Development and Evolution of Unisexual Flowers: A Review
Abstract
:1. Introduction
 | The adjective “hermaphrodite” is built upon the names of the Greek gods Hermes and Aphrodite, who, according to the Metamorphoses by the Roman poet Ovid [2], gave birth to a child, named Hermaphrodite after both his parents. The handsome young man was swimming in a lake when the naiad Salmacis fell in love with him, although her feelings were not reciprocal. The gods heard Salmacis’ prayers to be forever united to her beloved one, and merged both people into a single body, exhibiting both male and female sexes and physical attributes (Figure A). Hermaphrodite in mythology and in botany. (A) Salmacis and Hermaphrodite, from the Game of Mythology, by Stefano della Bella, 1644 (Metropolitan Museum of Art, New York, https://www.metmuseum.org/art/collection/search/412360 (accessed on 18 December 2021), Bequest of Phyllis Massar, 2011). An English translation of the short text would be “Salmacis and Hermaphrodite. The nymph Salmacis loved the handsome Hermaphrodite, son of Mercury and Venus. She pushed him into the water, and while kissing him, her wish to be united with him in the same body was granted”. (B) Hermaphroditic flower of Malus domestica (Suckow) Borkh. (Rosaceae; photograph: F. Jabbour). The numerous stamens surround the pentamerous gynoecium. |
| Flower Gender/Functional Sex | Sexual System When Functional Sex Is Found on: | |||
|---|---|---|---|---|
| Unisexual | Bisexual 3 | |||
| Female 1 | Male 2 | A Single Individual | Distinct Individuals | |
| ✓ | Hermaphroditism | |||
| ✓ | ✓ | Monoecy 4 | Dioecy | |
| ✓ | ✓ | Gynomonoecy | Gynodioecy | |
| ✓ | ✓ | Andromonoecy | Androdioecy | |
| ✓ | ✓ | ✓ | Trimonoecy 5 | Trioecy |
2. Meristem Development in Taxa with Unisexual Flowers, in an Evolutionary Framework
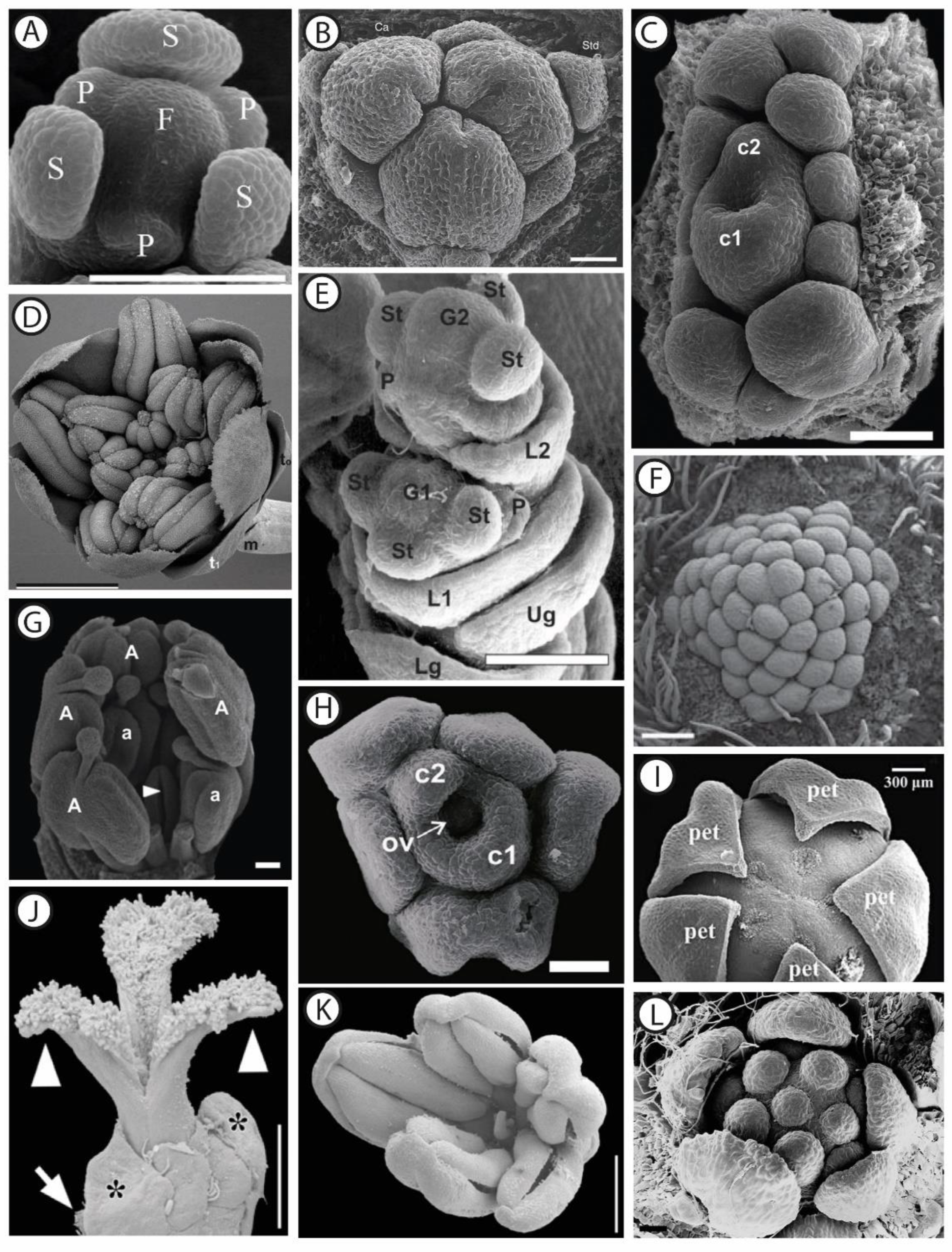
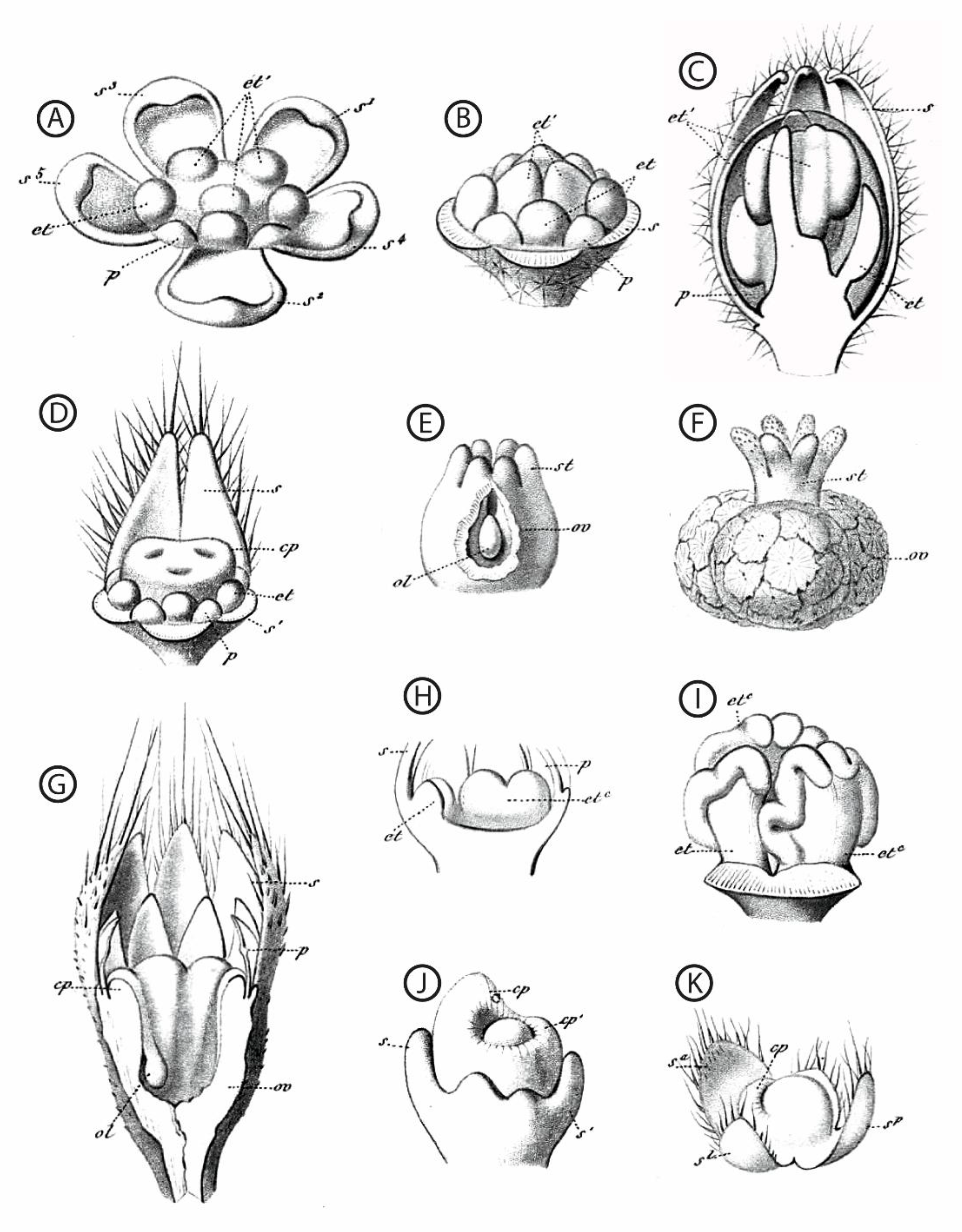
2.1. Morphological and Anatomical Description of Functionally Unisexual Flowers
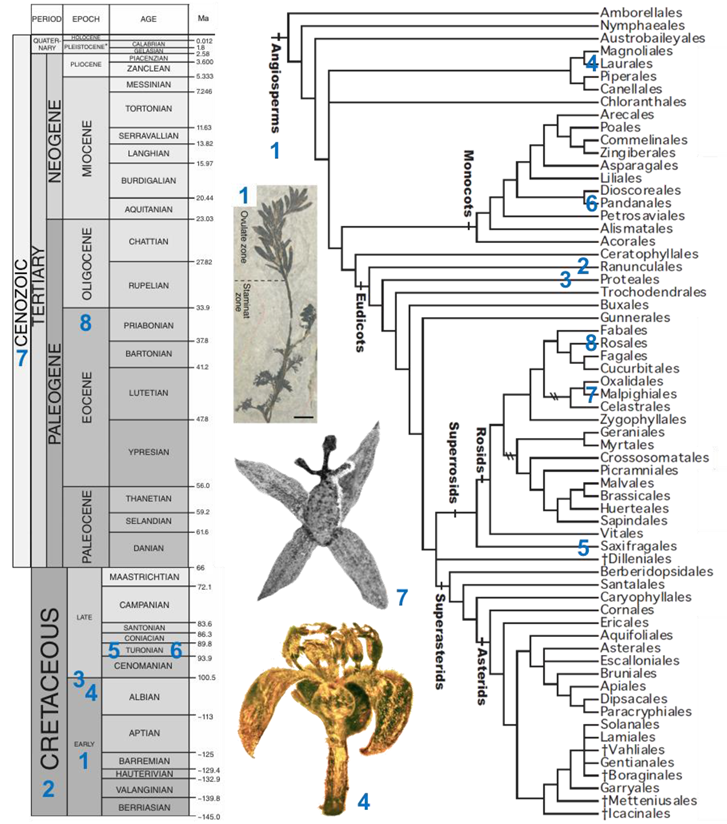
- Teixeira lusitanica von Balthazar, Pedersen and Friis (affinities with Ranunculales, Cretaceous, Portugal). A single male flower including 20 stamens with basifixed anthers was described [64].
- Platanaceae sp. (Proteales, Middle Cretaceous to Albian, USA). Pentamerous male (with five stamens) and female (with five carpels) flowers were described [65].
- Cascolaurus burmitis Poinar (Lauraceae, Laurales, Upper Albian, Myanmar, see associated picture). The male flower presents three whorls of three stamens each with, probably, nectar glands located on the stamens of the innermost whorl [66].
- Microaltingia apocarpela Zhou, Crepet, and Nixon (affinities with Hamamelidaceae, Saxifragales, Turonian, USA). Female flowers show a gynoecium consisting of a semi-inferior bilocular ovary, a style, and a capitate stigma [67].
- Mabelia connatifida Gandolfo, Nixon, et Crepet (Triuridaceae, Pandanales, Turonian, USA). Male flowers include a trimerous whorl of antetepalous stamens [68].
- Pseudosalix handleyi Boucher, Manchester, and Judd (Salicaceae, Malpighiales, Cenozoic, USA, see associated picture). The gynoecium of the female flower consists of a pistil with a single ovary. The androecium of male flowers includes ca. 30 stamens [69].
- Prunus s.l. (Rosaceae, Rosales, terminal Eocene, Ukraine). A fossil male flower, with 24 free stamens, was discovered [70].
2.2. Diversity of Ontogenic Pathways Leading to Functionally Unisexual Flowers
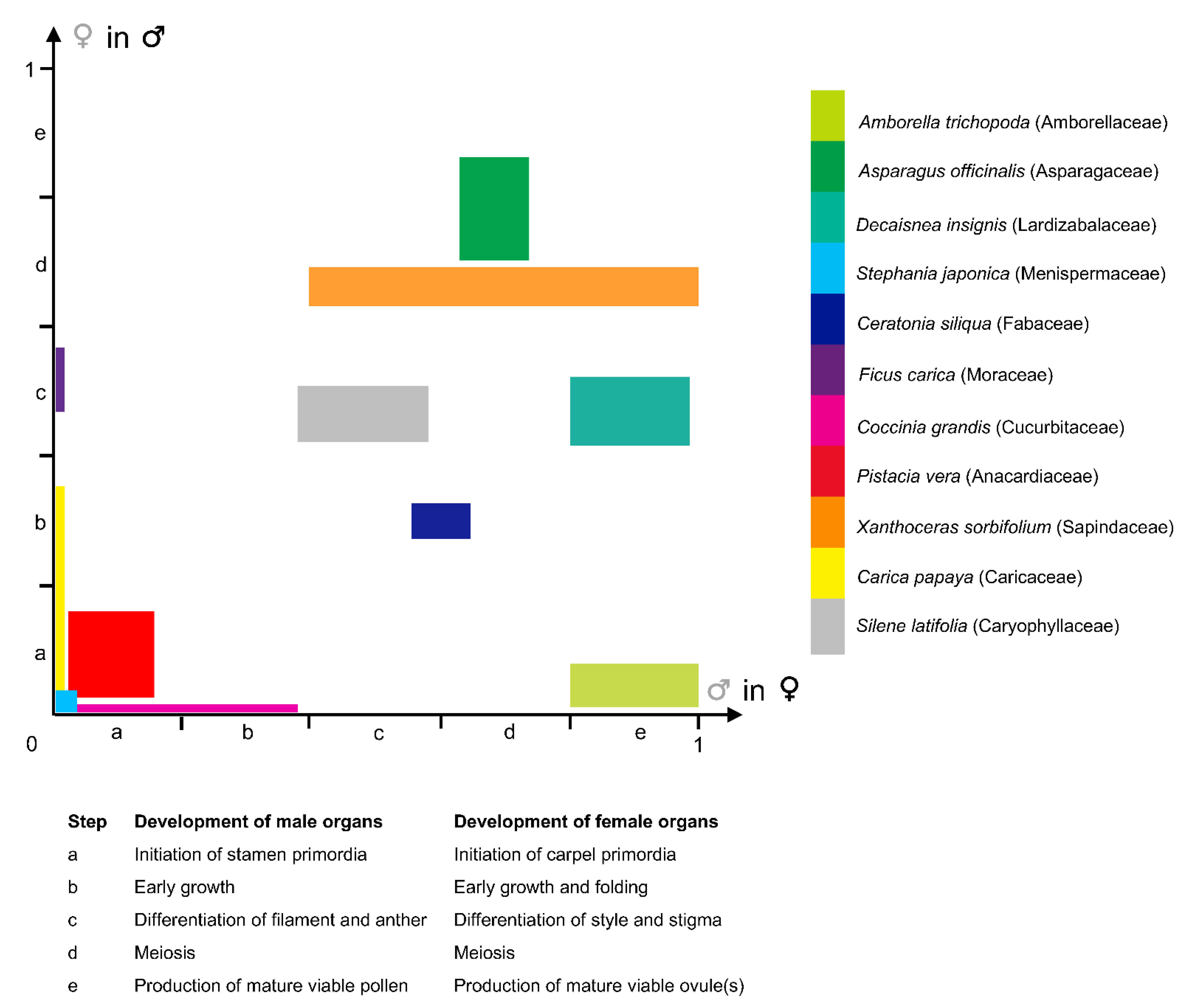
2.3. Expression of Gender
2.4. Phenotypic Plasticity of Sexual Systems and Sexual Instability
3. Molecular Bases of Floral Unisexuality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rudall, P.J.; Alves, M.; das Graças Sajo, M. Inside-out flowers of Lacandonia brasiliana (Triuridaceae) provide new insights into fundamental aspects of floral patterning. PeerJ 2016, 4, e1653. [Google Scholar] [CrossRef] [Green Version]
- Ovid. Les Métamorphoses (Bibliothèque Latine-Française)/Traduction Française de Gros, Refondue […] par M. Cabaret-Dupaty […] et Précédée d’une Notice sur Ovide par M. Charpentier; Frères, G., Ed.; Garnier Frères: Paris, France, 1866; Available online: https://gallica.bnf.fr/ark:/12148/bpt6k6138851t/f2.item (accessed on 19 December 2021).
- Barrett, S.C. The evolution of plant sexual diversity. Nat. Rev. Genet. 2002, 3, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.; Crowson, D. Mating systems in flowering plants. In Encyclopedia of Evolutionary Biology; Kliman, R., Ed.; Academic Press: Oxford, UK, 2016; pp. 473–479. [Google Scholar] [CrossRef]
- Henry, I.M.; Akagi, T.; Ryutaro, T.; Comai, L. One hundred ways to invent the sexes: Theoretical and observed paths to dioecy in plants. Annu. Rev. Plant Biol. 2018, 69, 553–575. [Google Scholar] [CrossRef] [Green Version]
- Beentje, H.J. The Kew Plant Glossary: An Illustrated Dictionary of Plant Terms; Royal Botanical Garden Kew: Kew, UK, 2010; ISBN 9781842464229. [Google Scholar]
- Willson, M.F.; Ågren, J. Differential floral rewards and pollination by deceit in unisexual flowers. Oikos 1989, 55, 23–29. [Google Scholar] [CrossRef]
- Humeau, L. Écologie et Évolution de la Dioécie et du Dimorphisme Sexuel de la Taille des Fleurs chez les «dombeya» (Sterculiacées) Endémiques de La Réunion. Ph.D. Thesis, Université de La Réunion, La Réunion, France, 1999. [Google Scholar]
- Wyatt, R.; Anderson, L.E. Breeding systems in bryophytes. In The Experimental Biology of Bryophytes; Dyer, A.F., Duckett, J.G., Eds.; Academic Press: London, UK, 1984; pp. 39–64. [Google Scholar]
- Renner, S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014, 101, 1588–1596. [Google Scholar] [CrossRef] [Green Version]
- Barrett, S.C. The evolution of mating strategies in flowering plants. Trends Plant Sci. 1998, 3, 335–341. [Google Scholar] [CrossRef]
- Cardoso, J.C.F.; Viana, M.L.; Matias, R.; Furtado, M.T.; de Souza Caetano, A.P.; Consolaro, H.; Garcia de Brito, V.L. Towards a unified terminology for angiosperm reproductive systems. Acta Bot. Bras. 2018, 32, 329–348. [Google Scholar] [CrossRef]
- Nadot, S.; Sannier, J.; Barfod, A.; Baker, W.J. Evolution of the palm androecium as revealed by character mapping on a supertree. In Flowers on the Tree of Life; Wanntorp, L., Ronse De Craene, L.P., Eds.; Royal Botanic Garden: Edinburgh, UK, 2011; pp. 156–180. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae, 4th ed.; Sumptibus Michaelis Antonii David (Typis Joannis Baptiste Coignard): Paris, France, 1744. [Google Scholar]
- Barrett, S.C.; Hough, J. Sexual dimorphism in flowering plants. J. Exp. Bot. 2013, 64, 67–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leins, P.; Erbar, C. Flower and fruit: Morphology, Ontogeny, Phylogeny, Function and Ecology; Schweizerbart Science Publishers: Stuttgart, Germany, 2010; ISBN 978-3-510-65261-7. [Google Scholar]
- Mitchell, C.H.; Diggle, P.K. The evolution of unisexual flowers: Morphological and functional convergence results from diverse developmental transitions. Am. J. Bot. 2005, 92, 1068–1076. [Google Scholar] [CrossRef]
- Meissner, S.T. Plant sexual reproduction: Perhaps the current plant two-sex model should be replaced with three-and four-sex models? Plant Reprod. 2021, 34, 175–189. [Google Scholar] [CrossRef]
- Rousseau, J.-J. Fragmens pour un Dictionnaire des Termes d’usage en Botanique. In Collection Complète des Œuvres de Jean-Jacques Rousseau (Tome septième); Du Peyrou, P.-A., Ed.; Société typographique de Genève: Genève, Switzerland, 1782; p. 510. [Google Scholar]
- Sauquet, H.; Von Balthazar, M.; Magallón, S.; Doyle, J.A.; Endress, P.K.; Bailes, E.J.; Barroso de Morais, E.; Bull-Hereñu, K.; Carrive, L.; Chartier, M.; et al. The ancestral flower of angiosperms and its early diversification. Nat. Commun. 2017, 8, 16047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilcher, D. Toward a new synthesis: Major evolutionary trends in the angiosperm fossil record. Proc. Natl. Acad. Sci. USA 2000, 97, 7030–7036. [Google Scholar] [CrossRef] [Green Version]
- Specht, C.D.; Bartlett, M.E. Flower evolution: The origin and subsequent diversification of the angiosperm flower. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 217–243. [Google Scholar] [CrossRef] [Green Version]
- Gerchen, J.F. Comparative genomics of transitions between combined and separate sexes in the plant genus Mercurialis. Ph.D. Thesis, Université de Lausanne, Lausanne, Switzerland, 2021. [Google Scholar]
- Endress, P.K. Diversity and Evolutionary Biology of Tropical Flowers; Cambridge University Press: Cambridge, UK, 1994; ISBN 9780521565103. [Google Scholar]
- Darwin, C.R. The Effects of Cross and Self Fertilisation in the Vegetable Kingdom; John Murray: London, UK, 1876. [Google Scholar]
- Darwin, C.R. The Different Forms of Flowers on Plants of the Same Species; D. Appleton and Company: New York, NY, USA, 1877. [Google Scholar]
- McDonnell, A.J.; Wetreich, H.B.; Cantley, J.T.; Jobson, P.; Martine, C.T. Solanum plastisexum, an enigmatic new bush tomato from the Australian Monsoon Tropics exhibiting breeding system fluidity. PhytoKeys 2019, 124, 39. [Google Scholar] [CrossRef]
- Sokoloff, D.D.; Remizowa, M.V.; Macfarlane, T.D.; Rudall, P.J. Classification of the early-divergent angiosperm family Hydatellaceae: One genus instead of two, four new species and sexual dimorphism in dioecious taxa. Taxon 2008, 57, 179–200. [Google Scholar] [CrossRef]
- Barabé, D.; Lacroix, C. Homeosis, morphogenetic gradient and the determination of floral identity in the inflorescences of Philodendron solimoesense (Araceae). Plant Syst. Evol. 1999, 219, 243–261. [Google Scholar] [CrossRef]
- Taylor, M.L.; Williams, J.H. Pollen tube development in two species of Trithuria (Hydatellaceae) with contrasting breeding systems. Sex. Plant Reprod. 2012, 25, 83–96. [Google Scholar] [CrossRef]
- Charlesworth, D. Plant sex determination and sex chromosomes. Heredity 2002, 88, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Garraud, C. Evolution de la gynodioécie-gynomonoécie: Approches Expérimentales chez Silene nutans & Approche Théorique. Ph.D. Thesis, Université Paris Sud, Paris, France, 2011. [Google Scholar]
- Yampolsky, C.; Yampolsky, H. Distribution of sex forms in phanerogamic flora. Biblio. Genet. 1922, 3, 1–62. [Google Scholar]
- Renner, S.S.; Ricklefs, R.E. Dioecy and its correlates in the flowering plants. Am. J. Bot. 1995, 82, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Pannell, J. Mixed genetic and environmental sex determination in an androdioecious population of Mercurialis annua. Heredity 1997, 78, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.M.; Forfang, A.S.; Báez, M. Stress-induced male sterility and mixed mating in the island plant Cedronella canariensis (Lamiaceae). Plant Syst. Evol. 1998, 212, 159–176. [Google Scholar] [CrossRef]
- Miller, J.S.; Diggle, P.K. Diversification of andromonoecy in Solanum section Lasiocarpa (Solanaceae): The roles of phenotypic plasticity and architecture. Am. J. Bot. 2003, 90, 707–715. [Google Scholar] [CrossRef]
- Wise, M.J.; Coffey, L.E.; Abrahamson, W.G. Nutrient stress and gall flies interact to affect floral-sex ratio in gynomonoecious Solidago altissima (Asteraceae). Am. J. Bot. 2008, 95, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S.; Müller, N.A. Plant sex chromosomes defy evolutionary models of expanding recombination suppression and genetic degeneration. Nat. Plants 2021, 7, 392–402. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, A.; Lyu, T.; Dimitrov, D.; Xu, X.; Freckleton, R.P.; Li, Y.; Su, X.; Li, Y.; Liu, Y.; et al. Global distribution and evolutionary transitions of angiosperm sexual systems. Ecol. Lett. 2021, 24, 1835–1847. [Google Scholar] [CrossRef]
- Neal, P.R.; Anderson, G.J. Are ‘mating systems’ ‘breeding systems’ of inconsistent and confusing terminology in plant reproductive biology? or is it the other way around? Plant Syst. Evol. 2005, 250, 173–185. [Google Scholar] [CrossRef]
- Fruchard, C.; Marais, G.A.B. The evolution of sex determination in plants. In Evolutionary Developmental Biology; Nuño de la Rosa, L., Müller, G.B., Eds.; Springer Publishing: New York, NY, USA, 2017; pp. 1–14. [Google Scholar] [CrossRef]
- Muyle, A.; Bachtrog, D.; Marais, G.A.; Turner, J.M. Epigenetics drive the evolution of sex chromosomes in animals and plants. Phil. Trans. R. Soc. B 2021, 376, 20200124. [Google Scholar] [CrossRef]
- Endress, P.K.; Igersheim, A. The reproductive structures of the basal angiosperm Amborella trichopoda (Amborellaceae). Int. J. Plant Sci. 2000, 161, S237–S248. [Google Scholar] [CrossRef]
- Zhou, Q.; Cai, Q.; Zheng, Y.; Wu, Z.; Mao, J. Floral development and the formation of functionally unisexual flowers in Xanthoceras sorbifolium (Sapindaceae), a morphologically andromonoecious tree endemic to northern China. Trees 2019, 33, 1571–1582. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, Z.; Li, J.; Ronse De Craene, L.; Wang, H. Floral development of Stephania (Menispermaceae): Impact of organ reduction on symmetry. Int. J. Plant Sci. 2012, 173, 861–874. [Google Scholar] [CrossRef]
- Castaño, F.; Stauffer, F.; Marquinez, X.; Crèvecoeur, M.; Collin, M.; Pintaud, J.C.; Tregear, J. Floral structure and development in the monoecious palm Gaussia attenuata (Arecaceae; Arecoideae). Ann. Bot. 2014, 114, 1483–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leme, F.M.; Staedler, Y.M.; Schönenberger, J.; Teixeira, S.P. Ontogeny and vascularization elucidate the atypical floral structure of Ampelocera glabra, a tropical species of Ulmaceae. Int. J. Plant Sci. 2018, 179, 461–476. [Google Scholar] [CrossRef]
- Buzgo, M.; Soltis, P.S.; Soltis, D.E. Floral developmental morphology of Amborella trichopoda (Amborellaceae). Int. J. Plant Sci. 2004, 165, 925–947. [Google Scholar] [CrossRef]
- Reinheimer, R.; Pozner, R.; Vegetti, A.C. Inflorescence, spikelet, and floral development in Panicum maximum and Urochloa plantaginea (Poaceae). Am. J. Bot. 2005, 92, 565–575. [Google Scholar] [CrossRef]
- Xu, F.; Ronse De Craene, L. Floral ontogeny of Annonaceae: Evidence for high variability in floral form. Ann. Bot. 2010, 106, 591–605. [Google Scholar] [CrossRef] [Green Version]
- Pedersoli, G.D.; Pádua Teixeira, S. Floral development of Parkia multijuga and Stryphnodendron adstringens, two andromonoecious mimosoid trees (Leguminosae). Int. J. Plant Sci. 2016, 177, 60–75. [Google Scholar] [CrossRef]
- Leme, F.M.; Schönenberger, J.; Staedler, Y.M.; Teixeira, S.P. Comparative floral development reveals novel aspects of structure and diversity of flowers in Cannabaceae. Bot. J. Linn. Soc. 2020, 193, 64–83. [Google Scholar] [CrossRef]
- Nuraliev, M.S.; Oskolski, A.A.; Sokoloff, D.D.; Remizowa, M.V. Flowers of Araliaceae: Structural diversity, developmental and evolutionary aspects. Plant Div. Evol. 2010, 128, 247–268. [Google Scholar] [CrossRef]
- Bachelier, J.B.; Endress, P.K. Development of inflorescences, cupules, and flowers in Amphipterygium and comparison with Pistacia (Anacardiaceae). Int. J. Plant Sci. 2007, 168, 1237–1253. [Google Scholar] [CrossRef]
- Thaowetsuwan, P.; Ritchie, S.; Riina, R.; Ronse De Craene, L. Divergent developmental pathways among staminate and pistillate flowers of some unusual Croton (Euphorbiaceae). Front. Ecol. Evol. 2020, 8, 253. [Google Scholar] [CrossRef]
- Payer, J.B. Traité D’organogénie Comparée de la Fleur; Masson: Paris, France, 1857. [Google Scholar]
- Condon, M.A.; Gilbert, L.E. Sex expression of Gurania and Psiguria (Cucurbitaceae): Neotropical vines that change sex. Am. J. Bot. 1988, 75, 875–884. [Google Scholar] [CrossRef]
- Kundu, B.C.; Guha, S. New species of Stephania and Rhaptonema (Menispermaceae). Bot. Not. 1976, 129, 257–265. [Google Scholar]
- Walker, J.D.; Geissman, J.W.; Bowring, S.A.; Babcock, L.E. (Compiler) Geologic Time Scale v. 5.0: Geological Society of America. 2018. Available online: https://www.geosociety.org/GSA/Education_Careers/Geologic_Time_Scale/GSA/timescale/home.aspx (accessed on 19 December 2021).
- APG IV. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Friis, E.M.; Doyle, J.A.; Endress, P.K.; Leng, Q. Archaefructus–angiosperm precursor or specialized early angiosperm? Trends Plant Sci. 2003, 8, 369–373. [Google Scholar] [CrossRef]
- Qiang, J.; Hongqi, L.; Bowe, L.M.; Yusheng, L.; Taylor, D.W. Early Cretaceous Archaefructus eoflora sp. nov. with bisexual flowers from Beipiao, Western Liaoning, China. Acta Geol. Sin. 2004, 78, 883–892. [Google Scholar] [CrossRef]
- von Balthazar, M.; Pedersen, K.R.; Friis, E.M. Teixeiria lusitanica, a new fossil flower from the Early Cretaceous of Portugal with affinities to Ranunculales. Plant Syst. Evol. 2005, 255, 55–75. [Google Scholar] [CrossRef]
- Crane, P.R.; Friis, E.M.; Pedersen, K.R. Lower Cretaceous angiosperm flowers: Fossil evidence on early radiation of dicotyledons. Science 1986, 232, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Poinar, G., Jr. A mid-Cretaceous Lauraceae flower, Cascolaurus burmitis gen. et sp. nov., in Myanmar amber. Cretac. Res. 2017, 71, 96–101. [Google Scholar] [CrossRef]
- Zhou, Z.K.; Crepet, W.L.; Nixon, K.C. The earliest fossil evidence of the Hamamelidaceae: Late Cretaceous (Turonian) inflorescences and fruits of Altingioideae. Am. J. Bot. 2001, 88, 753–766. [Google Scholar] [CrossRef] [Green Version]
- Gandolfo, M.A.; Nixon, K.C.; Crepet, W.L. Triuridaceae fossil flowers from the Upper Cretaceous of New Jersey. Am. J. Bot. 2002, 89, 1940–1957. [Google Scholar] [CrossRef]
- Boucher, L.D.; Manchester, S.R.; Judd, W.S. An extinct genus of Salicaceae based on twigs with attached flowers, fruits, and foliage from the Eocene Green River Formation of Utah and Colorado, USA. Am. J. Bot. 2003, 90, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokoloff, D.D.; Ignatov, M.S.; Remizowa, M.V.; Nuraliev, M.S.; Blagoderov, V.; Garbout, A.; Perkovsky, E.E. Staminate flower of Prunus s. l. (Rosaceae) from Eocene Rovno amber (Ukraine). J. Plant Res. 2018, 131, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Heslop-Harrison, J. Sex expression in flowering plants. Meristems and differentiation. Brookhaven Symp. Biol. 1964, 16, 109–125. [Google Scholar]
- Tucker, S.C. The developmental basis for sexual expression in Ceratonia siliqua (Leguminosae: Caesalpinioideae: Cassieae). Am. J. Bot. 1992, 79, 318–327. [Google Scholar] [CrossRef]
- Ainsworth, C. Boys and girls come out to play: The molecular biology of dioecious plants. Ann. Bot. 2000, 86, 211–221. [Google Scholar] [CrossRef]
- Tölke, E.D.; Demarco, D.; Carmello-Guerreiro, S.M.; Bachelier, J.B. Flower structure and development of Spondias tuberosa and Tapirira guianensis (Spondioideae): Implications for the evolution of the unisexual flowers and pseudomonomery in Anacardiaceae. Int. J. Plant Sci. 2021, 182, 747–762. [Google Scholar] [CrossRef]
- Grant, S.; Hunkirchen, B.; Saedler, H. Developmental differences between male and female flowers in the dioecious plant Silene latifolia. Plant J. 1994, 6, 471–480. [Google Scholar] [CrossRef]
- Lazarte, J.E.; Palser, B.F. Morphology, vascular anatomy and embryology of pistillate and staminate flowers of Asparagus officinalis. Am. J. Bot. 1979, 66, 753–764. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ren, Y. Comparative floral development in Lardizabalaceae (Ranunculales). Bot. J. Linn. Soc. 2011, 166, 171–184. [Google Scholar] [CrossRef]
- Beck, N.G.; Lord, E.M. Breeding system in Ficus carica, the common Figure I. Floral diversity. Am. J. Bot. 1988, 75, 1904–1912. [Google Scholar] [CrossRef]
- Ghadge, A.G.; Karmakar, K.; Devani, R.S.; Banerjee, J.; Mohanasundaram, B.; Sinha, R.K.; Sinha, S.; Banerjee, A.K. Flower development, pollen fertility and sex expression analyses of three sexual phenotypes of Coccinia grandis. BMC Plant Biol. 2014, 14, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hormaza, J.I.; Polito, V.S. Pistillate and staminate flower development in dioecious Pistacia vera (Anacardiaceae). Am. J. Bot. 1996, 83, 759–766. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P.; Smets, E.F. The floral development and anatomy of Carica papaya (Caricaceae). Canad. J. Bot. 1999, 77, 582–598. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. The experimental modification of sex expression in flowering plants. Biol. Rev. 1957, 32, 38–90. [Google Scholar] [CrossRef]
- Sattler, R. Organogenesis of Flowers: A Photographic Text-Atlas; University of Toronto Press: Toronto, ON, Canada, 1973; ISBN 0802018645. [Google Scholar]
- Gardner, R.O.; De Lange, P.J. Revision of Pennantia (Icacinaceae), a small isolated genus of Southern Hemisphere trees. J. Roy. Soc. N. Z. 2002, 32, 669–695. [Google Scholar] [CrossRef] [Green Version]
- Larue, C.; Austruy, E.; Basset, G.; Petit, R.J. Revisiting pollination mode in chestnut (Castanea spp.): An integrated approach. Bot. Lett. 2021, 168, 348–372. [Google Scholar] [CrossRef]
- Thien, L.B.; Sage, T.L.; Jaffré, T.; Bernhardt, P.; Pontieri, V.; Weston, P.H.; Malloch, D.; Azuma, H.; Graham, S.W.; McPherson, M.A.; et al. The population structure and floral biology of Amborella trichopoda (Amborellaceae). Ann. Missouri Bot. Gard. 2003, 90, 466–490. [Google Scholar] [CrossRef]
- Freeman, D.C.; Harper, K.T.; Ostler, W.K. Ecology of plant dioecy in the intermountain region of western North America and California. Oecologia 1979, 44, 410–417. [Google Scholar] [CrossRef]
- McArthur, E.D.; Freeman, D.C.; Luckinbill, L.S.; Sanderson, S.C.; Noller, G.L. Are trioecy and sexual lability in Atriplex canescens genetically based? evidence from clonal studies. Evolution 1992, 46, 1708–1721. [Google Scholar] [CrossRef]
- Delph, L.F.; Wolf, D.E. Evolutionary consequences of gender plasticity in genetically dimorphic breeding systems. New Phytol. 2005, 166, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Blake-Mahmud, J.; Struwe, L. Down to the wire: Late season changes in sex expression in a sexually labile tree species, Acer pensylvanicum (Sapindaceae). Trees 2018, 32, 549–557. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Chomicki, G.; Condamine, F.L.; de Vos, J.M.; Martins, A.C.; Smidt, E.C.; Klitgård, B.; Gerlach, G.; Heinrichs, J. Multiple geographical origins of environmental sex determination enhanced the diversification of Darwin’s favourite orchids. Sci. Rep. 2017, 7, 12878. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zheng, Y.; Lai, L. Observations on sexual reproduction in Xanthoceras sorbifolium (Sapindaceae). Acta Bot. Sin. 2017, 37, 14–22. [Google Scholar]
- Papadopoulou, E.; Grumet, R. Brassinosteriod-induced femaleness in cucumber and relationship to ethylene production. HortScience 2005, 40, 1763–1767. [Google Scholar] [CrossRef]
- Manzano, S.; Martínez, C.; García, J.M.; Megías, Z.; Jamilena, M. Involvement of ethylene in sex expression and female flower development in watermelon (Citrullus lanatus). Plant Physiol. Biochem. 2014, 85, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Scutt, C.P.; Li, Y.; Robertson, S.E.; Willis, M.E.; Gilmartin, P.M. Sex determination in dioecious Silene Iatifolia (Effects of the Y chromosome and the parasitic smut fungus (Ustilago violacea) on gene expression during flower development). Plant Physiol. 1997, 114, 969–979. [Google Scholar] [CrossRef] [Green Version]
- Freeman, D.C.; Wachocki, B.A.; Stender, M.J.; Goldschlag, D.E.; Michaels, H.J. Seed size and sex ratio in spinach: Application of the Trivers-Willard hypothesis to plants. Ecoscience 1994, 1, 54–63. [Google Scholar] [CrossRef]
- Stehlik, I.; Friedman, J.; Barrett, S.C. Environmental influence on primary sex ratio in a dioecious plant. Proc. Natl. Acad. Sci. USA 2008, 105, 10847–10852. [Google Scholar] [CrossRef] [Green Version]
- Pannell, J. Widespread functional androdioecy in Mercurialis annua L. (Euphorbiaceae). Biol. J. Linn. Soc. 1997, 61, 95–116. [Google Scholar] [CrossRef]
- Anger, N.; Fogliani, B.; Scutt, C.P.; Gâteblé, G. Dioecy in Amborella trichopoda: Evidence for genetically based sex determination and its consequences for inferences of the breeding system in early angiosperms. Ann. Bot. 2017, 119, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irish, E.E.; Nelson, T. Sex determination in monoecious and dioecious plants. Plant Cell 1989, 1, 737–744. [Google Scholar] [CrossRef]
- Dellaporta, S.L.; Calderon-Urrea, A. Sex determination in flowering plants. Plant Cell 1993, 5, 1241–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diggle, P.K.; Di Stilio, V.S.; Gschwend, A.R.; Golenberg, E.M.; Moore, R.C.; Russell, J.R.W.; Sinclair, J.P. Multiple developmental processes underlie sex differentiation in angiosperms. Trends Genet. 2011, 27, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S. Pathways for making unisexual flowers and unisexual plants: Moving beyond the “two mutations linked on one chromosome” model. Am. J. Bot. 2016, 103, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Sobral, R.; Silva, H.G.; Morais-Cecílio, L.; Costa, M.M. The quest for molecular regulation underlying unisexual flower development. Front. Plant Sci. 2016, 7, 160. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Sanderson, B.J.; Keefover-Ring, K.; Liu, J.; Ma, T.; Yin, T.; Smart, L.B.; DiFazio, S.P.; Olson, M.S. Pathways to sex determination in plants: How many roads lead to Rome? Curr. Opin. Plant Biol. 2020, 54, 61–68. [Google Scholar] [CrossRef]
- Montalvão, A.P.L.; Kersten, B.; Fladung, M.; Müller, N.A. The diversity and dynamics of sex determination in dioecious plants. Front. Plant Sci. 2020, 11, 580488. [Google Scholar] [CrossRef]
- Bateson, W. Mendel’s Principles of Heredity; Cambridge University Press: Cambridge, UK, 1902. [Google Scholar]
- Correns, C. Über die dominierenden Merkmale der Bastarde. Ber. Dtsch. Bot. Ges. 1903, 21, 133–147. [Google Scholar] [CrossRef]
- Correns, C. Die Bestimmung und Vererbung des Geschlechtes, nach Versuchen mit höheren Pflanzen. In Verhancll. d. Gesellschaft deutscher Naturforscher und Ärzte; Springer: Berlin/Heidelberg, Germany, 1907; pp. 794–802. [Google Scholar] [CrossRef]
- Volz, S.M.; Renner, S.S. Hybridization, polyploidy, and evolutionary transitions between monoecy and dioecy in Bryonia (Cucurbitaceae). Am. J. Bot. 2008, 95, 1297–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shull, G.H. Inheritance of sex in Lychnis. Bot. Gaz. 1910, 49, 110–125. [Google Scholar] [CrossRef]
- Shull, G.H. Hermaphrodite females in Lychnis dioica. Science 1912, 36, 482–483. [Google Scholar] [CrossRef]
- Shull, G.H. Sex-limited inheritance in Lychnis dioica L. Z. Indukt. Abstammungs-Vererbungsl. 1914, 12, 265–302. [Google Scholar] [CrossRef]
- Valleau, W.D. Inheritance of sex in the grape. Am. Nat. 1916, 50, 554–564. [Google Scholar] [CrossRef]
- Lewis, D. The evolution of sex in flowering plants. Biol. Rev. 1942, 17, 46–67. [Google Scholar] [CrossRef]
- Charlesworth, B.; Charlesworth, D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 1978, 112, 975–997. [Google Scholar] [CrossRef]
- Ming, R.; Bendahmane, A.; Renner, S.S. Sex chromosomes in land plants. Ann. Rev. Plant Biol. 2011, 62, 485–514. [Google Scholar] [CrossRef] [Green Version]
- Käfer, J.; Bewick, A.; Andres-Robin, A.; Lapetoule, G.; Harkess, A.; Caïus, J.; Fogliani, B.; Gâteblé, G.; Ralph, P.; dePamphilis, C.W.; et al. A derived ZW chromosome system in Amborella trichopoda, representing the sister lineage to all other extant flowering plants. New Phytol. 2021. [Google Scholar] [CrossRef]
- Ainsworth, C.; Crossley, S.; Buchanan-Wollaston, V.; Thangavelu, M.; Parker, J. Male and female flowers of the dioecious plant sorrel show different patterns of MADS box gene expression. Plant Cell 1995, 7, 1583–1598. [Google Scholar] [CrossRef]
- Shephard, H.L.; Parker, J.S.; Darby, P.; Ainsworth, C.C. Sexual development and sex chromosomes in hop. New Phytol. 2000, 148, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Juarez, C.; Banks, J.A. Sex determination in plants. Curr. Opin. Plant Biol. 1998, 1, 68–72. [Google Scholar] [CrossRef]
- Koizumi, A.; Amanai, Y.; Ishii, K.; Nichihara, K.; Kazama, Y.; Uchida, W.; Kawano, S. Floral development of an asexual and female-like mutant carrying two deletions in gynoecium-suppressing and stamen-promoting functional regions on the Y chromosome of the dioecious plant Silene latifolia. Plant Cell Physiol. 2007, 48, 1450–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef]
- Sather, D.N.; Jovanovic, M.; Golenberg, E.M. Functional analysis of B and C class floral organ genes in spinach demonstrates their role un sexual dimorphism. BMC Plant Biol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.-L.; Peng, Y.-B.; Cui, J.-X.; Gu, H.-T.; Xu, L.-Y.; Li, Y.-Q.; Xu, Z.-H.; Bai, S.-N. Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta 2004, 220, 230–240. [Google Scholar] [CrossRef]
- Hartley, R.D.; Neve, R.A. The effect of gibberellic acid on development and yield of Fuggle hops. J. Hort. Sci. 1966, 41, 53–56. [Google Scholar] [CrossRef]
- Weston, E.W. Changes in sex in the hop caused by plant growth substances. Nature 1960, 138, 81–82. [Google Scholar] [CrossRef]
- Song, Y.; Ma, K.; Ci, D.; Chen, Q.; Tian, J.; Zhang, D. Sexual dimorphic floral development in dioecious plants revealed by transcriptome, phytohormone, and DNA methylation analysis in Populus tomentosa. Plant Mol. Biol. 2013, 83, 559–576. [Google Scholar] [CrossRef]
- Maier, C.G.-A.; Chapman, K.D.; Smith, D.W. Phytoestrogens and floral development in dioecious Maclura pomifera (Raf.) Schneid. and Morus rubra L. (Moraceae). Plant Sci. 1997, 130, 27–40. [Google Scholar] [CrossRef]
- Switzenberg, J.A.; Little, H.A. Floral primordia-target ACS (1-aminocyclopropane-1-carboxylate synthase) expression in transgenic Cucumis melo implicates fine tuning of ethylene production mediating unisexual flower development. Planta 2014, 240, 797–808. [Google Scholar] [CrossRef]
- Boualem, A.; Troadec, C.; Camps, C.; Lemhemdi, A.; Morin, H.; Sari, M.-A.; Fraenkel-Zagouri, R.; Kovalski, I.; Dogimont, C.; Perl-Treves, R.; et al. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 2015, 350, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Jamilena, M. To be a male or a female flower, a question of ethylene in cucurbits. Curr. Opin. Plant Biol. 2021, 59, 101981. [Google Scholar] [CrossRef] [PubMed]
- Chartier, M.; von Balthazar, M.; Sontag, S.; Löfstrand, S.; Palme, T.; Jabbour, F.; Sauquet, H.; Schönenberger, J. Global patterns and a latitudinal gradient of flower disparity: Perspectives from the angiosperm order Ericales. New Phytol. 2021, 230, 821–831. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbour, F.; Espinosa, F.; Dejonghe, Q.; Le Péchon, T. Development and Evolution of Unisexual Flowers: A Review. Plants 2022, 11, 155. https://doi.org/10.3390/plants11020155
Jabbour F, Espinosa F, Dejonghe Q, Le Péchon T. Development and Evolution of Unisexual Flowers: A Review. Plants. 2022; 11(2):155. https://doi.org/10.3390/plants11020155
Chicago/Turabian StyleJabbour, Florian, Felipe Espinosa, Quentin Dejonghe, and Timothée Le Péchon. 2022. "Development and Evolution of Unisexual Flowers: A Review" Plants 11, no. 2: 155. https://doi.org/10.3390/plants11020155
APA StyleJabbour, F., Espinosa, F., Dejonghe, Q., & Le Péchon, T. (2022). Development and Evolution of Unisexual Flowers: A Review. Plants, 11(2), 155. https://doi.org/10.3390/plants11020155






