous. 75 Jahre Saatzucht Dr. h.c. Carsten Bad Schwartau: Lebendige Tradition—Im Dienste der Landwirtschaft; Bad Schwartau, Germany.
- Becker-Dillingen, J. Der Roggen. In Handbuch des Getreidebaues.; Becker-Dillingen, J., Ed.; Paul Parey: Berlin, Germany, 1927; pp. 103–190. [Google Scholar]
- Sun, S.; Zhou, Y.; Chen, J.; Shi, J.; Zhao, H.; Zhao, H.; Song, W.; Zhang, M.; Cui, Y.; Dong, X.; et al. Extensive intraspecific gene order and gene structural variations between Mo17 and other maize genomes. Nat. Genet. 2018, 50, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Comstock, R.E.; Robinson, H.F.; Harvey, P.H. A Breeding Procedure Designed To Make Maximum Use of Both General and Specific Combining Ability. Agron. J. 1949, 41, 360–367. [Google Scholar] [CrossRef]
- Geiger, H.H. Strategies of hybrid rye breeding. In Proceedings of the International Symposium on Rye Breeding & Genetics, Groß Lüsewitz, Germany, 28–30 June 2006; Wehling, P., Roux, S.R., Eds.; pp. 1–5. [Google Scholar]
- Haffke, S.; Wilde, P.; Schmiedchen, B.; Hackauf, B.; Roux, S.; Gottwald, M.; Miedaner, T. Toward a Selection of Broadly Adapted Germplasm for Yield Stability of Hybrid Rye under Normal and Managed Drought Stress Conditions. Crop. Sci. 2015, 55, 1026–1034. [Google Scholar] [CrossRef]
- Auinger, H.-J.; Schönleben, M.; Lehermeier, C.; Schmidt, M.; Korzun, V.; Geiger, H.H.; Piepho, H.-P.; Gordillo, A.; Wilde, P.; Bauer, E.; et al. Model training across multiple breeding cycles significantly improves genomic prediction accuracy in rye (Secale cereale L.). Theor. Appl. Genet. 2016, 129, 2043–2053. [Google Scholar] [CrossRef] [Green Version]
- Wilde, P.; Miedaner, T. Hybrid Rye Breeding. In The Rye Genome; Stein, N., Rabanus-Wallace, M.T., Eds.; Springer: Cham, Switzerland, 2021; pp. 13–41. [Google Scholar]
- Rembe, M.; Reif, J.C.; Ebmeyer, E.; Thorwarth, P.; Korzun, V.; Schacht, J.; Boeven, P.H.G.; Varenne, P.; Kazman, E.; Philipp, N.; et al. Reciprocal Recurrent Genomic Selection Is Impacted by Genotype-by-Environment Interactions. Front. Plant Sci. 2021, 12, 703419. [Google Scholar] [CrossRef]
- Cha, J.-K.; O’Connor, K.; Alahmad, S.; Lee, J.-H.; Dinglasan, E.; Park, H.; Lee, S.-M.; Hirsz, D.; Kwon, S.-W.; Kwon, Y.; et al. Speed vernalization to accelerate generation advance in winter cereal crops. Mol. Plant. 2022, 15, 1300–1309. [Google Scholar] [CrossRef]
- White, M.R.; Mikel, M.A.; Leon, N.; Kaeppler, S.M. Diversity and heterotic patterns in North American proprietary dent maize germplasm. Crop. Sci. 2020, 60, 100–114. [Google Scholar] [CrossRef]
- Wijnker, E.; de Jong, H. Managing meiotic recombination in plant breeding. Trends Plant Sci. 2008, 13, 640–646. [Google Scholar] [CrossRef]
- Lloyd, A. Crossover patterning in plants. Plant Reprod. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Haseneyer, G.; Schön, C.-C.; Ankerst, D.; Korzun, V.; Wilde, P.; Bauer, E. High levels of nucleotide diversity and fast decline of linkage disequilibrium in rye (Secale cereale L.) genes involved in frost response. BMC Plant Biol. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, M.; Unterseer, S.; Bauer, E.; de Leon, N.; Ordas, B.; Schön, C.-C. Is there an optimum level of diversity in utilization of genetic resources? Theor. Appl. Genet. 2017, 130, 2283–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voylokov, A.V. Prospects of using self-fertility in breeding rye populations varieties. Russ. J. Genet. 2007, 43, 1173–1180. [Google Scholar] [CrossRef]
- Döring, T.F.; Knapp, S.; Kovacs, G.; Murphy, K.; Wolfe, M.S. Evolutionary Plant Breeding in Cereals—Into a New Era. Sustainability 2011, 3, 1944–1971. [Google Scholar] [CrossRef] [Green Version]
- Langridge, P.; Reynolds, M. Breeding for drought and heat tolerance in wheat. Theor. Appl. Genet. 2021, 134, 1753–1769. [Google Scholar] [CrossRef]
- Fischer, S.; Melchinger, A.E.; Korzun, V.; Wilde, P.; Schmiedchen, B.; Möhring, J.; Piepho, H.-P.; Dhillon, B.S.; Würschum, T.; Reif, J.C. Molecular marker assisted broadening of the Central European heterotic groups in rye with Eastern European germplasm. Theor. Appl. Genet. 2010, 120, 291–299. [Google Scholar] [CrossRef]
- Hackauf, B.; Haffke, S.; Fromme, F.J.; Roux, S.R.; Kusterer, B.; Musmann, D.; Kilian, A.; Miedaner, T. QTL mapping and comparative genome analysis of agronomic traits including grain yield in winter rye. Theor. Appl. Genet. 2017, 130, 1801–1817. [Google Scholar] [CrossRef]
- Hori, K.; Shenton, M. Recent Advances in Molecular Research in Rice: Agronomically Important Traits. Int. J. Mol. Sci. 2020, 21, 5945. [Google Scholar] [CrossRef]
- Yao, W.; Li, G.; Yu, Y.; Ouyang, Y. funRiceGenes dataset for comprehensive understanding and application of rice functional genes. Gigascience 2018, 7, gix119. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tian, D.; Zhang, Z.; Hu, S.; Yu, J. Rice Genomics: Over the Past Two Decades and into the Future. Genom. Proteom. Bioinform. 2018, 16, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, J.; Chen, L.; Huang, X.; Cheng, Z.; Han, B.; Zhang, Q.; Wu, C. Rice Functional Genomics Research: Past Decade and Future. Mol. Plant. 2018, 11, 359–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, N.; Maji, S.; Waseem, M.; Thakur, P.; Kumar, V.; Parida, S.K.; Thakur, J.K. The Mediator subunit OsMED15a is a transcriptional co-regulator of seed size/weight-modulating genes in rice. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 194432. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Meng, X.; Guo, H.; Cheng, Q.; Jing, Y.; Chen, M.; Liu, G.; Wang, B.; Wang, Y.; Li, J.; et al. Targeting a gene regulatory element enhances rice grain yield by decoupling panicle number and size. Nat. Biotechnol. 2022, 40, 1403–1411. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, K.; Harberd, N.P.; Fu, X. Green Revolution DELLAs: From translational reinitiation to future sustainable agriculture. Mol. Plant. 2021, 14, 547–549. [Google Scholar] [CrossRef]
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [Green Version]
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef]
- Khush, G.S. Green revolution: The way forward. Nat. Rev. Genet. 2001, 2, 815–822. [Google Scholar] [CrossRef]
- Dzyubenko, N.I. Vavilov’s Collection of Worldwide Crop Genetic Resources in the 21st Century. Biopreserv. Biobank. 2018, 16, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Miedaner, T.; Haffke, S.; Siekmann, D.; Fromme, F.J.; Roux, S.R.; Hackauf, B. Dynamic quantitative trait loci (QTL) for plant height predict biomass yield in hybrid rye (Secale cereale L.). Biomass Bioenergy 2018, 115, 10–18. [Google Scholar] [CrossRef]
- Miedaner, T.; Hübner, M.; Korzun, V.; Schmiedchen, B.; Bauer, E.; Haseneyer, G.; Wilde, P.; Reif, J.C. Genetic architecture of complex agronomic traits examined in two testcross populations of rye (Secale cereale L.). BMC Genom. 2012, 13, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miedaner, T.; Müller, B.U.; Piepho, H.-P.; Falke, K.C. Genetic architecture of plant height in winter rye introgression libraries. Plant Breed. 2011, 130, 209–216. [Google Scholar] [CrossRef]
- Tenhola-Roininen, T.; Tanhuanpää, P. Tagging the dwarfing gene Ddw1 in a rye population derived from doubled haploid parents. Euphytica 2010, 172, 303–312. [Google Scholar] [CrossRef]
- Torop, A.A.; Dedyaev, V.G.; Tschaykin, V.V.; Dokuchaev, V.V. The results of rye breeding in the central-chernosem region of Russia. Plant Breed. Seed Sci. 2003, 47, 69–75. [Google Scholar]
- McLeod, J.G.; Gan, Y.T.; Payne, J.F. AC Remington winter rye. Can. J. Plant Sci. 2000, 80, 605–607. [Google Scholar] [CrossRef]
- Kobylyanskij, V.D. Initial material for solving the main problems encountered in breeding winter rye in the northwestern zone of the USSR. J. Agric. Sci. Finland 1988, 60, 215–221. [Google Scholar] [CrossRef]
- Litvinov, D.Y.; Chernook, A.G.; Kroupin, P.Y.; Bazhenov, M.S.; Karlov, G.I.; Avdeev, S.M.; Divashuk, M.G. A Convenient Co-Dominant Marker for Height-Reducing Ddw1 Allele Useful for Marker-Assisted Selection. Agriculture 2020, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Braun, E.-M.; Tsvetkova, N.; Rotter, B.; Siekmann, D.; Schwefel, K.; Krezdorn, N.; Plieske, J.; Winter, P.; Melz, G.; Voylokov, A.V.; et al. Gene Expression Profiling and Fine Mapping Identifies a Gibberellin 2-Oxidase Gene Co-segregating With the Dominant Dwarfing Gene Ddw1 in Rye (Secale cereale L.). Front. Plant Sci. 2019, 10, 857. [Google Scholar] [CrossRef]
- Laidig, F.; Feike, T.; Klocke, B.; Macholdt, J.; Miedaner, T.; Rentel, D.; Piepho, H.P. Long-term breeding progress of yield, yield-related, and disease resistance traits in five cereal crops of German variety trials. Theor. Appl. Genet. 2021, 134, 3805–3827. [Google Scholar] [CrossRef]
- Herelius, E. Residues of Straw Height Reducing Plant Growth Regulators in Cereal Products; SLU, Department of Molecular Sciences: Uppsala, Sweden, 2017. [Google Scholar]
- Börner, A.; Melz, G. Response of rye genotypes differing in plant height to exogenous gibberellic acid application. Arch. Züchtungsforsch. 1988, 18, 79–82. [Google Scholar]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Wüthrich, S.; Blösch, R.; Rindisbacher, A.; Cannarozzi, G.; Tadele, Z. Gibberellin Deficiency Confers Both Lodging and Drought Tolerance in Small Cereals. Front. Plant Sci. 2016, 7, 643. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.-F.; Ho, T.-H.D.; Liu, Y.-L.; Jiang, M.-J.; Hsieh, K.-T.; Chen, K.-T.; Yu, L.-C.; Lee, M.-H.; Chen, C.-Y.; Huang, T.-P.; et al. Ectopic expression of specific GA2 oxidase mutants promotes yield and stress tolerance in rice. Plant Biotechnol. J. 2017, 15, 850–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-I.; Li, P.-F.; Yang, C.-H. NAC-Like Gene GIBBERELLIN SUPPRESSING FACTOR Regulates the Gibberellin Metabolic Pathway in Response to Cold and Drought Stresses in Arabidopsis. Sci. Rep. 2019, 9, 19226. [Google Scholar] [CrossRef] [Green Version]
- Buss, W.; Ford, B.A.; Foo, E.; Schnippenkoetter, W.; Borrill, P.; Brooks, B.; Ashton, A.R.; Chandler, P.M.; Spielmeyer, W. Overgrowth mutants determine the causal role of gibberellin GA2oxidaseA13 in Rht12 dwarfism of wheat. J. Exp. Bot. 2020, 71, 7171–7178. [Google Scholar] [CrossRef]
- Börner, A.; Korzun, V.; Voylokov, A.V.; Worland, A.J.; Weber, W.E. Genetic mapping of quantitative trait loci in rye (Secale cereale L.). Euphytica 2000, 116, 203–209. [Google Scholar] [CrossRef]
- Kobylyanskij, V.D.; Babuzhina, D.I. Photosynthesis of different plant orpgans in short stem rye. In Proceedings of the International Symposium on Rye Breeding & Genetics, Groß Lüsewitz, Germany, 28–30 June 2006; Wehling, P., Roux, S.R., Eds.; pp. 62–65. [Google Scholar]
- Hyles, J.; Bloomfield, M.T.; Hunt, J.R.; Trethowan, R.M.; Trevaskis, B. Phenology and related traits for wheat adaptation. Heredity 2020, 125, 417–430. [Google Scholar] [CrossRef]
- Båga, M.; Bahrani, H.; Larsen, J.; Hackauf, B.; Graf, R.J.; Laroche, A.; Chibbar, R.N. Association mapping of autumn-seeded rye (Secale cereale L.) reveals genetic linkages between genes controlling winter hardiness and plant development. Sci. Rep. 2022, 12, 5793. [Google Scholar] [CrossRef]
- Reynolds, M.; Langridge, P. Physiological breeding. Curr. Opin. Plant Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Dawson, I.K.; Powell, W.; Hendre, P.; Bančič, J.; Hickey, J.M.; Kindt, R.; Hoad, S.; Hale, I.; Jamnadass, R. The role of genetics in mainstreaming the production of new and orphan crops to diversify food systems and support human nutrition. New Phytol. 2019, 224, 37–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Lammerts Bueren, E.T.; Struik, P.C.; van Eekeren, N.; Nuijten, E. Towards resilience through systems-based plant breeding. A review. Agron. Sustain. Dev. 2018, 38, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, M.A.; He, Y.; Zhou, M. Beyond a reference genome: Pangenomes and population genomics of underutilized and orphan crops for future food and nutrition security. New Phytol. 2022, 234, 1583–1597. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackauf, B.; Siekmann, D.; Fromme, F.J. Improving Yield and Yield Stability in Winter Rye by Hybrid Breeding. Plants 2022, 11, 2666. https://doi.org/10.3390/plants11192666
Hackauf B, Siekmann D, Fromme FJ. Improving Yield and Yield Stability in Winter Rye by Hybrid Breeding. Plants. 2022; 11(19):2666. https://doi.org/10.3390/plants11192666
Chicago/Turabian StyleHackauf, Bernd, Dörthe Siekmann, and Franz Joachim Fromme. 2022. "Improving Yield and Yield Stability in Winter Rye by Hybrid Breeding" Plants 11, no. 19: 2666. https://doi.org/10.3390/plants11192666
APA StyleHackauf, B., Siekmann, D., & Fromme, F. J. (2022). Improving Yield and Yield Stability in Winter Rye by Hybrid Breeding. Plants, 11(19), 2666. https://doi.org/10.3390/plants11192666





