Biotechnological Potential of Different Organs of Mistletoe (Viscum album L.) Collected from Various Host Tree Species in an Urban Area
Abstract
1. Introduction
2. Results
2.1. Variation in the Content of Phenolic Compounds
2.2. Variation in the Content of Triterpenic Acids
2.3. Variation in the Content of Organic Acids
2.4. Antioxidant Activity of Mistletoe Extracts
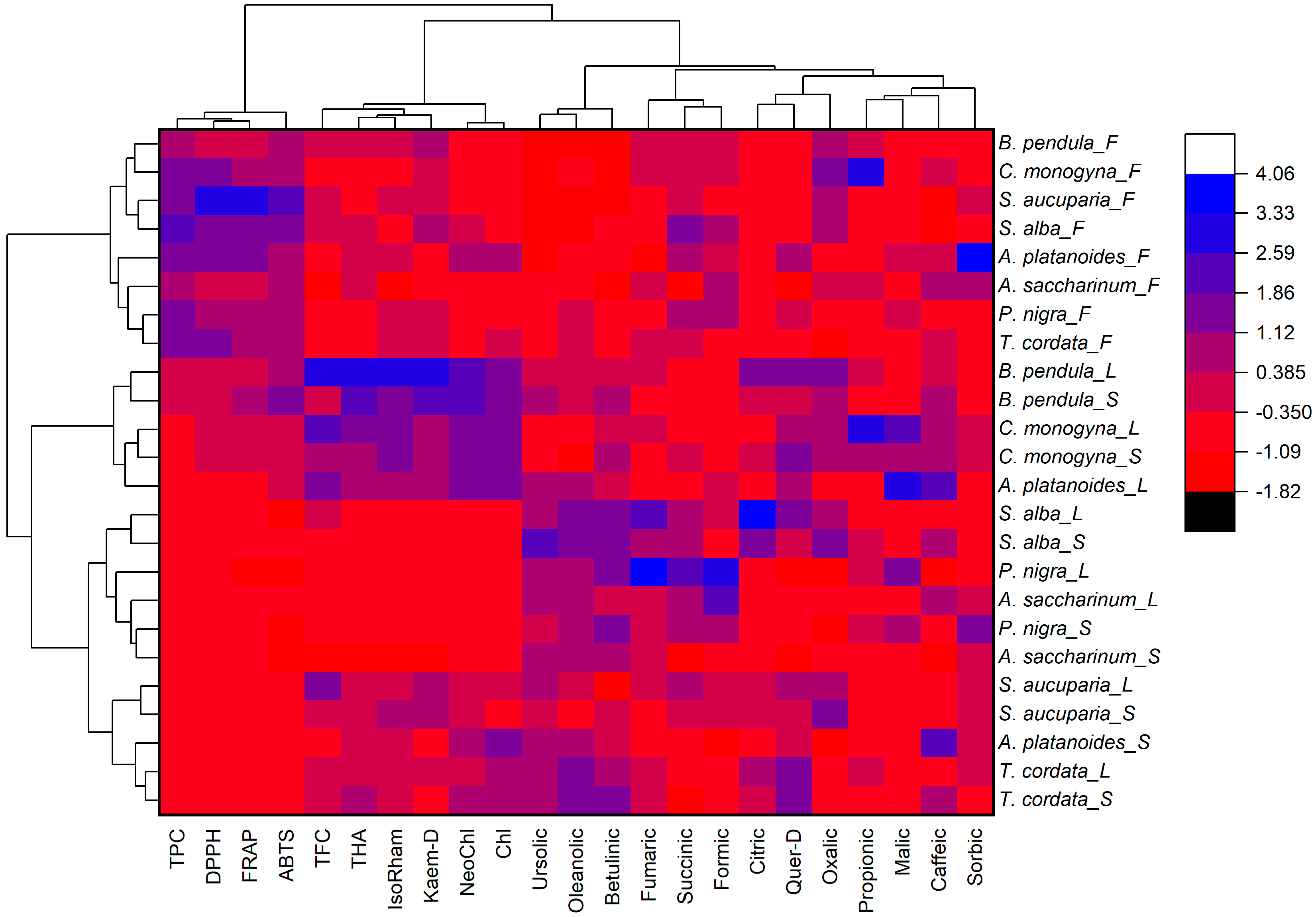
2.5. Heat Map and Cluster Analysis of the Mistletoe Samples and Phytochemical Parameters
3. Discussion
3.1. Impact of Host Tree Species on the Content of Phytochemicals in Mistletoe
3.2. Effect of Mistletoe Organ Type on the Content of Phytochemicals
3.3. Mistletoe as a Resource of Biologically Active Compounds
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Phenolic Compounds
4.2.1. Extract Preparation
4.2.2. Determination of Total Contents of Some Groups of Phenolic Compounds
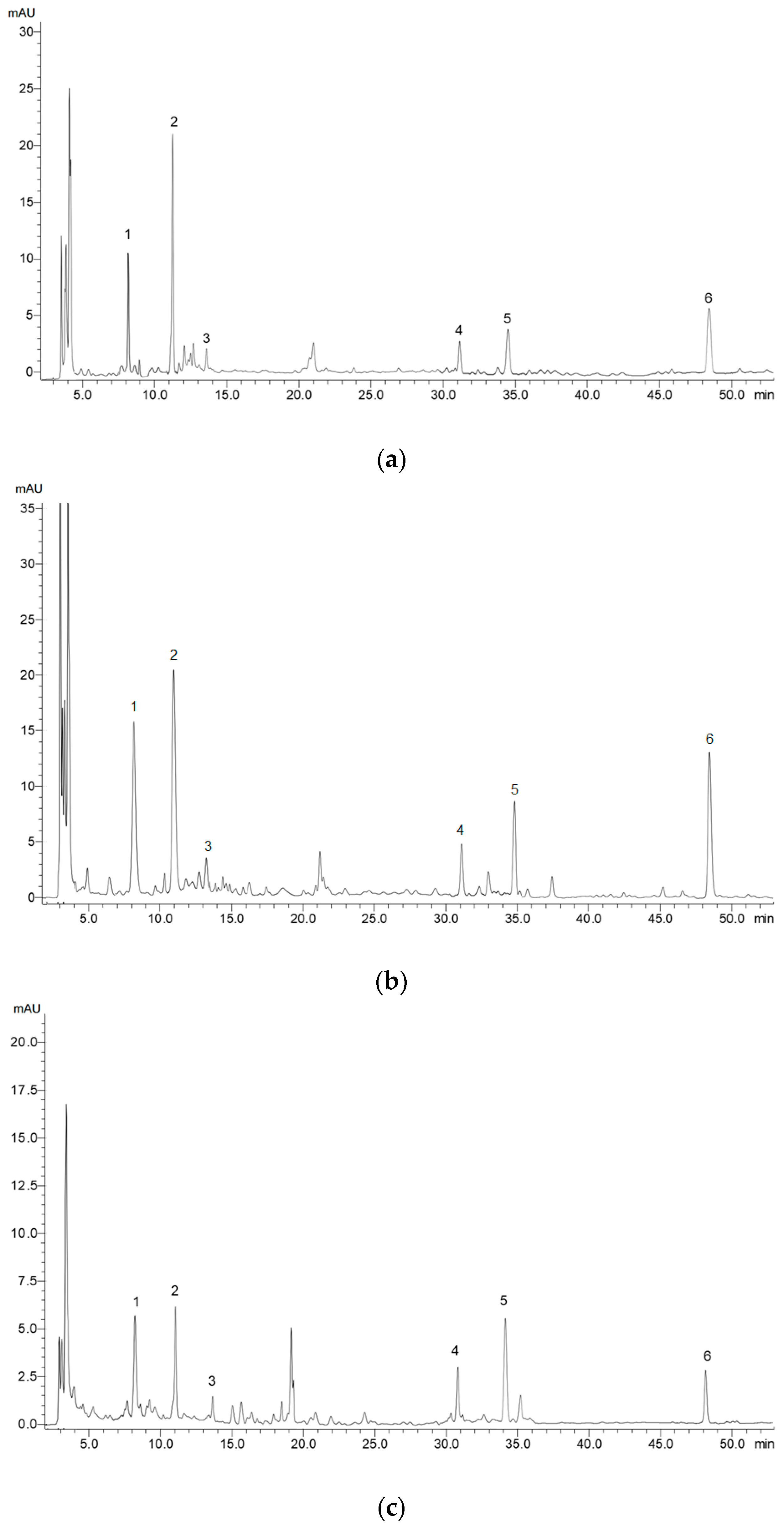
4.2.3. High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) Analysis of Individual Phenolic Compounds
4.3. Determination of Triterpenic Acids
4.3.1. Extract Preparation
4.3.2. High-Performance Thin Layer Chromatographic Analysis of Triterpenic Acids
4.4. Determination of Organic Acids
4.4.1. Extract Preparation
4.4.2. Capillary Electrophoretic Analysis of Organic Acids
4.5. Determination of Antioxidant Activity
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Lech, P.; Żółciak, A.; Hildebrand, R. Occurrence of European Mistletoe (Viscum album L.) on Forest Trees in Poland and Its Dynamics of Spread in the Period 2008–2018. Forests 2020, 11, 83. [Google Scholar] [CrossRef]
- Krasylenko, Y.; Sosnovsky, Y.; Atamas, N.; Popov, G.; Leonenko, V.; Janošíková, K.; Sytschak, N.; Rydlo, K.; Sytnyk, D. The European mistletoe (Viscum album L.): Distribution, host range, biotic interactions, and management worldwide with special emphasis on Ukraine. Botany 2020, 98, 499–516. [Google Scholar] [CrossRef]
- Skrypnik, L.; Maslennikov, P.; Feduraev, P.; Pungin, A.; Belov, N. Ecological and Landscape Factors Affecting the Spread of European Mistletoe (Viscum album L.) in Urban Areas (A Case Study of the Kaliningrad City, Russia). Plants 2020, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J. Mistletoe, Viscum album (Santalaceae), in Britain and Ireland; a discussion and review of current status and trends. Br. Irish Bot. 2021, 3, 419–454. [Google Scholar] [CrossRef]
- Varga, I.; Poczai, P.; Tiborcz, V.; Aranyi, N.R.; Baltazár, T.; Bartha, D.; Pejchal, M.; Hyvönen, J. Changes in the Distribution of European Mistletoe (Viscum album) in Hungary During the Last Hundred Years. Folia Geobot. 2014, 49, 559–577. [Google Scholar] [CrossRef]
- Szmidla, H.; Tkaczyk, M.; Plewa, R.; Tarwacki, G.; Sierota, Z. Impact of Common Mistletoe (Viscum album L.) on Scots Pine Forests—A Call for Action. Forests 2019, 10, 847. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Sikora, K. First report of the occurrence of Sphaeropsis visci on Mistletoe (Viscum album L.) in Poland. Balt. For. 2020, 26, 2–4. [Google Scholar] [CrossRef]
- Kotan, R.; Okutucu, A.; Ala Görmez, A.; Karagoz, K.; Dadasoglu, F.; Karaman, İ.; Hasanekoglu, İ.; Kordali, Ş. Parasitic Bacteria and Fungi on Common Mistletoe (Viscum album L.) and Their Potential Application in Biocontrol. J. Phytopathol. 2013, 161, 165–171. [Google Scholar] [CrossRef]
- Suveren, E.; Baxter, G.F.; Iskit, A.B.; Turker, A.U. Cardioprotective effects of Viscum album L. subsp. album (European misletoe) leaf extracts in myocardial ischemia and reperfusion. J. Ethnopharmacol. 2017, 209, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, Y.N.; Kim, J.-K.; Park, H.-Y.; Song, B.-S. Viscothionin purified from mistletoe (Viscum album var. coloratum Ohwi) induces insulin secretion from pancreatic beta cells. J. Ethnopharmacol. 2019, 234, 172–179. [Google Scholar] [CrossRef]
- Singh, B.N.; Saha, C.; Galun, D.; Upreti, D.K.; Bayry, J.; Kaveri, S. V European Viscum album: A potent phytotherapeutic agent with multifarious phytochemicals, pharmacological properties and clinical evidence. RSC Adv. 2016, 6, 23837–23857. [Google Scholar] [CrossRef]
- Nazaruk, J.; Orlikowski, P. Phytochemical profile and therapeutic potential of Viscum album L. Nat. Prod. Res. 2016, 30, 373–385. [Google Scholar] [CrossRef]
- Mavrikou, S.; Tsekouras, V.; Karageorgou, M.-A.; Moschopoulou, G.; Kintzios, S. Anticancer and biochemical effects of Viscum album L. protein extracts on HeLa cells. Plant Cell, Tissue Organ Cult. 2020, 140, 369–378. [Google Scholar] [CrossRef]
- Tsekouras, V.; Kintzios, S. Biotechnology of Viscum Album L. and Cancer Treatment: A Review. Curr. Bioact. Compd. 2020, 16, 738–746. [Google Scholar] [CrossRef]
- Freuding, M.; Keinki, C.; Micke, O.; Buentzel, J.; Huebner, J. Mistletoe in oncological treatment: A systematic review. J. Cancer Res. Clin. Oncol. 2019, 145, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Thronicke, A.; Schad, F.; Debus, M.; Grabowski, J.; Soldner, G. Viscum album L. Therapy in Oncology: An Update on Current Evidence. Complement. Med. Res. 2022, 29, 362–368. [Google Scholar] [CrossRef]
- Majeed, M.; Hakeem, K.R.; Rehman, R.U. Mistletoe lectins: From interconnecting proteins to potential tumour inhibiting agents. Phytomedicine Plus 2021, 1, 100039. [Google Scholar] [CrossRef]
- Yousefvand, S.; Fattahi, F.; Hosseini, S.M.; Urech, K.; Schaller, G. Viscotoxin and lectin content in foliage and fruit of Viscum album L. on the main host trees of Hyrcanian forests. Sci. Rep. 2022, 12, 10383. [Google Scholar] [CrossRef]
- de Oliveira Melo, M.N.; Oliveira, A.P.; Wiecikowski, A.F.; Carvalho, R.S.; de Lima Castro, J.; de Oliveira, F.A.G.; Pereira, H.M.G.; da Veiga, V.F.; Capella, M.M.A.; Rocha, L.; et al. Phenolic compounds from Viscum album tinctures enhanced antitumor activity in melanoma murine cancer cells. Saudi Pharm. J. 2018, 26, 311–322. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Li, Y.; Xue, X.; Huang, Y.; Luo, H.; Zhang, Y.; Lu, Z. Comparative Evaluation of Soluble and Insoluble-Bound Phenolics and Antioxidant Activity of Two Chinese Mistletoes. Molecules 2018, 23, 359. [Google Scholar] [CrossRef]
- Kleszken, E.; Timar, A.V.; Memete, A.R.; Miere, F.; Vicas, S.I. On Overview Of Bioactive Compounds, Biological And Pharmacological Effects Of Mistletoe (Viscum Album L). Pharmacophore 2022, 13, 10–26. [Google Scholar] [CrossRef]
- Twardziok, M.; Kleinsimon, S.; Rolff, J.; Jäger, S.; Eggert, A.; Seifert, G.; Delebinski, C.I. Multiple Active Compounds from Viscum album L. Synergistically Converge to Promote Apoptosis in Ewing Sarcoma. PLoS ONE 2016, 11, e0159749. [Google Scholar] [CrossRef]
- Strüh, C.M.; Jäger, S.; Schempp, C.M.; Scheffler, A.; Martin, S.F. Solubilized triterpenes from mistletoe show anti-tumor effects on skin-derived cell lines. Planta Med. 2008, 74, PD22. [Google Scholar] [CrossRef]
- Delebinski, C.I.; Twardziok, M.; Kleinsimon, S.; Hoff, F.; Mulsow, K.; Rolff, J.; Jäger, S.; Eggert, A.; Seifert, G. A Natural Combination Extract of Viscum album L. Containing Both Triterpene Acids and Lectins Is Highly Effective against AML In Vivo. PLoS ONE 2015, 10, e0133892. [Google Scholar] [CrossRef]
- Holandino, C.; de Oliveira Melo, M.N.; Oliveira, A.P.; da Costa Batista, J.V.; Capella, M.A.M.; Garrett, R.; Grazi, M.; Ramm, H.; Torre, C.D.; Schaller, G.; et al. Phytochemical analysis and in vitro anti-proliferative activity of Viscum album ethanolic extracts. BMC Complement. Med. Ther. 2020, 20, 215. [Google Scholar] [CrossRef]
- Hanousek Čiča, K.; Lukin, P.; Derewiaka, D.; Mrvčić, J.; Stanzer, D. Chemical Composition, Physical Properties, and Aroma Profile of Ethanol Macerates of Mistletoe (Viscum album). Beverages 2022, 8, 46. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R.; Gawlik-Dziki, U.; Lemieszek, M.K.; Rzeski, W. LC-ESI-MS/MS Identification of Biologically Active Phenolic Compounds in Mistletoe Berry Extracts from Different Host Trees. Molecules 2017, 22, 624. [Google Scholar] [CrossRef]
- Jäger, T.; Holandino, C.; Melo, M.N.d.O.; Peñaloza, E.M.C.; Oliveira, A.P.; Garrett, R.; Glauser, G.; Grazi, M.; Ramm, H.; Urech, K.; et al. Metabolomics by UHPLC-Q-TOF Reveals Host Tree-Dependent Phytochemical Variation in Viscum album L. Plants 2021, 10, 1726. [Google Scholar] [CrossRef]
- Majeed, M.; Pirzadah, T.B.; Mir, M.A.; Hakeem, K.R.; Alharby, H.F.; Alsamadany, H.; Bamagoos, A.A.; Rehman, R.U. Comparative Study on Phytochemical Profile and Antioxidant Activity of an Epiphyte, Viscum album L. (White Berry Mistletoe), Derived from Different Host Trees. Plants 2021, 10, 1191. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R. Impact of Harvest Conditions and Host Tree Species on Chemical Composition and Antioxidant Activity of Extracts from Viscum album L. Molecules 2021, 26, 3741. [Google Scholar] [CrossRef]
- Stefanucci, A.; Zengin, G.; Llorent-Martinez, E.J.; Dimmito, M.P.; Della Valle, A.; Pieretti, S.; Ak, G.; Sinan, K.I.; Mollica, A. Viscum album L. homogenizer-assisted and ultrasound-assisted extracts as potential sources of bioactive compounds. J. Food Biochem. 2020, 44, e13377. [Google Scholar] [CrossRef]
- Díaz-Limón, M.P.; Cano-Santana, Z.; Queijeiro-Bolaños, M.E. Mistletoe infection in an urban forest in Mexico City. Urban For. Urban Green. 2016, 17, 126–134. [Google Scholar] [CrossRef]
- Scalon, M.C.; Rossatto, D.R.; Franco, A.C. How does mistletoe infection affect seasonal physiological responses of hosts with different leaf phenology? Flora 2021, 281, 151871. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Pucek, K.; Szymczak, G.; Kocjan, R.; Luchowski, P. Evaluation of seasonal changes of triterpenic acid contents in Viscum album from different host trees. Pharm. Biol. 2017, 55, 1–4. [Google Scholar] [CrossRef]
- Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Biotransformation of Oleanane and Ursane Triterpenic Acids. Molecules 2020, 25, 5526. [Google Scholar] [CrossRef]
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2020, 29, 17–25. [Google Scholar] [CrossRef]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Zhao, J.-T.; Wang, W.-Q.; Fan, R.-H.; Rong, R.; Yu, Z.-G.; Zhao, Y.-L. Metabolomics-based comparative analysis of the effects of host and environment on Viscum coloratum metabolites and antioxidative activities. J. Pharm. Anal. 2022, 12, 243–252. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef] [PubMed]
- Medda, S.; Dessena, L.; Mulas, M. Monitoring of the PAL Enzymatic Activity and Polyphenolic Compounds in Leaves and Fruits of Two Myrtle Cultivars during Maturation. Agriculture 2020, 10, 389. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, H.; Kang, J.; Warusawitharana, H.K.; Chen, S.; Wang, K.; Yu, F.; Wu, Y.; He, P.; Tu, Y.; et al. Comparative Transcriptome and Phytochemical Analysis Provides Insight into Triterpene Saponin Biosynthesis in Seeds and Flowers of the Tea Plant (Camellia sinensis). Metabolites 2022, 12, 204. [Google Scholar] [CrossRef]
- Soursouri, A.; Hosseini, S.M.; Fattahi, F. Biochemical analysis of European mistletoe (Viscum album L.) foliage and fruit settled on Persian ironwood (Parrotia persica C. A. Mey.) and hornbeam (Carpinus betulus L.). Biocatal. Agric. Biotechnol. 2019, 22, 101360. [Google Scholar] [CrossRef]
- Szakiel, A.; Mroczek, A. Distribution of triterpene acids and their derivatives in organs of cowberry (Vaccinium vitis-idaea L.) plant. Acta Biochim. Pol. 2007, 54, 733–740. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Famiani, F. Organic acids in fruits: Metabolism, functions and contents. Hortic. Rev. (Am. Soc. Hortic. Sci). 2018, 45, 371–430. [Google Scholar] [CrossRef]
- Ochocka, J.R.; Piotrowski, A. Biologically active compounds from European mistletoe (Viscum album L.)1. Can. J. Plant Pathol. 2002, 24, 21–28. [Google Scholar] [CrossRef]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical Screening, Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Štefan, M.B.; Rodríguez, J.V.; Blažeković, B.; Kindl, M.; Vladimir-Knežević, S. Total hydroxycinnamic acids assay: Prevalidation and application on Lamiaceae species. Food Anal. Methods 2014, 7, 326–336. [Google Scholar] [CrossRef]
- Kaur, P.; Gupta, R.C.; Dey, A.; Kumar Pandey, D. Simultaneous quantification of oleanolic acid, ursolic acid, betulinic acid and lupeol in different populations of five Swertia species by using HPTLC-densitometry: Comparison of different extraction methods and solvent selection. Ind. Crops Prod. 2019, 130, 537–546. [Google Scholar] [CrossRef]
- Zipaev, D.V.; Makushin, A.N.; Kuraeva, J.G. Studies of organic acids in millet grain and products of its processing by capillary electrophoresis. Proc. BIO Web Conf. EDP Sci. 2020, 17, 39. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef] [PubMed]
| Factors | Level | TPC, mg GAE g–1 DW | TFC, mg QE g–1 DW | THA, mg CAE g–1 DW |
|---|---|---|---|---|
| Main Effects 1 | ||||
| Host tree species (S) | T. cordata | 11.43 ± 2.01 ab | 0.83 ± 0.16 bcd | 1.43 ± 0.23 bcd |
| A. platanoides | 13.17 ± 2.69 a | 0.97 ± 0.34 abc | 1.64 ± 0.26 bc | |
| A. saccharinum | 9.74 ± 1.63 b | 0.50 ± 0.10 d | 0.99 ± 0.19 d | |
| P. nigra | 10.43 ± 2.15 ab | 0.57 ± 0.10 cd | 0.89 ± 0.11 d | |
| S. alba | 12.57 ± 2.88 a | 0.77 ± 0.18 cd | 1.10 ± 0.20 cd | |
| C. monogyna | 13.44 ± 1.99 a | 1.24 ± 0.46 a | 1.83 ± 0.59 b | |
| S. aucuparia | 12.89 ± 2.69 a | 1.20 ± 0.33 ab | 1.24 ± 0.33 cd | |
| B. pendula | 12.34 ± 1.09 a | 1.26 ± 0.46 a | 2.48 ± 0.64 a | |
| Organ of mistletoe (O) | Stems | 7.16 ± 1.67 b | 0.78 ± 0.22 b | 1.58 ± 0.37 a |
| Leaves | 6.89 ± 1.48 b | 1.21 ± 0.55 a | 1.62 ± 0.42 a | |
| Fruits | 21.96 ± 3.59 a | 0.75 ± 0.17 b | 1.14 ± 0.21 b | |
| Significance | S | <0.001 * | <0.001 * | <0.001 * |
| O | <0.001 * | <0.001 * | <0.001 * | |
| S * O | <0.001 * | <0.001 * | <0.001 * |
| Factors | Level | Content of Individual Phenolic Compounds, mg g–1 DW | |||||
|---|---|---|---|---|---|---|---|
| NeoChl | Chl | Caff | IsoRham | Quer-D | Kaem-D | ||
| Main Effects 1 | |||||||
| Host tree species (S) | T. cordata | 0.23 ± 0.08 bc | 0.30 ± 0.07 bc | 0.032 ± 0.006 b | 0.056 ± 0.006 bcd | 0.10 ± 0.03 a | 0.085 ± 0.007 cde |
| A. platanoides | 0.37 ± 0.05 ab | 0.43 ± 0.06 a | 0.046 ± 0.011 a | 0.059 ± 0.013 bcd | 0.090 ± 0.007 a | 0.089 ± 0.025 bcd | |
| A. saccharinum | 0.10 ± 0.02 cd | 0.18 ± 0.03 d | 0.032 ± 0.012 b | 0.030 ± 0.004 e | 0.047 ± 0.006 c | 0.054 ± 0.007 e | |
| P. nigra | 0.09 ± 0.02 d | 0.11 ± 0.02 d | 0.021 ± 0.003 c | 0.044 ± 0.008 cde | 0.061 ± 0.016 bc | 0.066 ± 0.015 de | |
| S. alba | 0.11 ± 0.03 cd | 0.13 ± 0.03 d | 0.025 ± 0.009 bc | 0.042 ± 0.004 de | 0.084 ± 0.027 ab | 0.081 ± 0.037 cde | |
| C. monogyna | 0.34 ± 0.05 ab | 0.38 ± 0.08 ab | 0.035 ± 0.006 b | 0.074 ± 0.024 ab | 0.089 ± 0.028 ab | 0.12 ± 0.02 b | |
| S. aucuparia | 0.19 ± 0.03 cd | 0.18 ± 0.02 cd | 0.020 ± 0.004 c | 0.063 ± 0.010 bc | 0.083 ± 0.018 ab | 0.11 ± 0.02 bc | |
| B. pendula | 0.41 ± 0.06 a | 0.38 ± 0.06 ab | 0.032 ± 0.009 b | 0.088 ± 0.031 a | 0.090 ± 0.034 a | 0.17 ± 0.04 a | |
| Organ of mistletoe (O) | Stems | 0.28 ± 0.03 a | 0.29 ± 0.03 a | 0.033 ± 0.011 a | 0.059 ± 0.023 ab | 0.084 ± 0.025 a | 0.094 ± 0.05 a |
| Leaves | 0.27 ± 0.03 a | 0.30 ± 0.04 a | 0.031 ± 0.012 ab | 0.065 ± 0.029 a | 0.096 ± 0.031 a | 0.11 ± 0.05 a | |
| Fruits | 0.14 ± 0.01 b | 0.17 ± 0.01 b | 0.026 ± 0.009 b | 0.047 ± 0.008 b | 0.063 ± 0.016 b | 0.090 ± 0.02 a | |
| Significance | S | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| O | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| S * O | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| Factors | Level | Content of Triterpenic Acids, mg g–1 DW | ||
|---|---|---|---|---|
| Ursolic | Oleanolic | Betulinic | ||
| Main Effects 1 | ||||
| Host tree species (S) | T. cordata | 1.09 ± 0.27 a | 6.18 ± 0.89 a | 0.91 ± 0.39 ab |
| A. platanoides | 0.97 ± 0.37 ab | 4.39 ± 1.32 bc | 0.54 ± 0.18 bc | |
| A. saccharinum | 1.03 ± 0.33 ab | 4.50 ± 1.28 bc | 0.63 ± 0.24 bc | |
| P. nigra | 0.84 ± 0.23 ab | 4.96 ± 1.07 b | 1.04 ± 0.39 a | |
| S. alba | 1.12 ± 0.53 a | 4.58 ± 1.17 bc | 0.92 ± 0.26 ab | |
| C. monogyna | 0.64 ± 0.15 b | 2.35 ± 0.18 d | 0.51 ± 0.24 bc | |
| S. aucuparia | 0.80 ± 0.25 ab | 3.18 ± 1.12 cd | 0.27 ± 0.07 c | |
| B. pendula | 0.84 ± 0.35 ab | 2.97 ± 0.96 cd | 0.52 ± 0.21 bc | |
| Organ of mistletoe (O) | Stems | 1.14 ± 0.33 a | 4.77 ± 1.53 a | 0.97 ± 0.35 a |
| Leaves | 1.09 ± 0.15 a | 5.02 ± 1.47 a | 0.80 ± 0.27 b | |
| Fruits | 0.53 ± 0.11 b | 2.62 ± 0.89 b | 0.24 ± 0.08 c | |
| Significance | S | <0.001 * | <0.001 * | <0.001 * |
| O | <0.001 * | <0.001 * | <0.001 * | |
| S * O | <0.001 * | <0.001 * | <0.001 * | |
| Factors | Level | Content of Organic Acids, mg g–1 DW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Succinic | Citric | Oxalic | Formic | Fumaric | Propionic | Malic | Sorbic | ||
| Main Effects 1 | |||||||||
| Host tree species (S) | T. cordata | 3.09 ± 0.47 c | 1.27 ± 0.26 c | 0.29 ± 0.04 bc | 1.11 ± 0.27 b | 0.019 ± 0.001 bc | 0.23 ± 0.09 c | - | 0.047 ± 0.011 c |
| A. platanoides | 4.02 ± 0.52 bc | 1.71 ± 0.66 c | 0.26 ± 0.03 bc | 0.19 ± 0.01 c | 0.007 ± 0.001 c | - | 1.66 ± 0.33 a | 0.63 ± 0.08 a | |
| A. saccharinum | 3.32 ± 1.04 c | 2.97 ± 0.43 ab | 0.38 ± 0.10 b | 0.26 ± 0.06 c | 0.021 ± 0.004 b | 0.37 ± 0.07 bc | - | 0.30 ± 0.04 b | |
| P. nigra | 8.07 ± 0.68 a | 3.75 ± 0.36 a | 0.23 ± 0.07 c | 0.22 ± 0.01 c | 0.036 ± 0.009 a | 0.81 ± 0.19 b | 1.49 ± 0.26 a | 0.27 ± 0.05 b | |
| S. alba | 8.39 ± 0.47 a | 2.16 ± 0.46 bc | 0.90 ± 0.10 a | 3.10 ± 0.49 a | 0.031 ± 0.010 ab | 0.15 ± 0.04 c | - | - | |
| C. monogyna | 5.35 ± 0.95 bc | 1.65 ± 0.18 c | 0.92 ± 0.06 a | 0.44 ± 0.07 bc | 0.019 ± 0.004 bc | 3.66 ± 1.47 a | 1.75 ± 0.37 a | 0.061 ± 0.006 c | |
| S. aucuparia | 5.99 ± 1.20 ab | 1.94 ± 0.10 c | 0.90 ± 0.10 a | 0.83 ± 0.27 b | 0.016 ± 0.005 bc | - | - | 0.16 ± 0.02 bc | |
| B. pendula | 4.34 ± 1.21 bc | 1.53 ± 0.16 c | 0.94 ± 0.07 a | 1.33 ± 0.62 b | 0.017 ± 0.003 bc | 0.48 ± 0.11 bc | - | - | |
| Organ of mistletoe (O) | Stems | 4.16 ± 0.42 b | 1.51 ± 0.12 b | 0.60 ± 0.09 a | 0.89 ± 0.22 b | 0.017 ± 0.006 b | 0.54 ± 0.18 b | 0.35 ± 0.09 b | 0.20 ± 0.04 a |
| Leaves | 5.94 ± 0.49 a | 2.52 ± 0.27 a | 0.57 ± 0.05 a | 1.62 ± 0.59 a | 0.031 ± 0.012 a | 1.13 ± 0.37 a | 1.39 ± 0.34 a | 0.075 ± 0.009 b | |
| Fruits | 5.86 ± 0.46 a | 2.33 ± 0.51 a | 0.64 ± 0.06 a | 0.30 ± 0.06 c | 0.015 ± 0.007 b | 1.18 ± 0.21 a | - | 0.27 ± 0.09 a | |
| Significance | S | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| O | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| S * O | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| Factors | Level | Antioxidant Activity, mg TE g–1 | ||
|---|---|---|---|---|
| DPPH | ABTS | FRAP | ||
| Main Effects 1 | ||||
| Host tree species (S) | T. cordata | 5.63 ± 1.11 a | 18.72 ± 3.07 bc | 7.82 ± 1.14 ab |
| A. platanoides | 5.74 ± 1.02 a | 19.86 ± 2.76 abc | 7.67 ± 1.28 ab | |
| A. saccharinum | 2.58 ± 0.33 b | 16.80 ± 2.32 bc | 4.79 ± 0.61 b | |
| P. nigra | 4.14 ± 0.99 ab | 14.28 ± 3.06 c | 5.74 ± 1.29 b | |
| S. alba | 4.90 ± 1.41 ab | 19.5 ± 3.89 abc | 7.89 ± 1.67 ab | |
| C. monogyna | 6.23 ± 0.91 a | 21.85 ± 1.86 ab | 9.24 ± 0.85 a | |
| S. aucuparia | 6.67 ± 1.88 a | 20.50 ± 4.61 abc | 10.11 ± 2.36 a | |
| B. pendula | 4.66 ± 0.17 ab | 25.26 ± 2.34 a | 9.52 ± 0.66 a | |
| Organ of mistletoe (O) | Stems | 2.82 ± 0.17 b | 17.30 ± 2.13 b | 6.08 ± 0.51 b |
| Leaves | 2.77 ± 0.20 b | 16.31 ± 4.36 b | 4.34 ± 0.27 b | |
| Fruits | 9.62 ± 1.61 a | 25.16 ± 3.07 a | 13.12 ± 0.69 a | |
| Significance | S | <0.001 * | <0.001 * | <0.001 * |
| O | <0.001 * | <0.001 * | <0.001 * | |
| S * O | <0.001 * | <0.001 * | <0.001 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrypnik, L.; Feduraev, P.; Golovin, A.; Maslennikov, P.; Belov, N.; Matveev, M.; Pungin, A. Biotechnological Potential of Different Organs of Mistletoe (Viscum album L.) Collected from Various Host Tree Species in an Urban Area. Plants 2022, 11, 2686. https://doi.org/10.3390/plants11202686
Skrypnik L, Feduraev P, Golovin A, Maslennikov P, Belov N, Matveev M, Pungin A. Biotechnological Potential of Different Organs of Mistletoe (Viscum album L.) Collected from Various Host Tree Species in an Urban Area. Plants. 2022; 11(20):2686. https://doi.org/10.3390/plants11202686
Chicago/Turabian StyleSkrypnik, Liubov, Pavel Feduraev, Anton Golovin, Pavel Maslennikov, Nikolay Belov, Matvei Matveev, and Artem Pungin. 2022. "Biotechnological Potential of Different Organs of Mistletoe (Viscum album L.) Collected from Various Host Tree Species in an Urban Area" Plants 11, no. 20: 2686. https://doi.org/10.3390/plants11202686
APA StyleSkrypnik, L., Feduraev, P., Golovin, A., Maslennikov, P., Belov, N., Matveev, M., & Pungin, A. (2022). Biotechnological Potential of Different Organs of Mistletoe (Viscum album L.) Collected from Various Host Tree Species in an Urban Area. Plants, 11(20), 2686. https://doi.org/10.3390/plants11202686










