Apple Fruit Growth and Quality Depend on the Position in Tree Canopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Light Conditions
2.2. Photosynthetic Parameters
2.3. Fruit Share by Canopy Positions
2.4. Fruit Weight and Diameter
2.5. Fruit Blush and Colour
2.6. Fruit Maturation
2.7. Fruit Biochemical Content
3. Materials and Methods
3.1. Experimental Design
3.2. Light Measurements
3.3. Fruit Colour
3.4. Fruit Chemical Content
3.5. Fruit Maturity Indexes
3.6. Photosynthetic Parameters
3.7. Preparation of Apple Extracts for the Determination of Phenolic Compounds
3.8. Preparation of Apple Extracts for the Determination of Triterpenic Compounds
3.9. Preparation of Apple Extracts for the Determination of Anthocyanins
3.10. Preparation of Apple Extracts for the Determination of Sugars and Malic Acid
3.11. High Performance Liquid Chromatography (HPLC) for the Determination of Phenolic and Triterpenic Compounds
3.12. Ultra-High Performance Liquid Chromatography (UHPLC) for the Determination of Anthocyanin Compounds
3.13. High Performance Liquid Chromatography for the Determination of Sugars
3.14. High Performance Liquid Chromatography Method for the Determination of Malic Acid
3.15. Determination of Antioxidant Activity Using HPLC-ABTS Post-Column Assays
3.16. The Statistical Methods of Data Processing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kviklys, D.; Liaudanskas, M.; Janulis, V.; Viškelis, P.; Rubinskienė, M.; Lanauskas, J.; Uselis, N. Rootstock genotype determines phenol content in apple fruits. Plant Soil Environ. 2014, 60, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Uselis, N.; Viškelis, J.; Lanauskas, J.; Liaudanskas, M.; Janulis, V.; Kviklys, D. Planting distance affects apple tree growth, fruit yield and quality. Zemdirb.-Agric. 2020, 107, 367–372. [Google Scholar] [CrossRef]
- Uselis, N.; Viskelis, J.; Lanauskas, J.; Liaudanskas, M.; Janulis, V.; Kviklys, D. Effects of growth control on yield and fruit quality of the apple cultivar ‘Rubin’. Agric. Food Sci. 2020, 29, 257–264. [Google Scholar]
- Viškelis, J.; Uselis, N.; Liaudanskas, M.; Janulis, V.; Bielicki, P.; Univer, T.; Lepsis, J.; Kviklys, D. Triterpenic acid content in the fruit peel of Malus × domestica Borkh. depends on the growing technology. Zemdirb.-Agric. 2018, 105, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Lanauskas, J.; Kviklys, D.; Liaudanskas, M.; Janulis, V.; Uselis, N.; Viškelis, J.; Viškelis, P. Lower nitrogen nutrition determined higher phenolic content of organic apples. Hortic. Sci. 2017, 44, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Čeidaitė, A.; Sirtautas, R.; Duchovskis, P.; Kviklys, D. Effect of crop load on phytohormones, sugars, and biennial bearing in apple trees. Biol. Plant. 2016, 60, 394–400. [Google Scholar] [CrossRef]
- Viškelis, J.; Uselis, N.; Liaudanskas, M.; Lanauskas, J.; Bielicki, P.; Univer, T.; Lepsis, J.; Kviklys, D. Location effects across northeastern Europe on bioactive compounds in apple fruit. Agric. Food Sci. 2019, 28, 93–100. [Google Scholar] [CrossRef]
- Bastías, R.M.; Corelli-Grappadelli, L. Light quality management in fruit orchards: Physiological and technological aspects. Chil. J. Agric. Res. 2012, 72, 574–581. [Google Scholar] [CrossRef]
- Robinson, T.L. Can we manage light interception levels above 70% in apple orchards? Acta Hortic. 2017, 1177, 79–86. [Google Scholar] [CrossRef]
- Anthony, B.; Serra, S.; Musacchi, S. Optimization of Light Interception, Leaf Area and Yield in “WA38”: Comparisons among Training Systems, Rootstocks and Pruning Techniques. Agronomy 2020, 10, 689. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Lakso, A.N.; Robinson, T.L.; Lenz, F.; Denning, S.S. The bases of productivity in apple production systems: The role of light interception by different shoot types. J. Am. Soc. Hortic. Sci. 1996, 121, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Kalcsits, L.; Mattheis, J.; Giordani, L.; Reid, M.; Mullin, K. Fruit canopy positioning affects fruit calcium and potassium concentrations, disorder incidence, and fruit quality for ‘Honeycrisp’ apple. Can. J. Plant Sci. 2019, 99, 761–771. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Losciale, P.; Zibordi, M.; Corelli-Grappadelli, L. The positive effects of skin transpiration peach fruit growth. J. Plant Physiol. 2010, 167, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, G.; Dichio, B.; Xiloyannis, C.; Celano, G. Light influences transpiration and calcium accumulation in fruit of kiwi plants (Actinidia deliciosa var. deliciosa). Plant Sci. 2006, 170, 520–527. [Google Scholar] [CrossRef]
- Johnson, S.R.; Lakso, A.N. Carbon balance model of a growing apple shoot. I: Development of the model. J. Am. Soc. Hortic. Sci. 1986, 111, 160–164. [Google Scholar]
- Robinson, T.L.; Seeley, E.J.; Barritt, B.H. Effect of light environment and spur age on ‘Delicious’ apple fruit size and quality. J. Am. Soc. Hortic. Sci. 1983, 108, 855–861. [Google Scholar]
- Hamadziripi, E.; Theron, K.I.; Muller, M.; Steyn, W.J. The effect of canopy position on apple fruit eating and appearance preferences by consumers. Acta Hortic. 2013, 1007, 487–493. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Corelli-Grappadelli, L.; Lakso, A.N. Is maximizing orchard light interception always the best choice? Acta Hortic. 2007, 732, 507–518. [Google Scholar] [CrossRef]
- Racskó, J.; Nagy, J.; Szabó, Z.; Major, M.; Nyéki, J. The impact of location, row direction, plant density and rootstock on the sunburn damage of apple cultivars. Int. J. Hortic. Sci. 2005, 11, 19–30. [Google Scholar] [CrossRef]
- Chiang, C.; Olsen, J.E.; Basler, D.; Bånkestad, D.; Hoch, G. Latitude and Weather Influences on Sun Light Quality and the Relationship to Tree Growth. Forests 2019, 10, 610. [Google Scholar] [CrossRef] [Green Version]
- Tustin, D.S.; Cashmore, W.M.; Bensley, R.B. The influence of orchard row canopy discontinuity on irradiance and leaf area distribution in apple trees. J. Hortic. Sci. Biotechnol. 1998, 73, 289–297. [Google Scholar] [CrossRef]
- Fouché, J.R.; Roberts, S.C.; Midgley, S.J.E.; Steyn, W.J. Peel colour and blemishes in ‘Granny Smith’ apples in relation to canopy light environment. HortScience 2010, 45, 899–905. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Calcerrada, J.; Limousin, J.-M.; Martin-StPaul, N.K.; Jaeger, C.; Rambal, S. Gas exchange and leaf aging in an evergreen oak: Causes and consequences for leaf carbon balance and canopy respiration. Tree Physiol. 2012, 32, 464–477. [Google Scholar] [CrossRef]
- Bielczynski, L.W.; Łącki, M.K.; Hoefnagels, I.; Gambin, A.; Croce, R. Leaf and Plant Age Affects Photosynthetic Performance and Photoprotective Capacity. Plant Physiol. 2017, 175, 1634–1648. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, B.N.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal favonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Overbeck, V.; Schmitz, M.; Tartachnyk, I.; Blanke, M. Identifcation of light availability in diferent sweet cherry orchards under cover by using non-destructive measurements with a DualexTM. Eur. J. Agron. 2018, 93, 50–56. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Lakso, A.N. Apple Tree Physiology-Implications for Orchard and Tree Management. In Proceedings of the 43rd Annual IDFTA Conference, Napier, New Zealand, 6–9 February 2000; pp. 82–88. [Google Scholar]
- Laužikė, K.; Uselis, N.; Kviklys, D.; Samuolienė, G. Orchard Planting Density and Tree Development Stage Affects Physiological Processes of Apple (Malus domestica Borkh.) Tree. Agronomy 2020, 10, 1912. [Google Scholar] [CrossRef]
- Cronin, G.; Lodge, D. Effects of Light and Nutrient Availability on the Growth, Allocation, Carbon/nitrogen Balance, Phenolic Chemistry, and Resistance to Herbivory of Two Freshwater Macrophytes. Oecologia 2003, 137, 32–41. [Google Scholar] [CrossRef]
- Dallabetta, N.; Costa, F.; Giordan, M.; Guerra, A.; Pasqualini, J.; Wehrens, R.; Costa, G. A 3D digitizing tool to determine fruit quality distribution within the canopy. Acta Hortic. 2017, 1160, 19–26. [Google Scholar] [CrossRef]
- Corelli-Grappadelli, L.; Lakso, A.N. Fruit development in deciduous tree crops as affected by physiological factors and environmental conditions. Acta Hortic. 2004, 636, 425–441. [Google Scholar] [CrossRef]
- Hamadziripi, E.T.; Theron, K.I.; Muller, M.; Steyn, W.J. Apple Compositional and Peel Color Differences Resulting from Canopy Microclimate Affect Consumer Preference for Eating Quality and Appearance. HortScience 2014, 49, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Dallabetta, N.; Costa, F.; Guerra, A.; Pasqualini, J.; Giordan, M.; Zorer, R.; Wehrens, R.; George, E.; Magnanini, E.; Costa, G. Effects of light availability and training systems on apple fruit quality. Acta Hortic. 2017, 1160, 237–244. [Google Scholar] [CrossRef]
- Cocetta, G.; Beghi, R.; Mignani, I.; Spinardi, A. Nondestructive Apple Ripening Stage Determination Using the Delta Absorbance Meter at Harvest and after Storage. HortTechnology 2017, 27, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Palmer, J.; Harker, R.; Tustin, S.; Johnston, J. Fruit dry matter concentration: A new quality metric for apples. J. Sci. Food Agric. 2010, 90, 2586–2594. [Google Scholar] [CrossRef]
- Nilsson, T.; Gustavsson, K.E. Postharvest physiology of ‘Aroma’ apples in relation to position on the tree. Posthar. Biol. Techn. 2007, 43, 36–46. [Google Scholar] [CrossRef]
- Lewallen, K.S.; Marini, R.P. Relationship between flesh firmness and ground colour in peach as influenced by light and canopy position. J. Amer. Soc. Hortic. Sci. 2003, 128, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Flore, J.A.; Layne, D.R. Photoassimilate production and distribution in cherry. HortScience 1999, 34, 1015–1019. [Google Scholar] [CrossRef] [Green Version]
- Solomakhin, A.; Blanke, M.M. Can coloured hailnets improve taste (sugar, sugar: Acid ratio), consumer appeal (colouration) and nutritional value (anthocyanin, vitamin C) of apple fruit. Food Sci. Technol. 2010, 43, 1277–1284. [Google Scholar] [CrossRef]
- Endrizzi, I.; Torri, L.; Corollaro, L.; Demattè, L.; Aprea, E.; Charles, M.; Biasioli, F.; Gasperi, F. A conjoint study on apple acceptability: Sensory characteristics and nutritional information. Food Qual. Pref. 2015, 40, 39–48. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, P.M.; Cheng, L.L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Zhou, R.; Cheng, L.; Dandekar, A.M. Down-regulation of sorbitol dehydrogenase and upregulation of sucrose synthase in shoot tips of the transgenic apple trees with decreased sorbitol synthesis. J. Exp. Bot. 2006, 57, 3647–3657. [Google Scholar] [CrossRef]
- Beruter, J. Carbohydrate metabolism in two apple genotypes that differ in malate accumulation. J. Plant Physiol. 2004, 161, 1011–1029. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. Effects of location within the tree canopy on carbohydrates, organic acids, amino acids and phenolic compounds in the fruit peel and flesh from three apple (Malus × domestica) cultivars. Hortic. Res. 2014, 1, 14019. [Google Scholar] [CrossRef] [Green Version]
- Weber, S.; Damerow, L.; Kunz, A.; Blanke, M. Anthocyanin synthesis and light utilisation can be enhanced by reflective mulch—Visualisation of light penetration into a tree canopy. J. Plant Physiol. 2019, 233, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Steyn, W.J.; Holcroft, D.M.; Wand, S.J.E. Red colour development and loss in pears. Acta Hortic. 2005, 671, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.K.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Chagne, D.; Rowan, D.D.; Troggio, M.; Iglesias, I.; Allen, A.C. High temperature reduces apple fruit colour via regulation of the anthocyanin complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef]
- Ryu, S.; Han, H.H.; Jeong, J.H.; Kwon, Y.; Han, J.H.; Do, G.R.; Choi, I.M.; Lee, H.J. Night temperatures affect fruit coloration and expressions of anthocyanin biosynthetic genes in ‘Hongro’ apple fruit skins. Eur. J. Hortic. Sci. 2017, 82, 232–238. [Google Scholar] [CrossRef]
- Honda, C.; Moriya, S. Anthocyanin Biosynthesis in Apple Fruit. Hortic. J. 2018, 87, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wang, C.; Zhang, W.; Liu, S.; Wang, W.; Yu, X.; Song, T.; Yu, M.; Yu, W.; Qu, S. The R2R3-type MYB transcription factor MdMYB90-like is responsible for the enhanced skin color of an apple bud sport mutant. Hortic Res. 2021, 8, 156. [Google Scholar] [CrossRef]
- Awad, M.A.; Wagenmakers, P.S.; de Jager, A. Effects of light on flavonoid and chlorogenic acid levels in the skin of ‘Jonagold‘ apples. Sci. Hortic. 2001, 88, 289–298. [Google Scholar] [CrossRef]
- Jakopic, J.; Stampar, F.; Veberic, R. The influence of exposure to light on the phenolic content of ‘Fuji’apple. Sci. Hortic. 2009, 123, 234–239. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Pantelidis, G. Effects of position on canopy and harvest time on fruit physico-chemical and antioxidant properties in different apple cultivars. Sci. Hortic. 2011, 129, 752–760. [Google Scholar] [CrossRef]
- Unuk, T.; Tijskens, L.M.; Germšek, B.; Zadravec, P.; Vogrin, A.; Hribar, J.; Simčič, M.; Tojnko, S. Effect of location in the canopy on the colour development of three apple cultivars during growth. J. Sci. Food Agric. 2012, 92, 2450–2458. [Google Scholar] [CrossRef]
- Curry, E. Effects of 1-MCP applied postharvest on epicuticular wax of apples (Malus domestica Borkh.) during storage. J. Sci. Food Agric. 2008, 88, 996–1006. [Google Scholar] [CrossRef]
- Tahir, I.I.; Johansson, E.; Olsson, M.E. Improvement of apple quality and storability by a combination of heat treatment and controlled atmosphere storage. HortScience 2009, 44, 1648–1654. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T. Advances in apple culture worldwide. Rev. Bras. Frutic. 2011, 33, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Tustin, D.S.; Breen, K.C.; van Hooijdonk, B.M. Light utilisation, leaf canopy properties and fruiting responses of narrow-row, planar cordon apple orchard planting systems—A study of the productivity of apple. Sci. Hort. 2022, 294, 110778. [Google Scholar] [CrossRef]
- Hess, P.; Kunz, A.; Blanke, M.M. Innovative strategies for the use of reflective foils for fruit colouration to reduce plastic use in orchards. Sustainability 2021, 13, 73. [Google Scholar] [CrossRef]
- Funke, K.; Blanke, M. Spatial and Temporal Enhancement of Colour Development in Apples Subjected to Reflective Material in the Southern Hemisphere. Horticulturae 2021, 7, 2. [Google Scholar] [CrossRef]
- Valiuškaitė, A.; Uselis, N.; Kviklys, D.; Lanauskas, J.; Rasiukevičiūtė, N. Effect of sustainable plant protection and apple tree management on fruit quality and yield. Zemdirb.-Agric. 2017, 104, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Liaudanskas, M.; Viškelis, P.; Jakštas, V.; Raudonis, R.; Kviklys, D.; Milašius, A.; Janulis, V. Application of an Optimized HPLC Method for the Detection of Various Phenolic Compounds in Apples from Lithuanian Cultivars. J. Chem. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Liaudanskas, M.; Kviklys, D.; Zymonė, K.; Raudonis, R.; Viškelis, J.; Uselis, N.; Janulis, V. Detection and analysis of triterpenic compounds in apple extract. Int. J. Food Prop. 2018, 21, 1716–1727. [Google Scholar] [CrossRef] [Green Version]
- Vilkickyte, G.; Motiekaityte, V.; Vainoriene, R.; Liaudanskas, M.; Raudone, L. Development, validation, and application of UPLC-PDA method for anthocyanins profiling in Vaccinium, L. berries. J. Berry Res. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Raudonis, R.; Raudonė, L.; Jakštas, V.; Janulis, V. Comparative evaluation of post-column free radical scavenging and ferric reducing antioxidant power assays for screening of antioxidants in strawberries. J. Chromatogr. A 2012, 1233, 8–15. [Google Scholar] [CrossRef]
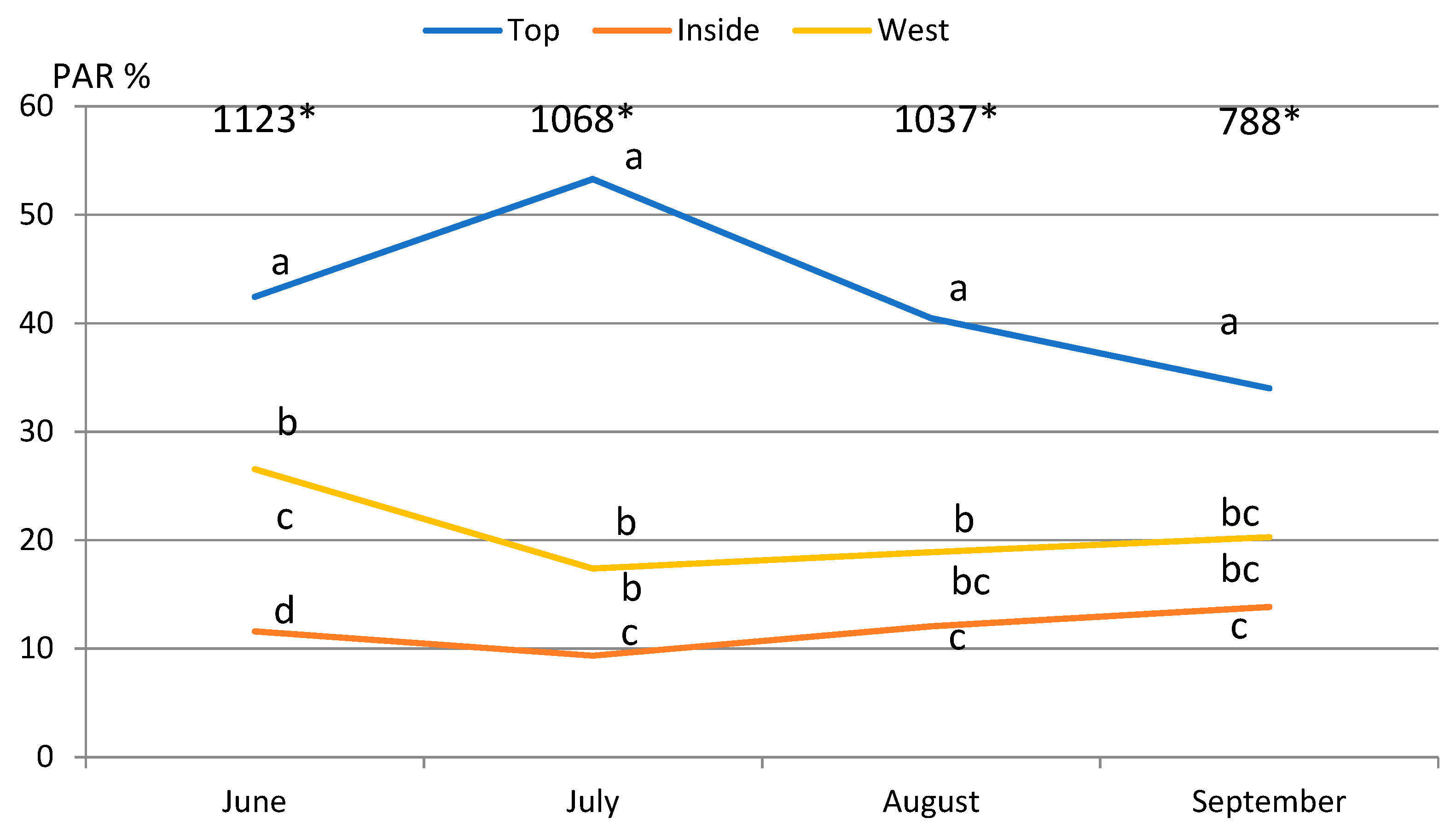

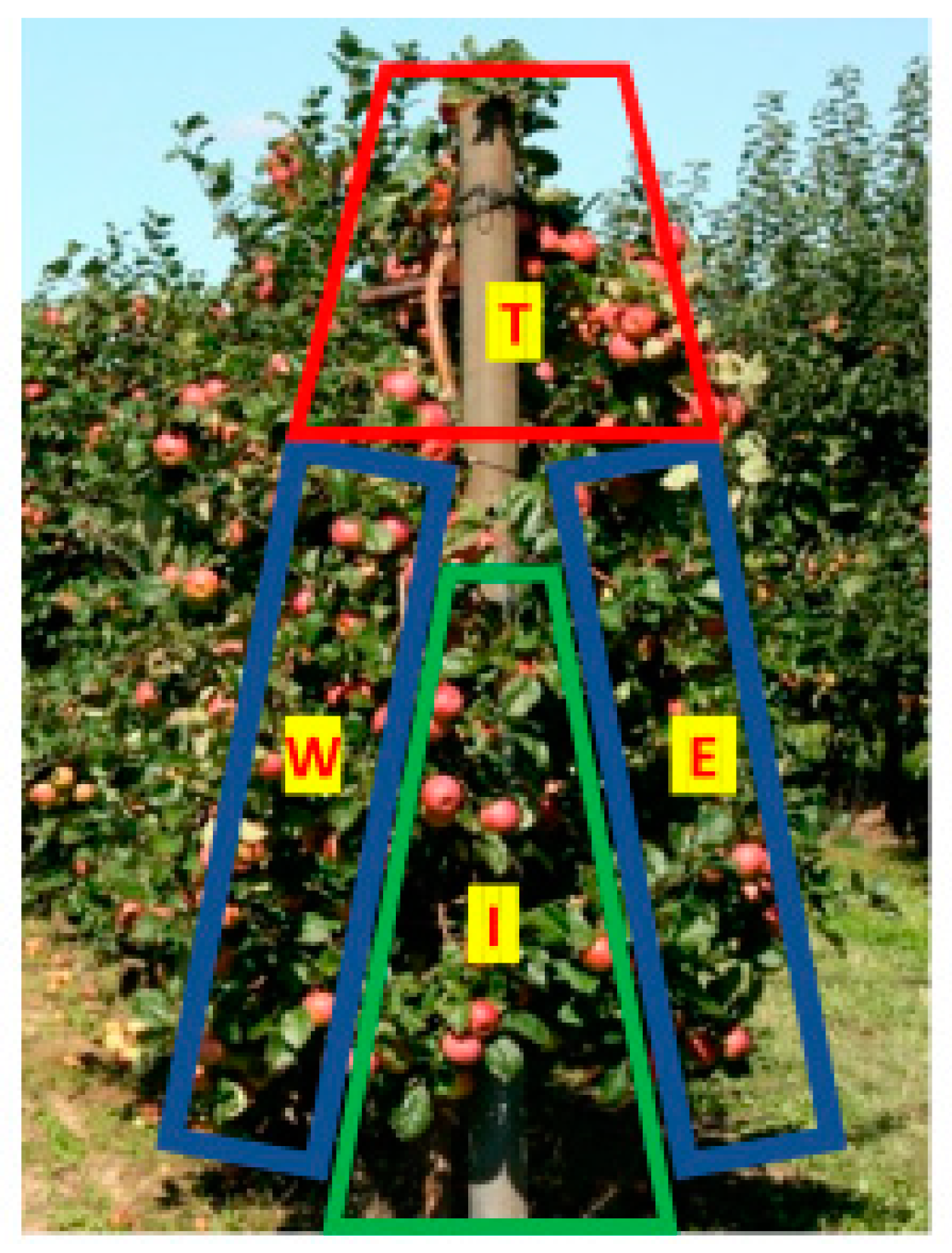
| Parameter | Canopy Position | |||
|---|---|---|---|---|
| Top | Inside | East | West | |
| Photosynthetic rate, µmol CO2 m−2 s−1 | 18.35 ± 1.09 a * | 10.79 ± 0.75 d | 16.34 ± 1.00 b | 12.92 ± 0.65 c |
| Stomatal conductance, mol H2O m−2 s−1 | 0.50 ± 0.05 a | 0.30 ± 0.04 c | 0.47 ± 0.05 ab | 0.43 ± 0.05 ab |
| Intercellular CO2, µmol CO2 mol−1 | 300.2 ± 8.47 a | 312.4 ± 9.74 a | 306.9 ± 7.25 a | 320.6 ± 3.45 a |
| Transpiration rate, mmol H2O m−2 s−1 | 5.23 ± 0.21 a | 3.83 ± 0.38 c | 5.02 ± 0.28 ab | 4.81 ± 0.30 b |
| Chlorophyll index | 40.5 ± 1.04 a | 31.3 ± 3.10 c | 34.2 ± 0.87 b | 37.6 ± 2.21 ab |
| Flavonol index | 1.89 ± 012 a | 1.38 ± 0.16 c | 1.77 ± 0.04 ab | 1.72 ± 0.14 b |
| Nitrogen balance index | 21.3 ± 1.36 b | 23.1 ± 2.71 a | 19.3 ± 0.62 c | 22.1 ± 2.27 ab |
| Parameter | Canopy Position | |||
|---|---|---|---|---|
| Top | Inside | East | West | |
| Fruit yield, kg | 8.1 ± 0.9 a * | 6.3 ± 1.2 b | 7.3 ± 1.0 ab | 7.2 ± 0.8 ab |
| Fruit weight, g | 190 ± 18.5 a | 144 ± 12.4 c | 158 ± 17.3 b | 157 ± 18.2 b |
| Share of fruits with diameter >65 mm, % | 96 ± 3.5 a | 63 ± 8.2 c | 81 ± 5.6 b | 82 ± 5.9 b |
| Fruit blush, % | 67 ± 5.2 a | 22 ± 8.6 c | 35 ± 7.2 cb | 49 ± 8.3 b |
| Fruit firmness, kg cm−2 | 8.5 ± 1.01 a | 8.2 ± 1.14 a | 8.3 ± 1.21 a | 8.6 ± 0.94 a |
| IAD | 0.95 ± 0.08 a | 0.83 ± 0.06 b | 0.94 ± 0.08 a | 0.99 ± 0.06 a |
| Starch index | 7.9 ± 1.3 a | 8.1 ± 1.1 a | 8.2 ± 1.5 a | 8.3 ± 1.2 a |
| Streif index | 0.056 ± 0.011 a | 0.055 ± 0.019 a | 0.059 ± 0.023 a | 0.057 ± 0.021 a |
| Respiration rate, mg CO2· Kg·h−1 | 2.2 ± 0.34 a | 2.5 ± 0.22 a | 2.3 ± 0.20 a | 2.6 ± 0.32 a |
| Colour Coordinate | Canopy Position | |||
|---|---|---|---|---|
| Top | Inside | East | West | |
| L* | 33.6 ± 1.35 c * | 55.7 ± 2.11 a | 39.7 ± 1.56 b | 38.8 ± 2.01 b |
| a* | 31.8 ± 1.78 a | 18.6 ± 2.31 b | 32.1 ± 2.25 a | 32.1 ± 2.32 a |
| b* | 13.6 ± 1.25 c | 29.3 ± 2.06 a | 17.4 ± 1.51 b | 16.5 ± 1.09 b |
| C* | 35.4 ± 1.32 b | 38.1 ± 2.01 a | 36. 9 ± 1.07 a | 36.1 ± 1.53 ab |
| h° | 22.8 ± 1.51 c | 63.6 ± 2.35 a | 29.6 ± 2.22 b | 27.5 ± 2.58 b |
| Canopy Position | ||||
|---|---|---|---|---|
| Top | Inside | East | West | |
| Dry matter, % | 15.2 ± 0.24 a * | 14.4 ± 0.42 b | 14.9 ± 0.36 ab | 15.2 ± 0.27 a |
| Total sugar content, % | 12.4 ± 0.09 a | 11.7 ±0.29 b | 12 ± 0.17 ab | 12.1 ± 0.14 ab |
| Soluble solid content, % | 13.5 ± 0.15 a | 12.9 ± 0.32 a | 13.3 ± 0.36 a | 13.5 ± 0.20 a |
| Compound | Canopy Position | |||
|---|---|---|---|---|
| Top | Inside | East | West | |
| Fructose | 447.2 ± 22.36 a * | 505.6 ± 25.28 a | 460.6 ± 23.03 a | 493.9 ± 24.69 a |
| Glucose | 82.9 ± 4.15 b | 101.6 ± 5.08 a | 87.5 ± 4.38 b | 90.3 ± 4.52 ab |
| Sucrose | 260.6 ± 13.03 a | 229.2 ± 11.46 b | 256.4 ± 12.82 a | 251.5 ± 12.57 a |
| Sorbitol | 23.2 ± 1.16 a | 15.4 ± 0.77 c | 18.9 ± 0.95 b | 23.4 ± 1.17 a |
| Malic acid | 40.2 ± 2.01 b | 45.8 ± 2.29 a | 42.7 ± 2.14 ab | 40.1 ± 2.01 b |
| Compound | Canopy Position | |||
|---|---|---|---|---|
| Top | Inside | East | West | |
| Total anthocyanin content in apple peel, µg g−1 DW | 177.35 ± 28.56 a * | 19.33 ± 7.42 c | 40.86 ± 14.91 bc | 63.24 ± 12.67 b |
| Total phenolic content in whole apple, mg g−1 DW | 2.41 ± 0.23 a | 1.95 ± 0.12 b | 2.18 ± 0.19 ab | 2.09 ± 0.13 b |
| Total triterpene content content in apple peel, mg g−1 DW | 12.65 ± 1.15 b | 14.81 ± 1.22 a | 13.52 ± 1.95 ab | 13.01 ± 1.48 b |
| Radical scavenging activity (TEABTS, μmol/g) | 5.86 ± 1.10 a | 4.20 ± 0.94 b | 4.56 ± 0.88 ab | 5.34 ± 1.07 a |
| Compound | Canopy Position | |||
|---|---|---|---|---|
| Top | Inside | East | West | |
| Cyanidin-3-galactoside | 150.84 ± 21.05 a * | 15.34 ± 1.88 c | 30.05 ± 8.68 bc | 55.81 ± 3.66 b |
| Cyanidin-3-glucoside | 0.17 ± 0.15 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.51 ± 0.57 a |
| Cyanidin-3-arabinoside | 2.91 ± 1.76 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.26 ± 0.45 b |
| Cyanidin | 3.16 ± 0.24 a | 2.18 ± 0.14 b | 2.19 ± 0.14 b | 2.50 ± 0.37 b |
| Compound | Canopy Position | |||
|---|---|---|---|---|
| Top | Inside | East | West | |
| Hyperoside | 0.18 ± 0.009 a * | 0.04 ± 0.002 d | 0.11 ± 0.006 b | 0.10 ± 0.005 c |
| Isoquercitrin | 0.04 ± 0.002 a | 0.02 ± 0.001 c | 0.03 ± 0.001 b | 0.03 ± 0.002 b |
| Rutin | 0.03 ± 0.001 a | 0.01 ± 0.001 c | 0.02 ± 0.001 b | 0.02 ± 0.001 b |
| Reynoutrin | 0.04 ± 0.002 b | 0.01 ± 0.000 d | 0.03 ± 0.001 c | 0.05 ± 0.003 a |
| Avicularin | 0.10 ± 0.005 a | 0.04 ± 0.002 c | 0.08 ± 0.004 b | 0.09 ± 0.005 a |
| Quercitrin | 0.11 ± 0.005 a | 0.06 ± 0.003 c | 0.09 ± 0.004 b | 0.06 ± 0.003 c |
| Procyanidin B1 | 0.02 ± 0.001 b | 0.03 ± 0.002 a | 0.03 ± 0.001 b | 0.02 ± 0.001 c |
| Procyanidin B2 | 0.48 ± 0.024 a | 0.39 ± 0.019b c | 0.41 ± 0.021 b | 0.36 ± 0.018 c |
| Procyanidin C1 | 0.17 ± 0.008 a | 0.15 ± 0.007 b | 0.17 ± 0.008 a | 0.16 ± 0.008 ab |
| (+)-Catechin | 0.04 ± 0.002 a | 0.04 ± 0.002 a | 0.04 ± 0.002 a | 0.03 ± 0.002 a |
| (−)-Epicatechin | 0.29 ± 0.014 a | 0.28 ± 0.014 a | 0.30 ± 0.015 a | 0.29 ± 0.015 a |
| Phlorizin | 0.13 ± 0.006 a | 0.11 ± 0.005 b | 0.12 ± 0.006 ab | 0.10 ± 0.005 b |
| Chlorogenic acid | 0.79 ± 0.039 a | 0.78 ± 0.039 a | 0.77 ± 0.038 a | 0.76 ± 0.038 a |
| Compound | Fruit Position in Apple Tree Canopy | |||
|---|---|---|---|---|
| Top | Inside | East | West | |
| Corosolic acid | 1.25 ± 0.063 b * | 1.44 ± 0.072 a | 1.34 ± 0.067 ab | 1.41 ± 0.071 ab |
| Betulinic acid | 0.10 ± 0.005 c | 0.14 ± 0.007 a | 0.12 ± 0.006 b | 0.09 ± 0.004 c |
| Oleonolic acid | 1.89 ± 0.095 ab | 2.13 ± 0.107 a | 1.95 ± 0.098 ab | 1.77 ± 0.088 b |
| Ursolic acid | 8.65 ± 0.433 b | 10.05 ± 0.502 a | 8.90 ± 0.445 ab | 8.65 ± 0.432 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kviklys, D.; Viškelis, J.; Liaudanskas, M.; Janulis, V.; Laužikė, K.; Samuolienė, G.; Uselis, N.; Lanauskas, J. Apple Fruit Growth and Quality Depend on the Position in Tree Canopy. Plants 2022, 11, 196. https://doi.org/10.3390/plants11020196
Kviklys D, Viškelis J, Liaudanskas M, Janulis V, Laužikė K, Samuolienė G, Uselis N, Lanauskas J. Apple Fruit Growth and Quality Depend on the Position in Tree Canopy. Plants. 2022; 11(2):196. https://doi.org/10.3390/plants11020196
Chicago/Turabian StyleKviklys, Darius, Jonas Viškelis, Mindaugas Liaudanskas, Valdimaras Janulis, Kristina Laužikė, Giedrė Samuolienė, Nobertas Uselis, and Juozas Lanauskas. 2022. "Apple Fruit Growth and Quality Depend on the Position in Tree Canopy" Plants 11, no. 2: 196. https://doi.org/10.3390/plants11020196






