Cytogenetics and Consequences of Polyploidization on Different Biotic-Abiotic Stress Tolerance and the Potential Mechanisms Involved
Abstract
1. Introduction
2. Role of Polyploidy in Modern Plant Breeding
3. Induction of Polyploids
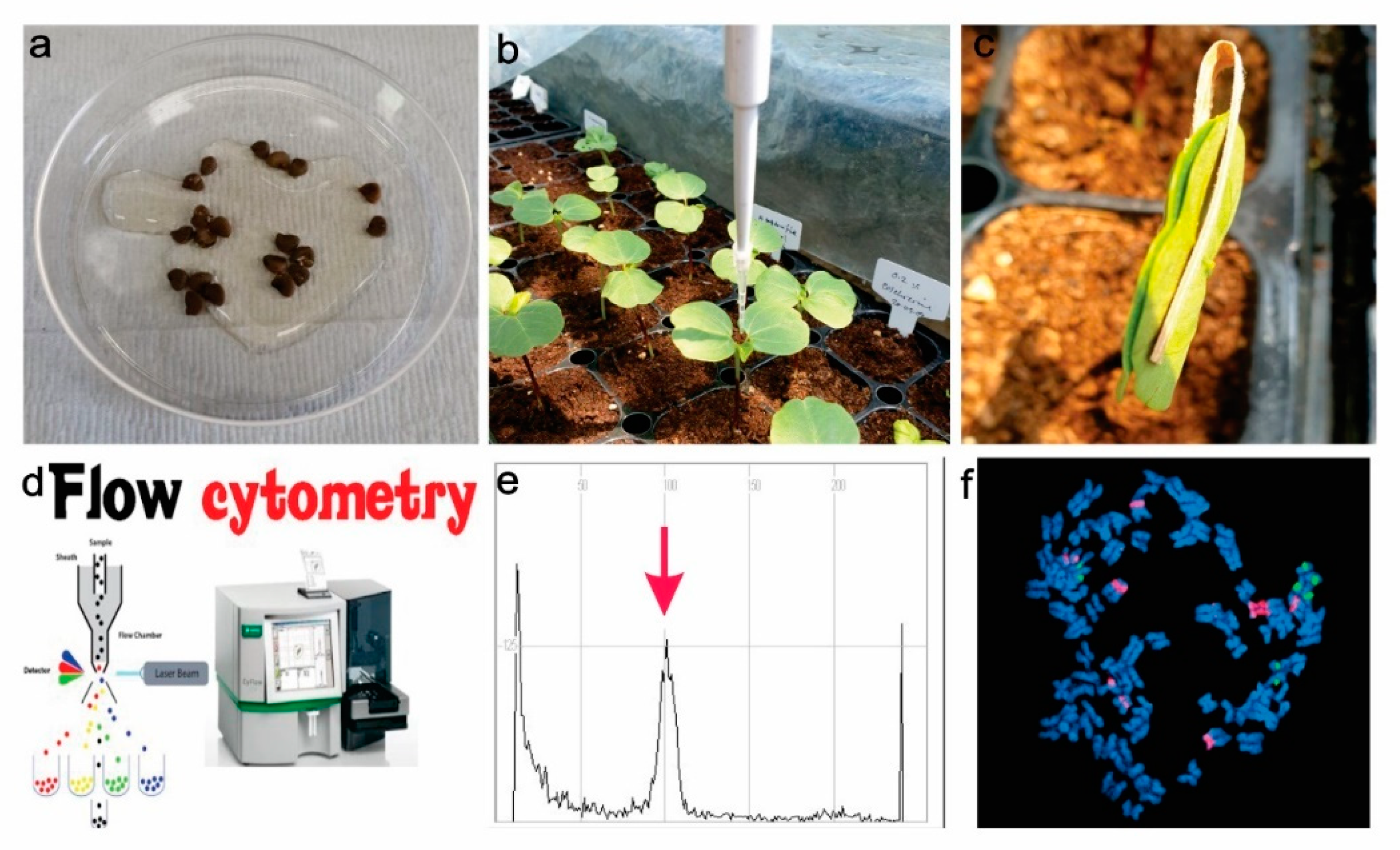
4. Cytogenetic Evaluation of Induced Polyploids
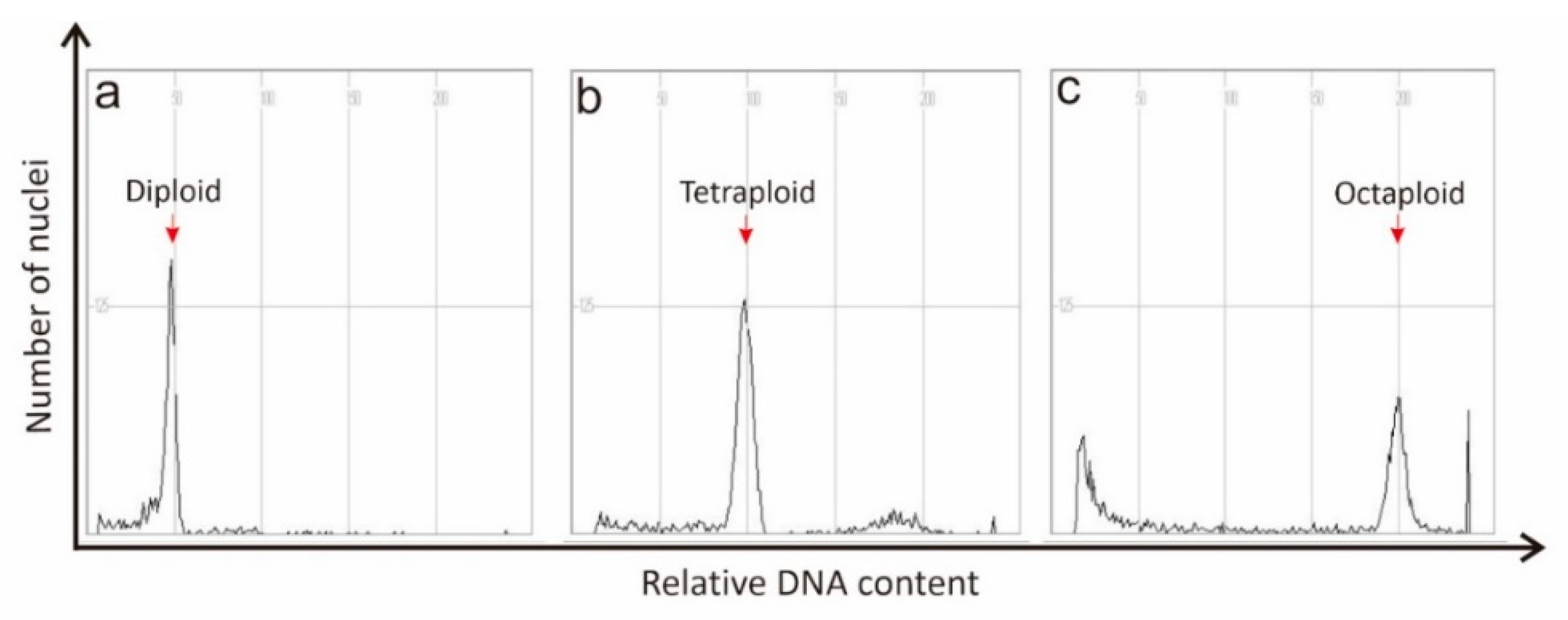
4.1. Flow Cytometry
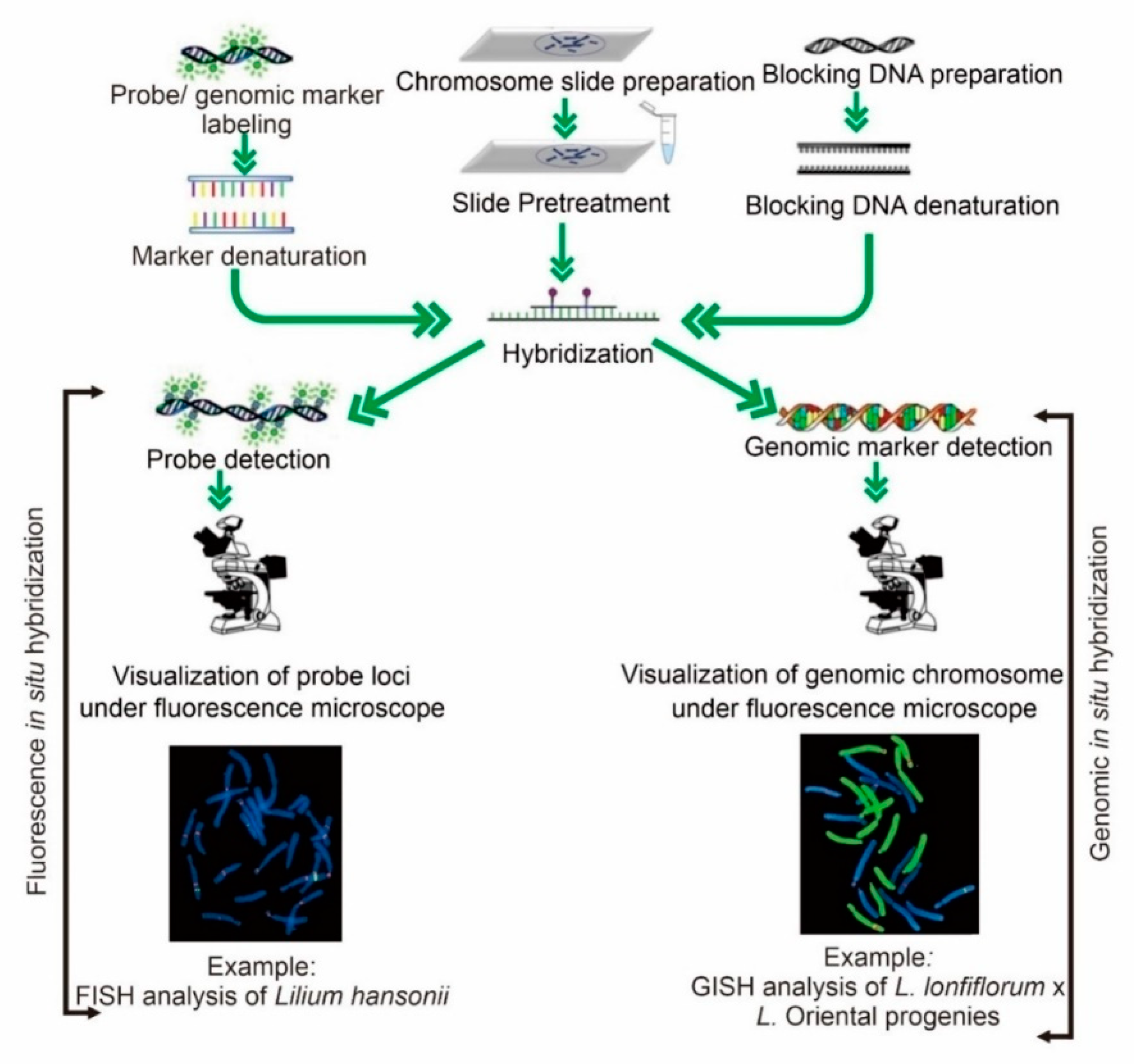
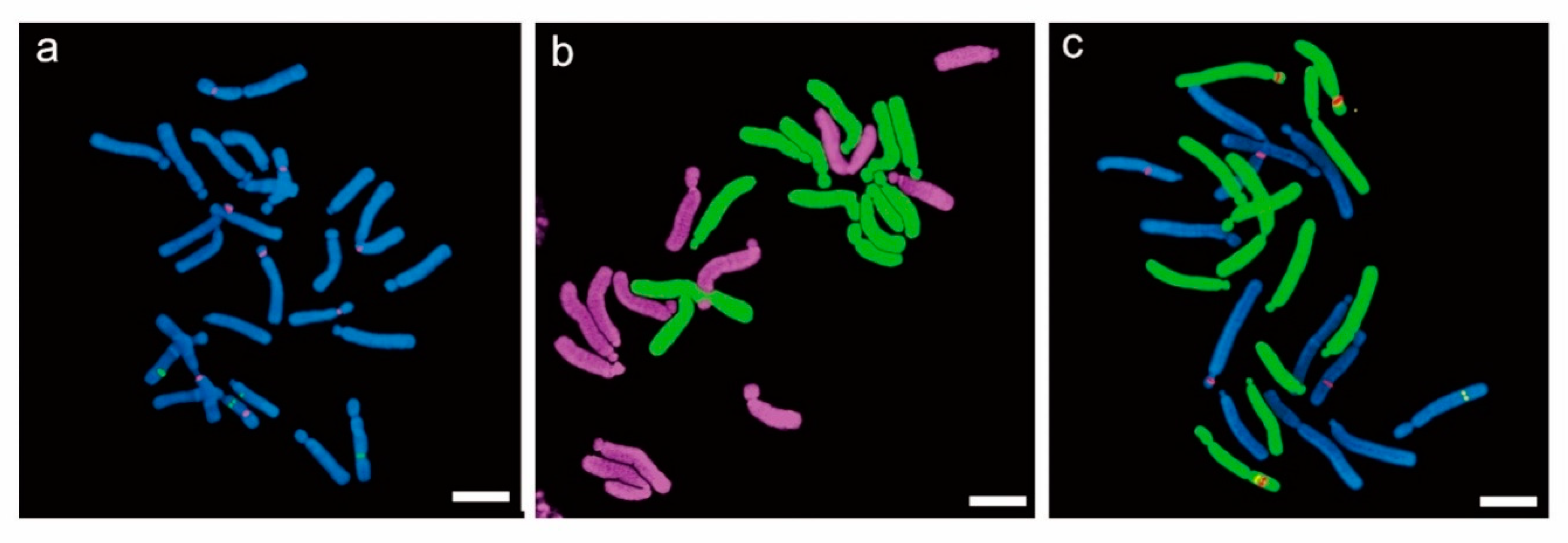
4.2. In Situ Hybridization
5. Effect of Polyploidization at the Morphological and Molecular Level
6. Effect of Polyploidization on Abiotic Stresses
6.1. Salinity Induced Stress Alleviation
6.2. Drought Stress Alleviation
6.3. Temperature Stress Alleviation
7. Effect of Polyploidization on Plant Biotic Stresses
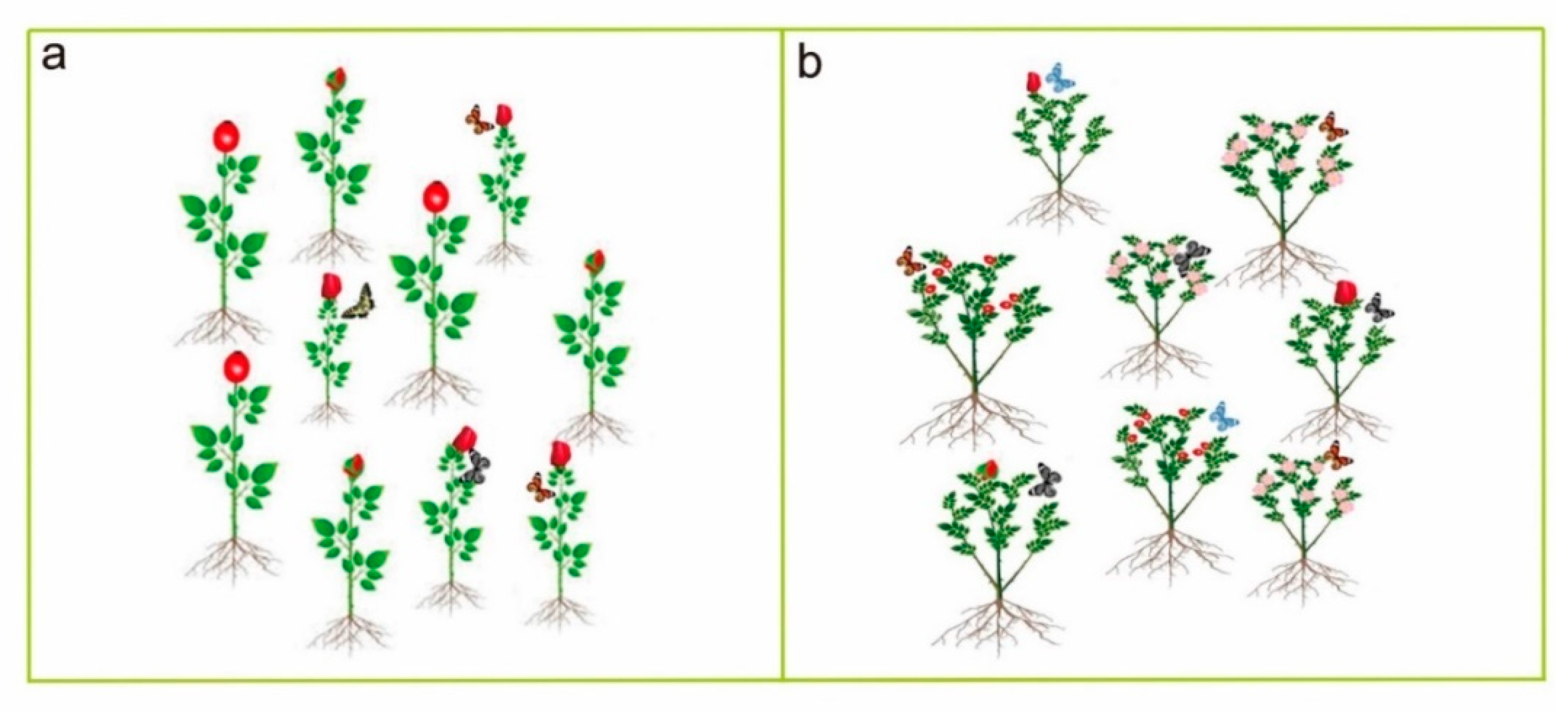
7.1. Polyploid-Insect Interaction
7.2. Polyploidy and Pathogen Resistance
8. Challenges of Polyploidization
8.1. Changes in Cellular Architecture
8.2. Mitotic and Meiotic Abnormalities
8.3. Epigenetic Instability
9. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, K.L.; Wendel, J.F. Exploring the genomic mysteries of polyploidy in cotton. Biol. J. Linn. Soc. 2004, 82, 573–581. [Google Scholar] [CrossRef]
- Lee, J.; Grant, D.; Vallejos, C.E.; Shoemaker, R. Genome organization in dicots. II. Arabidopsis as a ‘bridging species’ to resolve genome evolution events among legumes. Theor. Appl. Genet. 2001, 103, 765–773. [Google Scholar] [CrossRef]
- Udall, J.A.; Wendel, J.F. Polyploidy and crop improvement. Crop Sci. 2006, 46, S-3–S-14. [Google Scholar] [CrossRef]
- Henry, I.M.; Dilkes, B.P.; Miller, E.S.; Burkart-Waco, D.; Comai, L. Phenotypic consequences of aneuploidy in Arabidopsis thaliana. Genetics 2010, 186, 1231–1245. [Google Scholar] [CrossRef]
- Pavlíková, Z.; Paštová, L.; Münzbergová, Z. Synthetic polyploids in Vicia cracca: Methodology, effects on plant performance and aneuploidy. Plant Syst. Evol. 2017, 303, 827–839. [Google Scholar] [CrossRef]
- Shoemaker, R.; Polzin, K.; Labate, J.; Specht, J.; Brummer, E.; Olson, T.; Young, N.; Concibido, V.; Wilcox, J.; Tamulonis, J.P.; et al. Genome duplication in soybean (Glycine subgenus soja). Genetics 1996, 144, 329–338. [Google Scholar] [CrossRef]
- Chai, J.; Su, Y.; Huang, F.; Liu, S.; Tao, M.; Murphy, R.W. The gap in research on polyploidization between plants and vertebrates: Model systems and strategic challenges. Sci. Bull. 2015, 60, 1471–1478. [Google Scholar] [CrossRef]
- Islam, M.M.; Yesmin, R.; Jung, M.J.; Kim, H.Y.; Kim, C.K.; Lim, K.B. Investigation of the morphological and cytogenetic variations of an intraspecific Asiatic lily hybrid using 5S and 18S rDNA probes. Hortic. Environ. Biotechnol. 2020, 61, 339–346. [Google Scholar] [CrossRef]
- Nei, M.; Nozawa, M. Roles of mutation and selection in speciation: From Hugo de Vries to the modern genomic era. Genome Biol. Evol. 2011, 3, 812–829. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Davis, D.; Birchler, J.A. Dosage effects on gene expression in a maize ploidy series. Genetics 1996, 142, 1349–1355. [Google Scholar] [CrossRef]
- Paterson, A.H. Polyploidy, evolutionary opportunity, and crop adaptation. Genetica 2005, 123, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Stuessy, T.; Weiss-Schneeweiss, H. What drives polyploidization in plants? New Phytol. 2019, 223, 1690–1692. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Haerum, B.K.; Clark, A.G. Evolutionary changes in cis and trans gene regulation. Nature 2004, 430, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; Wagler, T.N.; Quijada, P.; Doebley, J. A distant upstream enhancer at the maize domestication gene tb1 has pleiotropic effects on plant and inflorescent architecture. Nat. Genet. 2006, 38, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Endrizzi, J.; Turcotte, E.; Kohel, R. Genetics, cytology, and evolution of Gossypium. Adv. Genet. 1985, 23, 271–375. [Google Scholar]
- Wendel, J.F.; Cronn, R.C. Polyploidy and the evolutionary history of cotton. Adv. Agron. 2003, 78, 139–186. [Google Scholar]
- Bae, S.J.; Islam, M.M.; Kim, H.Y.; Lim, K.B. Induction of Tetraploidy in Watermelon with Oryzalin Treatments. Hortic. Sci. Technol. 2020, 38, 385–393. [Google Scholar]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.B.; Miller, C.; Ramanna, M.; van Tuyl, J. Nitrous oxide N2O incudes 2n gametes in sterile F1 hybrids of Oriental × Asiatic lilies (Lilium) and leads to intergenomic recombination. Euphytica 2006, 148, 303–309. [Google Scholar] [CrossRef]
- Jo, Y.K.; Mazharul, I.M.; Kim, C.K.; Kim, H.Y.; Lim, K.B. Morphological Characteristics and FISH Analysis of Hibiscus F1 Hybrids and Parental Lines. Hortic. Sci. Technol. 2019, 37, 630–639. [Google Scholar]
- Lim, K.B.; Barba-Gonzalez, R.; Zhou, S.; Ramanna, M.; Van Tuyl, J.M. Interspecific hybridization in lily (Lilium): Taxonomic and commercial aspects of using species hybrids in breeding. Floric. Ornam. Plant Biotechnol. 2008, 5, 138–145. [Google Scholar]
- Mazharul, I.; Reshma, Y.; Jung, J.; Mohammad, D.; Lim, K. Cytogenetic assessment of Lilium longiflorum × L. hansonii revealed by genomic in situ hybridization (GISH). Acta Hortic. 2019, 1237, 79–86. [Google Scholar] [CrossRef]
- Van Tuyl, J.M.; Arens, P. Lilium: Breeding history of the modern cultivar assortment. Acta Hortic. 2010, 900, 223–230. [Google Scholar] [CrossRef]
- Ahmad, P.; Sharma, S. Salt stress and phyto-biochemical responses of plants. Plant Soil Environ. 2008, 54, 89–99. [Google Scholar]
- Castillo, E.; To Phuc, T.; Ismail, A.M.; Inubushi, K. Response to salinity in rice: Comparative effects of osmotic and ionic stresses. Plant Prod. Sci. 2007, 10, 159–170. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Raja, S.; Ravikrishna, R.; Kommalapati, R.; Valsaraj, K. Monitoring of fogwater chemistry in the gulf coast urban industrial corridor: Baton Rouge (Louisiana). Environ. Monit. Assess. 2005, 110, 99–120. [Google Scholar] [CrossRef]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.C.; Asanuma, K.I.; Kusutani, A.; Toyota, T.M. Leaf gas exchange properties of potato under different temperature and soil moisture at different growth stages. Environ. Control. Biol. 2000, 38, 229–239. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Vollenweider, P.; Günthardt-Goergv, M.S. Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ. Pollut. 2005, 137, 455–465. [Google Scholar] [CrossRef]
- DeLucia, E.H.; Nabity, P.D.; Zavala, J.A.; Berenbaum, M.R. Climate change: Resetting plant-insect interactions. Plant Physiol. 2012, 160, 1677–1685. [Google Scholar] [CrossRef]
- Rasmann, S.; Pellissier, L.; Defossez, E.; Jactel, H.; Kunstler, G. Climate-driven change in plant–insect interactions along elevation gradients. Funct. Ecol. 2014, 28, 46–54. [Google Scholar] [CrossRef]
- Tobin, P.C.; Nagarkatt, S.; Loeb, G.; Saunders, M.C. Historical and projected interactions between climate change and insect voltinism in a multivoltine species. Glob. Chang. Biol. 2008, 14, 951–957. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009, 10, 725–732. [Google Scholar] [CrossRef]
- Crow, J.F. Hitoshi Kihara, Japan’s pioneer geneticist. Genetics 1994, 137, 891. [Google Scholar] [CrossRef]
- Touchell, D.H.; Palmer, I.E.; Ranney, T.G. In vitro Ploidy Manipulation for Crop Improvement. Front. Plant Sci. 2020, 11, 722. [Google Scholar] [CrossRef]
- Forrester, N.J.; Rebolleda-Gómez, M.; Sachs, J.L.; Ashman, T.L. Polyploid plants obtain greater fitness benefits from a nutrient acquisition mutualism. New Phytol. 2020, 227, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Katepa-Mupondwa, F.M.; Christie, B.R.; Michaels, T.E. An improved breeding strategy for autotetraploid alfalfa (Medicago sativa L.). Euphytica 2002, 123, 139–146. [Google Scholar] [CrossRef]
- Barton, N.H. The role of hybridization in evolution. Mol. Ecol. 2008, 10, 551–568. [Google Scholar] [CrossRef]
- Acquaah, G. Principles of Plant Genetics and Breeding; Wiley-Blackwell: Malden, UK, 2007. [Google Scholar]
- Chen, Z.J. Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 2010, 15, 57–71. [Google Scholar] [CrossRef]
- Huang, G.; Zhu, Y.X. Plant polyploidy and evolution. J. Integr. Plant Biol. 2019, 61, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Salman Minkov, A.; Sabath, N.; Mayrose, I. Whole-genome duplication as a key factor in crop domestication. Nat. Plants 2016, 2, 16115. [Google Scholar] [CrossRef] [PubMed]
- Van Tuyl, J.; Lim, K.B.; Ramanna, M. Interspecific hybridization and introgression. In Breeding for Ornamentals: Classical and Molecular Approaches; Vainstain, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 85–103. [Google Scholar]
- Gaul, H. Present aspects of induced mutations in plant breeding. Euphytica 1958, 7, 275–289. [Google Scholar] [CrossRef]
- Winterfeld, G.; Ley, A.; Hoffmann, M.H.; Paule, J.; Röser, M.J. Dysploidy and polyploidy trigger strong variation of chromosome numbers in the prayer-plant family (Marantaceae). Plant Syst. Evol. 2020, 306, 36. [Google Scholar] [CrossRef]
- Simioni, C.; Schifino Wittmann, M.T.; Dall’Agnol, M. Sexual polyploidization in red clover. Sci. Agric. 2006, 63, 26–31. [Google Scholar] [CrossRef][Green Version]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, X.; Cheng, F. Plant Polyploidy: Origin, Evolution, and Its Influence on Crop Domestication. Hortic. Plant J. 2019, 5, 231–239. [Google Scholar] [CrossRef]
- Otto, S.P.; Whitton, J. Polyploid incidence and evolution. Annu. Rev. Genet. 2000, 34, 401–437. [Google Scholar] [CrossRef]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Ramsey, J.; Schemske, D.W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef]
- Blakeslee, A.F.; Avery, A.G. Methods of inducing doubling of chromosomes in plants: By treatment with colchicine. J. Hered. 1937, 28, 393–411. [Google Scholar] [CrossRef]
- Chauvin, J.E.; Souchet, C.; Dantec, J.; Ellissèche, D. Chromosome doubling of 2x Solanum species by oryzalin: Method development and comparison with spontaneous chromosome doubling in vitro. Plant Cell Tissue Organ Cult. 2003, 73, 65–73. [Google Scholar] [CrossRef]
- Song, P.; Kang, W.; Peffley, E.B. Chromosome doubling of Allium fistulosum × A. cepa interspecific F1 hybrids through colchicine treatment of regenerating callus. Euphytica 1997, 93, 257–262. [Google Scholar] [CrossRef]
- Omran, S.; Guerra Sanz, J.; Cardenas, J.G. Methodology of tetraploid induction and expression of microsatellite alleles in triploid watermelon. In Proceedings of the IXth Eucarpia Meeting on Genetics and Breeding of Cucurbitaceae, Avignon, France, 21–24 May 2008; pp. 381–384. [Google Scholar]
- Awoleye, F.; Van Duren, M.; Dolezel, J.; Novak, F. Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 1994, 76, 195–202. [Google Scholar] [CrossRef]
- Dunn, B.L.; Lindstrom, J.T. Oryzalin-induced chromosome doubling in Buddleja to facilitate interspecific hybridization. HortScience 2007, 42, 1326–1328. [Google Scholar] [CrossRef]
- Henny, R.J.; Holm, J.R.; Chen, J.; Scheiber, M. In vitro induction of tetraploids in Dieffenbachia × ‘Star Bright M-1′ by colchicine. HortScience 2009, 44, 646–650. [Google Scholar] [CrossRef]
- Teng, E.; Leonhardt, K. In vitro and in vivo polyploidization of Dracaena with oryzalin. Acta Hortic 2007, 813, 509–516. [Google Scholar] [CrossRef]
- Meyer, E.M.; Touchell, D.H.; Ranney, T.G. In vitro shoot regeneration and polyploid induction from leaves of Hypericum species. HortScience 2009, 44, 1957–1961. [Google Scholar] [CrossRef]
- Reshma, Y.; Mazharul, I.M.; Kim, H.Y.; Kim, C.K.; Lim, K.B. Role of Growth Regulators in the Somatic Organogenesis of Haworthia Inflorescences in Vitro. Hortic. Sci. Technol. 2020, 38, 394–404. [Google Scholar]
- Zhang, F.; Xue, H.; Lu, X.; Zhang, B.; Wang, F.; Ma, Y.; Zhang, Z. Autotetraploidization enhances drought stress tolerance in two apple cultivars. Trees 2015, 29, 1773–1780. [Google Scholar] [CrossRef]
- Kermani, M.; Sarasan, V.; Roberts, A.; Yokoya, K.; Wentworth, J.; Sieber, V. Oryzalin-induced chromosome doubling in Rosa and its effect on plant morphology and pollen viability. Theor. Appl. Genet. 2003, 107, 1195–1200. [Google Scholar] [CrossRef]
- Allum, J.; Bringloe, D.; Roberts, A. Chromosome doubling in a Rosa rugosa Thunb. hybrid by exposure of in vitro nodes to oryzalin: The effects of node length, oryzalin concentration and exposure time. Plant Cell Rep. 2007, 26, 1977–1984. [Google Scholar] [CrossRef]
- Rose, J.; Kubba, J.; Tobutt, K. Induction of tetraploidy in Buddleia globosa. Plant Cell Tissue Organ Cult. 2000, 63, 121–125. [Google Scholar] [CrossRef]
- Thao, N.T.P.; Ureshino, K.; Miyajima, I.; Ozaki, Y.; Okubo, H. Induction of tetraploids in ornamental Alocasia through colchicine and oryzalin treatments. Plant Cell Tissue Organ Cult. 2003, 72, 19–25. [Google Scholar] [CrossRef]
- Lu, C.; Bridgen, M.P. Chromosome doubling and fertility study of Alstroemeria aurea × A. caryophyllaea. Euphytica 1997, 94, 75–81. [Google Scholar] [CrossRef]
- Xavier de Mello e Silva, P.A.K.; Callegari-Jacques, S.; Bodanese-Zanettini, M.H. Induction and identification of polyploids in Cattleya intermedia Lindl. (Orchidaceae) by in vitro techniques. Cienc. Rural 2000, 30, 105–111. [Google Scholar] [CrossRef]
- Takamura, T.; Lim, K.B.; Van Tuyl, J. Effect of a new compound on the mitotic polyploidization of Lilium longiflorum and Oriental hybrid lilies. Acta Hortic. 2001, 572, 37–42. [Google Scholar] [CrossRef]
- Takamura, T. Cyclamen. Flower Breed. Genet. 2007, 2007, 459–478. [Google Scholar]
- Chauvin, J.; Label, A.; Kermarrec, M. In vitro chromosome-doubling in tulip (Tulipa gesneriana L.). J. Hortic. Sci. Biotechnol 2005, 80, 693–698. [Google Scholar] [CrossRef]
- Ascough, G.D.; Van Staden, J.; Erwin, J.E. Effectiveness of colchicine and oryzalin at inducing polyploidy in Watsonia lepida NE Brown. HortScience 2008, 43, 2248–2251. [Google Scholar] [CrossRef]
- Cohen, D.; Yao, J.L. In vitro chromosome doubling of nine Zantedeschia cultivars. Plant Cell Tissue Organ Cult. 1996, 47, 43–49. [Google Scholar] [CrossRef]
- Chen, L.L.; Gao, S.L. In vitro tetraploid induction and generation of tetraploids from mixoploids in Astragalus membranaceus. Sci. Hortic 2007, 112, 339–344. [Google Scholar] [CrossRef]
- de Carvalho, J.F.R.P.; de Carvalho, C.R.d.P.; Otoni, W.C. In vitro induction of polyploidy in annatto (Bixa orellana). Plant Cell Tissue Organ Cult. 2005, 80, 69–75. [Google Scholar] [CrossRef]
- Rubuluza, T.; Nikolova, R.; Smith, M.; Hannweg, K. In vitro induction of tetraploids in Colophospermum mopane by colchicine. S. Afr. J. Bot. 2007, 73, 259–261. [Google Scholar] [CrossRef]
- Roy, A.; Leggett, G.; Koutoulis, A. In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep. 2001, 20, 489–495. [Google Scholar] [CrossRef]
- Adaniya, S.; Shirai, D. In vitro induction of tetraploid ginger (Zingiber officinale Roscoe) and its pollen fertility and germinability. Sci. Hortic. 2001, 88, 277–287. [Google Scholar] [CrossRef]
- Alix, K.; Gérard, P.R.; Schwarzacher, T.; Heslop-Harrison, J. Polyploidy and interspecific hybridization: Partners for adaptation, speciation and evolution in plants. Ann. Bot 2017, 120, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Dewey, D.R. Some applications and misapplications of induced polyploidy to plant breeding. Polyploidy 1980, 13, 445–470. [Google Scholar]
- Van Tuyl, J.M.; Lim, K.B. Interspecific hybridisation and polyploidisation as tools in ornamental plant breeding. Acta Hortic. 2003, 612, 13–22. [Google Scholar] [CrossRef]
- Kamstra, S.A.; De Jeu, M.J.; Kuipers, A.G.; Jacobsen, E. Homoeologous chromosome pairing in the distant hybrid Alstroemeria aurea × A. inodora and the genome composition of its backcross derivatives determined by fluorescence in situ hybridization with species-specific probes. Heredity 1999, 82, 69–78. [Google Scholar] [CrossRef]
- Lim, K.B.; Wennekes, J.; Jong, J.H.; Jacobsen, E.; Van Tuyl, J.M. Karyotype analysis of Lilium longiflorum and Lilium rubellum by chromosome banding and fluorescence in situ hybridisation. Genome 2001, 44, 911–918. [Google Scholar] [CrossRef]
- Takahashi, C.; Leitch, I.; Ryan, A.; Bennett, M.; Brandham, P. The use of genomic in situ hybridization (GISH) to show transmission of recombinant chromosomes by a partially fertile bigeneric hybrid, Gasteria lutzii × Aloe aristata (Aloaceae), to its progeny. Chromosoma 1997, 105, 342–348. [Google Scholar]
- Veilleux, R. Diploid and polyploid gametes in crop plants: Mechanisms of formation and utilization in plant breeding. Plant Breed Rev. 1985, 3, 253–288. [Google Scholar]
- Brownfield, L.; Köhler, C. Unreduced gamete formation in plants: Mechanisms and prospects. J. Exp. Bot 2011, 62, 1659–1668. [Google Scholar] [CrossRef]
- Bretagnolle, F.A.; Thompson, J.D. Gametes with the somatic chromosome number: Mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 1995, 129, 1–22. [Google Scholar] [CrossRef]
- Lim, K.B.; Barba Gonzalez, R.; Zhou, S.; Ramanna, M.; Van Tuyl, J.M. Meiotic polyploidization with homoeologous recombination induced by caffeine treatment in interspecific lily hybrids. Korean J. Genet. 2005, 27, 219. [Google Scholar]
- Mohammad, D.D.; Mazharul, I.M.; Ann, T.C.; Kim, H.Y.; Lim, K.B. Phenotypic Characteristics and Karyotype Analysis of Hibiscus sabdariffa var. sabdariffa by Fluorescence in Situ Hybridization (FISH). Hortic. Sci. Technol. 2020, 38, 695–704. [Google Scholar]
- Duque, R.E.; Phan, S.; Hudson, J.L.; Till, G.; Ward, P. Functional defects in phagocytic cells following thermal injury. Application of flow cytometric analysis. Am. J. Pathol 1985, 118, 116. [Google Scholar] [PubMed]
- Sabharwal, P.; Doležel, J. Interspecific hybridization in Brassica: Application of flow cytometry for analysis of ploidy and genome composition in hybrid plants. Biol. Plant 1993, 35, 169–177. [Google Scholar] [CrossRef]
- Suda, J.; Krahulcová, A.; Trávníek, P.; Krahulec, F. Ploidy level versus DNA ploidy level: An appeal for consistent terminology. Taxon 2006, 55, 447–450. [Google Scholar] [CrossRef]
- He, Y.; Sun, Y.; Zheng, R.; Ai, Y.; Cao, Z.; Bao, M. Induction of tetraploid male sterile Tagetes erecta by colchicine treatment and its application for interspecific hybridization. Hortic. Plant J. 2016, 2, 284–292. [Google Scholar]
- Younis, A.; Ramzan, F.; Hwang, Y.J.; Lim, K.B. FISH and GISH: Molecular cytogenetic tools and their applications in ornamental plants. Plant Cell Rep. 2015, 34, 1477–1488. [Google Scholar] [CrossRef]
- Andres, R.J.; Kuraparthy, V. Development of an improved method of mitotic metaphase chromosome preparation compatible for fluorescence in situ hybridization in cotton. J. Cotton Sci. 2013, 17, 149–156. [Google Scholar]
- Lim, K.B.; De Jong, H.; Yang, T.J.; Park, J.Y.; Kwon, S.J.; Kim, J.S. Characterization of rDNAs and tandem repeats in the heterochromatin of Brassica rapa. Mol. Cells 2005, 19, 436–444. [Google Scholar]
- Gan, Y.; Liu, F.; Chen, D.; Wu, Q.; Qin, Q.; Wang, C. Chromosomal Locations of 5S and 45S rDNA in Gossypium genus and its phylogenetic implications revealed by FISH. PLoS ONE 2013, 8, e68207. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Chen, Q.; Zhang, L.; Tang, H.; Luo, Y. Phylogenetic insight into subgenera Idaeobatus and Malachobatus (Rubus, Rosaceae) inferring from ISH analysis. Mol. Cytogenet. 2015, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Budylin, M.; Kan, L.Y.; Romanov, V.; Khrustaleva, L. GISH study of advanced generation of the interspecific hybrids between Allium cepa L. and Allium fistulosum L. with relative resistance to downy mildew. Russ. J. Genet. 2014, 50, 387–394. [Google Scholar] [CrossRef]
- Książczyk, T.; Taciak, M.; Zwierzykowski, Z. Variability of ribosomal DNA sites in Festuca pratensis, Lolium perenne, and their intergeneric hybrids, revealed by FISH and GISH. J. Appl. Genet. 2010, 51, 449–460. [Google Scholar] [CrossRef]
- Kwon, M.J.; Ramzan, F.; Ahn, Y.J.; Hwang, Y.J.; Kang, Y.I.; Kim, C.K. Chromosomal analysis of Lilium longiflorum × Asiatic hybrids using GISH (genomic in situ hybridization). Hortic. Environ. Biotechnol. 2017, 58, 591–600. [Google Scholar] [CrossRef]
- Ramzan, F.; Younis, A.; Lim, K.B. Application of genomic in situ hybridization in horticultural science. Int. J. Genom. 2017, 129, 7561909. [Google Scholar] [CrossRef] [PubMed]
- Allario, T.; Brumos, J.; Colmenero-flores, J.M.; Iglesias, D.J.; Pina, J.A.; Navarro, L. Tetraploid Rangpur lime rootstock increases drought tolerance via enhanced constitutive root abscisic acid production. Plant Cell Environ. 2013, 36, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.Y.; Lucero, M.E.; Sanderson, S.C.; Zacharias, E.H.; Holbrook, N.M. Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 2013, 197, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Sun, H.; Li, L.; Bell, R.L. In vitro colchicine-induced polyploid plantlet production and regeneration from leaf explants of the diploid pear (Pyrus communis L.) cultivar, ‘Fertility’. J. Hortic. Sci. Biotechnol. 2009, 84, 548–552. [Google Scholar] [CrossRef]
- Levin, D.A. The role of chromosomal change in plant evolution. Syst. Bot. 2004, 29, 460–461. [Google Scholar]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Baig, M.M.Q.; Quresh, A.A.; Shah, M.K.N. Induction and identification of colchicine induced polyploidy in Gladiolus grandiflorus ‘White Prosperity’. Folia Hortic. 2018, 30, 307–319. [Google Scholar] [CrossRef]
- Vichiato, M.R.; Vichiato, M.; Pasqual, M.; Rodrigues, F.A.; Castro, D.M. Morphological effects of induced polyploidy in Dendrobium nobile Lindl.(Orchidaceae). Crop Breed. Appl. Biotechnol. 2014, 14, 154–159. [Google Scholar] [CrossRef]
- Khalid, M.F.; Hussain, S.; Anjum, M.A.; Ahmad, S.; Ali, M.A.; Ejaz, S. Better salinity tolerance in tetraploid vs diploivolkamer lemon seedlings is associated with robust antioxidant and osmotic adjustment mechanisms. J. Plant Physiol. 2020, 244, 153071. [Google Scholar] [CrossRef]
- Ari, E.; Djapo, H.; Mutlu, N.; Gurbuz, E.; Karaguzel, O. Creation of variation through gamma irradiation and polyploidization in Vitex agnus-castus L. Sci. Hortic 2015, 195, 74–81. [Google Scholar] [CrossRef]
- Tan, F.Q.; Tu, H.; Liang, W.J.; Long, J.M.; Wu, X.M.; Zhang, H.Y. Comparative metabolic and transcriptional analysis of a doubled diploid and its diploid citrus rootstock (C. junos cv. Ziyang xiangcheng) suggests its potential value for stress resistance improvement. BMC Plant Biol. 2015, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Amah, D.; Van Biljon, A.; Maziya-Dixon, B.; Labuschagne, M.T.; Swennen, R. Effects of in vitro polyploidization on agronomic characteristics and fruit carotenoid content; implications for banana genetic improvement. Front. Plant Sci. 2019, 10, 1450. [Google Scholar] [CrossRef]
- Prabhukumar, K.; Thomas, V.; Sabu, M.; Prasanth, M.; Mohanan, K. Induced mutation in ornamental gingers (Zingiberaceae) using chemical mutagens viz. colchicine, acridine and ethyl methane sulphonate. J. Hortic. For. Biotechnol. 2015, 19, 18–27. [Google Scholar]
- Xue, H.; Zhang, B.; Tian, J.R.; Chen, M.M.; Zhang, Y.Y.; Zhang, Z.H.; Ma, Y. Comparison of the morphology, growth and development of diploid and autotetraploid ‘Hanfu’apple trees. Sci. Hortic. 2017, 225, 277–285. [Google Scholar] [CrossRef]
- Van Laere, K.; França, S.C.; Vansteenkiste, H.; Van Huylenbroeck, J.; Steppe, K.; Van Labeke, M.C. Influence of ploidy level on morphology, growth and drought susceptibility in Spathiphyllum wallisii. Acta Physiol. Plant. 2011, 33, 1149–1156. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, X. Cytological characteristics of numerically unreduced pollen production in Populus tomentosa Carr. Euphytica 2010, 173, 151–159. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Y.; Guo, X.; Xu, L.; Lei, P.; Luo, Q.; Liu, J.; Li, W.; Tao, L.; Meng, F. The lectin gene TRpL1 of tetraploid Robinia pseudoacacia L. response to salt stress. J. For. Res. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, N.; Yamashita, S.; Ohta, K.; Hosoki, T. Morphological characteristics and their inheritance in colchicine-induced Salvia polyploids. J. Jpn. Soc. Hortic. Sci. 2008, 77, 186–191. [Google Scholar] [CrossRef]
- Ranney, T.G. Polyploidy: From evolution to new plant development. Comb. Proc. Int. Plant Propagators’ Soc. 2006, 2006, 137–142. [Google Scholar]
- Faria, R.T.D.; Takahashi, L.S.; Lone, A.B. UEL 6: Nova cultivar de Dendrobium. Hortic. Bras. 2009, 27, 114–115. [Google Scholar] [CrossRef][Green Version]
- Schepper, S.D.; Leus, L.; Mertens, M.; Debergh, P.; Bockstaele, E.V.; Loose, M.D. Somatic polyploidy and its consequences for flower coloration and flower morphology in azalea. Plant Cell Rep. 2001, 20, 583–590. [Google Scholar] [CrossRef]
- Obute, G.C.; Ndukwu, B.; Chukwu, O.F. Targeted mutagenesis in Vigna unguiculata (L.) Walp. and Cucumeropsis mannii (NAUD) in Nigeria. Afr. J. Biotechnol. 2007, 6, 2467–2472. [Google Scholar] [CrossRef]
- Wu, J.H.; Ferguson, A.R.; Murray, B.G.; Jia, Y.; Datson, P.M.; Zhang, J. Induced polyploidy dramatically increases the size and alters the shape of fruit in Actinidia chinensis. Ann. Bot. 2012, 109, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Samatadze, T.E.; Yurkevich, O.Y.; Khazieva, F.M.; Basalaeva, I.V.; Konyaeva, E.A.; Burova, A.E.; Zoshchuk, S.A.; Morozov, A.I.; Amosova, A.V.; Muravenko, O.V. Agro-Morphological and Cytogenetic Characterization of Colchicine-Induced Tetraploid Plants of Polemonium caeruleum L. (Polemoniaceae). Plants 2022, 11, 2585. [Google Scholar] [CrossRef]
- Khaing, T.; Perera, A.; Sumanasinghe, V.; Wijesundara, D. Improvement of Gymnostachyum species by induced mutation. Trop. Agric. Res. 2007, 19, 265–272. [Google Scholar]
- Majdi, M.; Karimzadeh, G.; Malboobi, M.A.; Omidbaigi, R.; Mirzaghaderi, G. Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): Morphological, physiological, cytological, and phytochemical changes. Hort. Sci. 2010, 45, 16–21. [Google Scholar] [CrossRef]
- Serrano-Fuentes, M.K.; Gómez-Merino, F.C.; Cruz-Izquierdo, S.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Gamma Radiation (60Co) Induces Mutation during In Vitro Multiplication of Vanilla (Vanilla planifolia Jacks. ex Andrews). Horticulturae 2022, 8, 503. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Hafiz, I.A.; Silvestri, C. Studies on colchicine induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Niazian, M.; Nalousi, A.M. Artificial polyploidy induction for improvement of ornamental and medicinal plants. Plant Cell Tissue Organ Cult. 2020, 107, 447–469. [Google Scholar] [CrossRef]
- Rathod, A.; Patil, S.; Taksande, P.; Karad, G.; Kalamkar, V.; Jayade, V. Effect of colchicine on morphological and biometrical traits in African marigold. J. Soils Crops 2018, 28, 72–80. [Google Scholar]
- Jones, K.D.; Reed, S.M.; Rinehart, T.A. Analysis of ploidy level and its effects on guard cell length, pollen diameter, and fertility in Hydrangea macrophylla. HortScience 2007, 42, 483–488. [Google Scholar] [CrossRef]
- Alexander, L. Ploidy level influences pollen tube growth and seed viability in interploidy crosses of Hydrangea macrophylla. Front. Plant Sci. 2020, 11, 100. [Google Scholar] [CrossRef]
- Cheniclet, C.; Rong, W.Y.; Causse, M.; Frangne, N.; Bolling, L.; Carde, J.P. Cell expansion and endoreduplication show a large genetic variability in pericarp and contribute strongly to tomato fruit growth. Plant Physiol. 2005, 139, 1984–1994. [Google Scholar] [CrossRef]
- Cohen, H.; Fait, A.; Tel Zur, N. Morphological, cytological and metabolic consequences of autopolyploidization in Hylocereus (Cactaceae) species. BMC Plant Biol. 2013, 13, 173. [Google Scholar] [CrossRef]
- Jokari, S.; Shekafandeh, A.; Jowkar, A. In vitro tetraploidy induction in Mexican lime and sour orange and evaluation of their morphological and physiological characteristics. Plant Cell Tiss Org. 2022, 6, 1–8. [Google Scholar] [CrossRef]
- Hassan, J.; Miyajima, I.; Ozaki, Y.; Mizunoe, Y.; Sakai, K.; Zaland, W.J.P. Tetraploid induction by colchicine treatment and crossing with a diploid reveals less-seeded fruit production in Pointed Gourd (Trichosanthes dioica Roxb). Plants 2020, 9, 370. [Google Scholar] [CrossRef]
- Zlesak, D.C.; Thill, C.A.; Anderson, N.O. Trifluralin-mediated polyploidization of Rosa chinensis minima (Sims) Voss seedlings. Euphytica 2005, 141, 281–290. [Google Scholar] [CrossRef]
- Cai, X.; Cao, Z.; Xu, S.; Deng, Z. Induction, regeneration and characterization of tetraploids and variants in Tapestry caladium. Plant Cell Tissue Organ Cult. 2015, 120, 689–700. [Google Scholar] [CrossRef]
- Luo, Z.; Iaffaldano, B.J.; Cornish, K. Colchicine induced polyploidy has the potential to improve rubber yield in Taraxacum koksaghyz. Ind. Crops Prod. 2018, 112, 75–81. [Google Scholar] [CrossRef]
- Pan-pan, H.; Wei Xu, L.; Hui Hui, L.; Xu, X.Z. In vitro induction and identification of autotetraploid of Bletilla striata (Thunb.) Reichb. f. by colchicine treatment. Plant Cell Tissue Organ Cult. 2018, 132, 425–432. [Google Scholar] [CrossRef]
- Ye, Y.; Tong, J.; Shi, X.; Yuan, W.; Li, G. Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Sci. Hortic. 2010, 124, 95–101. [Google Scholar] [CrossRef]
- Thatayaone, M.; Saji, G.; Meagle, J.; Kuruvila, B. Biochemical and nutritional characteristics of some commercial banana (Musa spp.) cultivars of Kerala. Plant Sci. Today 2022, 9, 681–686. [Google Scholar] [CrossRef]
- Do Amaral, C.M.; Dos Santos-Serejo, J.D.A.; Silva, S.D.O.E.; Da Silva Ledo, C.A.; Amorim, E.P. Agronomic characterization of autotetraploid banana plants derived from ‘Pisang Lilin’(AA) obtained through chromosome doubling. Euphytica 2015, 202, 435–443. [Google Scholar] [CrossRef]
- Jadrná, P.; Plavcová, O.; Kobza, F. Morphological changes in colchicine--treated Pelargonium× hortorum LH Bailey greenhouse plants. Hortic. Sci. 2011, 37, 27–33. [Google Scholar] [CrossRef]
- Manzoor, S.A.; Riaz, A.; Zafar, T.; Hassan, M.; Umar, H.M.; Hassan, J.; Alam, W.; Muhammad, S.; Mahmood, M.; Sohail, H.; et al. Improving growth performance of jatropha curcas by inducing polyploidy through colchicine treatment. Am. J. Plant Sci. 2016, 7, 769–772. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Ruter, J.M. Development and Evaluation of diploid and polyploid Hibiscus moscheutos. HortScience 2017, 52, 676–681. [Google Scholar] [CrossRef]
- Padoan, D.; Mossad, A.; Chiancone, B.; Germana, M.A.; Khan, P.S. Ploidy levels in Citrus clementine affects leaf morphology, stomatal density and water content. Theor. Exp. Plant Physiol. 2013, 25, 83–290. [Google Scholar] [CrossRef]
- Wong, C.; Murray, B.G. Variable changes in genome size associated with different polyploid events in Plantago (Plantaginaceae). J. Hered. 2012, 103, 711–719. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lattier, J.; Chen, H.; Contreras, R.N. Variation in genome size, ploidy, stomata, and rDNA signals in Althea. J. Am. Soc. Hortic. Sci. 2019, 144, 130–140. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, J.; Sun, K.; Chang, D.; Bai, S.; Shen, Y. Ploidy level and DNA content of Erianthus arundinaceus as determined by flow cytometry and the association with biological characteristics. PLoS ONE 2016, 11, e0151948. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lei, T.; Meng, F.; Wei, C.; Li, X.; Guo, H. Polyploidy index and its implications for the evolution of polyploids. Front. Genet. 2019, 10, 807. [Google Scholar] [CrossRef]
- Nair, N.V.; Praneetha, M. Cyto-morphological studies on three Erianthus arundinaceus (Retz.) Jeswiet accessions from the Andaman-Nicobar Islands, India. Cytologia 2006, 71, 107–109. [Google Scholar] [CrossRef][Green Version]
- Manchanda, G.; Garg, N. Salinity and its effects on the functional biology of legumes. Acta Physiol. Plant. 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.; Wani, A.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Tu, Y.; Jiang, A.; Gan, L.; Hossain, M.; Zhang, J.; Peng, B. Genome duplication improves rice root resistance to salt stress. Rice 2014, 7, 15. [Google Scholar] [CrossRef]
- Jiang, A.; Gan, L.; Tu, Y.; Ma, H.; Zhang, J.; Song, Z.; He, Y.; Cai, D.; Xue, X. The effect of genome duplication on seed germination and seedling growth of rice under salt stress. Aust. J. Crop Sci. 2013, 7, 1814. [Google Scholar]
- Jiang, J. Fluorescence in situ hybridization in plants: Recent developments and future applications. Chromosome Res. 2019, 27, 153–165. [Google Scholar] [CrossRef]
- Meng, H.B.; Jiang, S.S.; Hua, S.J.; Lin, X.Y.; Li, Y.L.; Guo, W.L. Comparison between a tetraploid turnip and its diploid progenitor (Brassica rapa L.): The adaptation to salinity stress. Agr. Sci. China 2011, 10, 363–375. [Google Scholar] [CrossRef]
- Saleh, B.; Allario, T.; Dambier, D.; Ollitrault, P.; Morillon, R. Tetraploid citrus rootstocks are more tolerant to salt stress than diploid. C. R. Biol. 2008, 331, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.; Bomblies, K. Meiosis in autopolyploid and allopolyploid Arabidopsis. Curr. Opin. Plant Biol. 2016, 30, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Abdolinejad, R.; Shekafandeh, A. Tetraploidy Confers Superior in vitro Water-Stress Tolerance to the Fig Tree (Ficus carica) by Reinforcing Hormonal, Physiological, and Biochemical Defensive Systems. Front. Plant Sci. 2022, 12, 796215. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Xia, X.; Li, Y.; Chen, J. Chromosome doubling mediates superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Hortic. Res. 2020, 7, 40. [Google Scholar] [CrossRef]
- Del Pozo, J.C.; Ramirez-parra, E. Deciphering the molecular bases for drought tolerance in A rabidopsis autotetraploids. Plant Cell Environ. 2014, 37, 2722–2737. [Google Scholar] [CrossRef]
- Yang, P.M.; Huang, Q.C.; Qin, G.Y.; Zhao, S.P.; Zhou, J.G. Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica 2014, 52, 193–202. [Google Scholar] [CrossRef]
- Akinroluyo, O.K.; Jasukune, K.; Kemesyte, V.; Statkeviciute, G. Drought stress response of Westerwolths ryegrass (Lolium multiflorum ssp. multiflorum) cultivars differing in their ploidy level. Zemdirbyste 2020, 107, 161–170. [Google Scholar] [CrossRef]
- Li, W.D.; Biswas, D.K.; Xu, H.; Xu, C.Q.; Wang, X.Z.; Liu, J.K.; Jiang, G.M. Photosynthetic responses to chromosome doubling in relation to leaf anatomy in Lonicera japonica subjected to water stress. Funct. Plant Biol. 2009, 36, 783–792. [Google Scholar] [CrossRef]
- Godfree, R.C.; Marshall, D.J.; Young, A.G.; Miller, C.H.; Mathews, S. Empirical evidence of fixed and homeostatic patterns of polyploid advantage in a keystone grass exposed to drought and heat stress. R. Soc. Open Sci. 2017, 4, 170934. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, S.; Chen, Y.; Guan, Z.; Yin, D.; Chen, F. In vitro induced tetraploid of Dendranthema nankingense (Nakai) Tzvel. shows an improved level of abiotic stress tolerance. Sci. Hortic. 2011, 127, 411–419. [Google Scholar] [CrossRef]
- Xu, X.; Lu, J.; Bradley, F. Applications of polyploids in muscadine grape (Vitis Rotundifolia Michx.) breeding. Acta Hortic. 2010, 1046, 411–417. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Z.; Wang, J.; Chen, T.; Gao, J.; Zheng, J. Induction of new tetraploid genotypes and heat tolerance assessment in Asparagus officinalis L. Sci. Hortic. 2020, 264, 109168. [Google Scholar] [CrossRef]
- Yin, C.; Li, P.; Li, H.; Xu, L.; Zhao, J.; Shan, T. Enhancement of diosgenin production in Dioscorea zingiberensis seedling and cell cultures by beauvericin from the endophytic fungus Fusarium redolens Dzf2. J. Med. Plant Res. 2011, 5, 6550–6554. [Google Scholar]
- Arvanitis, L.; Wiklund, C.; Münzbergova, Z.; Dahlgren, J.P.; Ehrlén, J. Novel antagonistic interactions associated with plant polyploidization influence trait selection and habitat preference. Ecol. Lett. 2010, 13, 330–337. [Google Scholar] [CrossRef]
- Edger, P.P.; Heidel Fischer, H.M.; Bekaert, M.; Rota, J.; Glöckner, G.; Platts, A.E. The butterfly plant arms-race escalated by gene and genome duplications. Proc. Natl. Acad. Sci. USA 2015, 112, 8362–8366. [Google Scholar] [CrossRef]
- Thompson, J.N.; Cunningham, B.M.; Segraves, K.A.; Althoff, D.M.; Wagner, D. Plant polyploidy and insect/plant interactions. Am. Nat. 1997, 150, 730–743. [Google Scholar] [CrossRef]
- Kao, R.H. Implications of polyploidy in the host plant of a dipteran seed parasite. West. N. Am. Nat. 2008, 68, 225–230. [Google Scholar] [CrossRef][Green Version]
- Nuismer, S.L.; Thompson, J.N. Plant polyploidy and non-uniform effects on insect herbivores. Proc. Royal Soc. B 2001, 268, 1937–1940. [Google Scholar] [CrossRef]
- Gross, K.; Schiestl, F.P. Are tetraploids more successful? Floral signals, reproductive success and floral isolation in mixed-ploidy populations of a terrestrial orchid. Ann. Bot. 2015, 115, 263–273. [Google Scholar] [CrossRef]
- Segraves, K.A.; Anneberg, T.J. Species interactions and plant polyploidy. Am. J. Bot. 2016, 103, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yan, Z.; Zhao, S.; Gu, Q.; Li, L. Study on Resistance to Fusarium Wilt in Different Polyploidy of Watermelons. J. Changjiang Veg. 2009, 18. [Google Scholar]
- Gonda, I.; Milavski, R.; Adler, C.; Abu-Abied, M.; Tal, O.; Faigenboim, A.; Chaimovitsh, D.; Dudai, N. Genome-based high-resolution mapping of fusarium wilt resistance in sweet basil. Plant Sci. 2022, 11, 111316. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Zhang, Z.; Fu, J.; Ma, Y. Characterization of fungi resistance in two autotetraploid apple cultivars. Sci. Hortic 2017, 220, 27–35. [Google Scholar] [CrossRef]
- Gottula, J.; Lewis, R.; Saito, S.; Fuchs, M. Allopolyploidy and the evolution of plant virus resistance. BMC Evol. Biol. 2014, 14, 149. [Google Scholar] [CrossRef][Green Version]
- Yuan, J.; Wang, F.; Liu, T.; Chen, W. Expression and suppression of leaf rust resistance genes in amphidiploids from crosses of diploids and tetraploids. Acta Phytopathol. Sin. 2007, 5, 8. [Google Scholar]
- Zhu, Z.; Zhou, R.; Dong, Y.C.; Jia, J. Analysis of powdery mildew resistance genes in some tetraploid wheat-Aegilops amphidiploids and their parents. Plant Genet. Resour. 2003, 2, 137–143. [Google Scholar]
- Coate, J.E.; Doyle, J.J. Quantifying whole transcriptome size, a prerequisite for understanding transcriptome evolution across species: An example from a plant allopolyploid. Genome Biol. Evol. 2010, 2, 534–546. [Google Scholar] [CrossRef] [PubMed]
- De Godoy, L.M.; Olsen, J.V.; Cox, J.; Nielsen, M.L.; Hubner, N.C.; Fröhlich, F. Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature 2008, 455, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubešová, M. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot 2012, 109, 19–45. [Google Scholar] [CrossRef]
- Mitchell, S.E.; Rogers, E.S.; Little, T.J.; Read, A.F. Host-parasite and genotype-by-environment interactions: Temperature modifies potential for selection by a sterilizing pathogen. Evolution 2005, 59, 70–80. [Google Scholar] [CrossRef]
- Melaragno, J.E.; Mehrotra, B.; Coleman, A.W. Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 1993, 5, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Olmo, E. Nucleotype and cell size in vertebrates: A review. Basic Appl. Histochem 1983, 27, 227. [Google Scholar]
- Pacey, E.K.; Maherali, H.; Husband, B.C. Polyploidy increases storage but decreases structural stability in Arabidopsis thaliana. Current Biology 2022. [CrossRef] [PubMed]
- Petersen, K.K.; Hagberg, P.; Kristiansen, K.; Forkmann, G. In vitro chromosome doubling of Miscanthus sinensis. Plant Breed 2002, 121, 445–450. [Google Scholar] [CrossRef]
- Pelé, A.; Rousseau-Gueutin, M.; Chèvre, A.M. Speciation success of polyploid plants closely relates to the regulation of meiotic recombination. Front. Plant Sci. 2018, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Bharadwa, J.; Dinesh, N. Polyploidy in crop improvement and evolution. In Plant Biology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 619–638. [Google Scholar]
- Mayer, V.W.; Aguilera, A. High levels of chromosome instability in polyploids of Saccharomyces cerevisiae. Mutat. Res.-Fund. Mol. M. 1990, 231, 177–186. [Google Scholar] [CrossRef]
- Klinner, U.; Böttcher, F. Mitotically unstable polyploids in the yeast Pichia guilliermondii. J. Basic MicroBiol. 1992, 32, 331–338. [Google Scholar] [CrossRef]
- Doyle, G. Aneuploidy and inbreeding depression in random mating and self-fertilizing autotetraploid populations. Theor. Appl. Genet. 1986, 72, 799–806. [Google Scholar] [CrossRef]
- Müntzing, A. Cyto-genetic properties and practical value of tetraploid rye. Hereditas 2010, 37, 17–84. [Google Scholar] [CrossRef]
- Bomblies, K.; Madlung, A. Polyploidy in the Arabidopsis genus. Chromosome Res. 2014, 22, 117–134. [Google Scholar] [CrossRef]
- Papp, I.; Iglesias, V.; Moscone, E.; Michalowski, S.; Spiker, S.; Park, Y.D. Structural instability of a transgene locus in tobacco is associated with aneuploidy. Plant J. 1996, 10, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, C.; Yang, H.; Hou, J.; Ji, T.; Cheng, J.; Veitia, R.A.; Birchler, J.A. The Gene Balance Hypothesis: Epigenetics and Dosage Effects in Plants. Plant Epigenetics Epigenomics 2020, 2093, 161–171. [Google Scholar]
- Scheid, O.M.; Afsar, K.; Paszkowski, J. Formation of stable epialleles and their paramutation-like interaction in tetraploid Arabidopsis thaliana. Nat. Genet. 2003, 34, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Scheid, O.M.; Jakovleva, L.; Afsar, K.; Maluszynska, J.; Paszkowski, J. A change of ploidy can modify epigenetic silencing. Proc. Natl. Acad. Sci. USA 1996, 93, 7114–7119. [Google Scholar] [CrossRef] [PubMed]


| Plants | Treatment | Most Successful Method | References |
|---|---|---|---|
| Vegetables | |||
| Allium | Callus | Colchicine 2.5 mM, 1/2 days | [60] |
| Citrullus lanatus | Germinating seedlings | 2,6-Dinitroaniline 65.5 µM, 24 h | [61] |
| Manihot esculenta | Axillary node cuttings | Colchicine 5 mM, 48 h | [62] |
| Smallanthus songifolius | Nodal segments | Oryzalin 25 µM, 8–48 h | [63] |
| Ornamentals | |||
| Buddleja | Nodal sections | Oryzalin 25 µM, 3 days | [64] |
| Dieffenbachia | Shoot clumps | Colchicine 1.25 mM, 24 h | [65] |
| Dracaena deremensis | Callus | Oryzalin 144.5 µM, 48 h | [66] |
| Hypericum | Callus | Oryzalin 30 µM, 3–9 days | [67] |
| Lagerstroemia indica | Nodal buds | Colchicine 750 µM 24 h | [68] |
| Rhododendron | Micro-shoots | Oryzalin 150 µM, 24 h | [69] |
| Rosa | Shoots tips, nodal sections | Oryzalin 5 µM, 1 day | [70] |
| Rosa rugosa | 2 or 10mm nodes | Oryzalin 2.5 µM, 48 h | [71] |
| Syringa | Nodal sections | Colchicine 0.05–0.25 mM, 1–2 Days | [72] |
| Alocasia | Shoot tips | Oryzalin 289 µM, 24 h | [73] |
| Alstroemeria | Plantlets | Colchicine 5–15 mM, 6–24 h | [74] |
| Cattleya | PLB | Colchicine 1.25 mM, 8 days | [75] |
| Cyclamen | Tuber segments | Colchicine 0.25 mM, 4 days | [76] |
| Lilium longiflorum | Scale | Surflan (0.1 mM oryzalin), 3 h | [77] |
| Tulipa gesneriana | Flower stem dices | Oryzalin 2.88–120 µM, 2–24 h | [78] |
| Watsonia lepida | Shoots | Oryzalin 120 µM, 24 h | [79] |
| Zantedeschia | Shoot cultures | Colchicine 1.25 mM, 1–4 days | [80] |
| Aromatic, medicinal plants | |||
| Astragalus membranaceus | Apical buds | Colchicine 5 mM, 36 h | [81] |
| Bixa orellana | Cotyledonary nodes from seedlings | Oryzalin 15 µM, 15 days | [82] |
| Colophospermum mopane | Seeds | Colchicine 2.5 mM, 48 h | [83] |
| Dioscorea zingiberensis | Apical buds | Colchicine 3.75 mM, 24 h | [53] |
| Humulus lupulus | Apical buds | Colchicine 1.25 mM, 48 h | [84] |
| Zingiber officinale | Shoot tips | Colchicine 5mM, 8 days | [85] |
| Induced Polyploid | Effect (Increased/Decreased) | References |
|---|---|---|
| Plant height | Increase | [52,54,115] |
| Root length and number | Increase | [86,116] |
| Number of leaves/plants | Increase/decrease | [117] |
| Leaf area | Increase/decrease | [17,118] |
| Leaf size | Increase | [17,112] |
| Stomata number/leaf | Decrease | [17,119,120] |
| Stomata size | Increase | [17,115,119] |
| Flower size, number | Increase | [52,112,117,118,119,120,121,122,123,124,125] |
| Pollen size | Increase | [123] |
| Fruit size, number | Increase | [98,116,124] |
| Seed size | Increase | [98,116] |
| Seeds/fruit | Decrease | [17,39] |
| Stress | Inducing Method/Organism | Crop | Adaptation | Mechanism | References |
|---|---|---|---|---|---|
| Salinity | NaCl induced salinity in laboratory | Orange | Better adaptation | 1. NPK, proline content was higher in tetraploid than diploid. 2. MDA and H2O2 content was lower in tetraploid than in diploid. | [167] |
| Turnip | Better adaptation | 1. 100% increase in seed germination in tetraploid in highest saline level 200 (m.mol L−1). 2. Shoot and roots length reduced in diploid under salt stress condition compared to tetraploid. 3. At highest level of salinity, 74.7% diploid, and 64.4% tetraploid seedlings were injured. 4. Chlorophyll content reduced by 11.9% and 40.3% in tetraploid and diploid, respectively. 5. K+ concentration was stable in tetraploid (16:10, 15:10 K+/Na+ but reduced in diploid (46:100, 48:100) in root and shoot, respectively. | [165] | ||
| Rice | Better adaptation | 1. Proline concentration was higher in tetraploid (23.3% higher than diploid). 2. MDA content was lower in tetraploid than in diploid. | [163] | ||
| Lemon Seedling | Better adaptation | 1. Malondialdehyde and hydrogen peroxide was greater in the leaves and roots of diploid seedlings. 2. Antioxidative enzymes (peroxidase, ascorbate peroxidase, glutathione reductase, and catalase) were higher in tetraploid. | [167] | ||
| Rice | Better adaptation | 1. Mortality rates of tetraploids were lower than diploid. 2. Proline content was increased in tetraploid. | [164] | ||
| Hoagland solution in green house pot | Citrus | Better adaptation | Lower accumulations of chloride ions in leaves of the tetraploid plants as compared to diploid. | [166] | |
| Drought | Laboratory condition induced by polyethylene glycol | Apple | Better adaptation | 1. Relative water content (RWC) was higher in tetraploid than diploid (after 3 h of treatment 81.76% and 63.84%, respectively, and after 6h of treatment 69.89% and 48.16%, respectively, in tetraploid and diploid cultivar). 2. Lower level of MDA content in tetraploid indicated membrane integrity under drought stress. 3. Less expression of aquaporin genes in drought stress was shown in tetraploid. | [168] |
| Controlled environment, drought condition by limited water | A solanaceous plant | Better adaptation | 1. Tetraploid plants grew normally, and leaves remained turgid where diploid plants died in drought stress. 2. Higher chlorophyll content and lower H2O2 synthesis were shown in tetraploid than diploid (less oxidative damage). | [169] | |
| Limited Water supply | Arabidopsis | Better adaptation | 1. Tetraploid stomatal pore is 20% bigger than diploid due to the bigger size of the guard cells. 2. Higher survival rates in tetraploid. 3. ABA induced stomatal closure happened in tetraploid leaves. 4. ROS increased in cellular levels and affect stomatal aperture. 5. Polyploidy induced gene, which helps in stress adaptation. | [170] | |
| Limited Water supply | Rice | Better adaptation | 1. MDA content was lower in tetraploid rice. 2. Phosphoenolpyruvate carboxylase (PEPC) alleviates photosynthesis inhibition. 3. Tetraploid showed more PEPC activities in drought stress. 4. Higher superoxide dismutase (SOD), POD (peroxidase), CAT (Catalase) was shown, ROS scavenging was more, and cell membrane damage was less in tetraploid rice. | [171] | |
| Both controlled and field trial | Westerwolths rye grass | Better adaptation | 1. 30–40% more phenolic content and higher antiradical activities, better stress adaptation found in tetraploid. 2. More biomass in tetraploid. | [172] | |
| Laboratory | Honeysuckle plant | Better adaptation | 1. No photosynthesis in diploid, 80% reduction in tetraploid. 2. Higher MDA in diploid. | [173] | |
| Temperature | Heat Stress (42 °C) | Dioscorea zingiberensis | Better adaptation | 1. Relative electrolyte leakage (%) and MDA content was lower in tetraploid than diploid in heat stress condition. 2. ROS production rate was higher in diploid and antioxidant enzymes such SOD, CAT, and APX were higher in tetraploid. 3. Glutathione-ascorbate and AsA declined slowly in tetraploid were drastically in diploid. | [123] |
| Drought and Heat Stress (52 °C), field condition | Keystone grass | Better adaptation | 1. 20% heavier seeds in tetraploid under stress condition. 2. Genome duplication and reproductive flexibility jointly contributes to stress alleviation. 3. Homeostatic maintenance of reproductive output under increasing abiotic stress. 4. Fixed differences in seed size and morphology that increase propagule fitness and mobility. | [174] | |
| Laboratory condition | Dendranthema nankingense | Lower heat stress adaptability | 1. Higher cold stress adaptability in tetraploid but lower heat stress adaptability. 2. Tetraploid did not show much morphological change with diploid. | [175] | |
| Laboratory condition (39 °C day/30 °C night | Dioscorea zingiberensis | Better adaptation | 1. Activation transcriptomic response in tetraploid (19 bands silenced and 47 bands activated) where in diploid 32 silenced and 28 activated. 2. Activation transcriptomic responses may confer tolerance in heat stress in tetraploid. | [176] | |
| 96 h long stress at 45 °C. | Asparagus officinalis | Better adaptation | 1. During heat stress MDA decreased by 42% in tetraploid, SOD increased by 81%, POD increased by 119%, and PRO content increased by 63% compared to diploid. | [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.M.; Deepo, D.M.; Nasif, S.O.; Siddique, A.B.; Hassan, O.; Siddique, A.B.; Paul, N.C. Cytogenetics and Consequences of Polyploidization on Different Biotic-Abiotic Stress Tolerance and the Potential Mechanisms Involved. Plants 2022, 11, 2684. https://doi.org/10.3390/plants11202684
Islam MM, Deepo DM, Nasif SO, Siddique AB, Hassan O, Siddique AB, Paul NC. Cytogenetics and Consequences of Polyploidization on Different Biotic-Abiotic Stress Tolerance and the Potential Mechanisms Involved. Plants. 2022; 11(20):2684. https://doi.org/10.3390/plants11202684
Chicago/Turabian StyleIslam, Md Mazharul, Deen Mohammad Deepo, Saifullah Omar Nasif, Abu Bakar Siddique, Oliul Hassan, Abu Bakar Siddique, and Narayan Chandra Paul. 2022. "Cytogenetics and Consequences of Polyploidization on Different Biotic-Abiotic Stress Tolerance and the Potential Mechanisms Involved" Plants 11, no. 20: 2684. https://doi.org/10.3390/plants11202684
APA StyleIslam, M. M., Deepo, D. M., Nasif, S. O., Siddique, A. B., Hassan, O., Siddique, A. B., & Paul, N. C. (2022). Cytogenetics and Consequences of Polyploidization on Different Biotic-Abiotic Stress Tolerance and the Potential Mechanisms Involved. Plants, 11(20), 2684. https://doi.org/10.3390/plants11202684










