In Vitro Sprouted Plantlets of Citrullus colocynthis (L.) Schrad Shown to Possess Interesting Levels of Cucurbitacins and Other Bioactives against Pathogenic Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.1.1. C. colocynthis Seeds Provenience
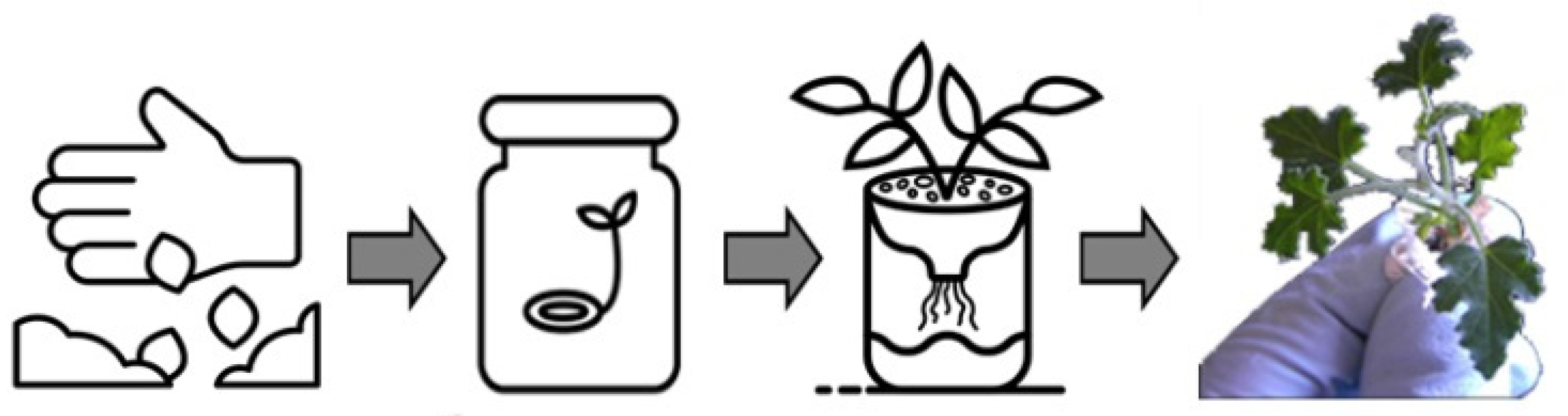
2.1.2. Seeds Germination and Plantlets Achievement
2.2. Preparation of Crude Extracts
2.3. Crude Extracts Characterization
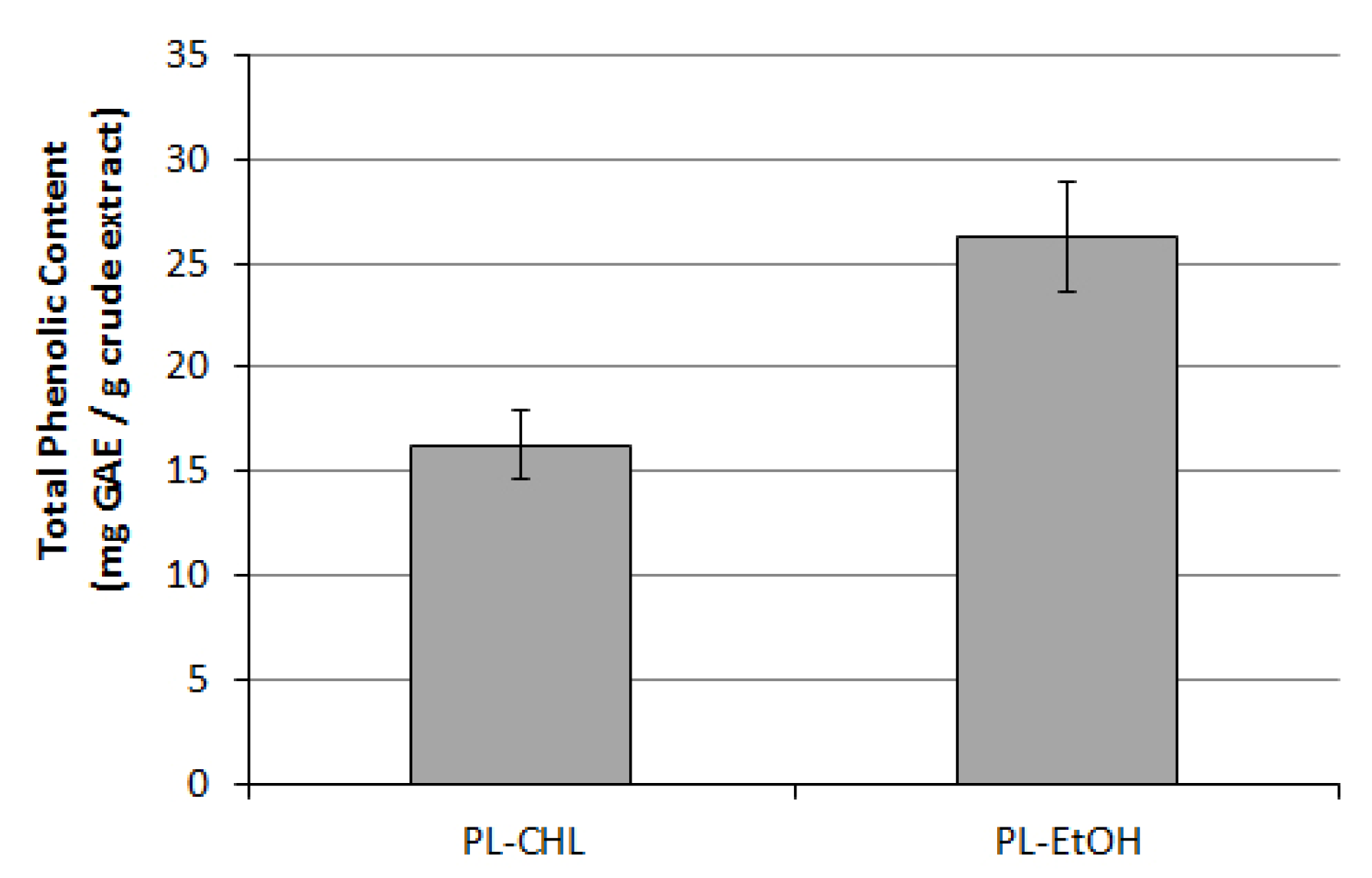
2.3.1. Determination of Total Phenolics Content
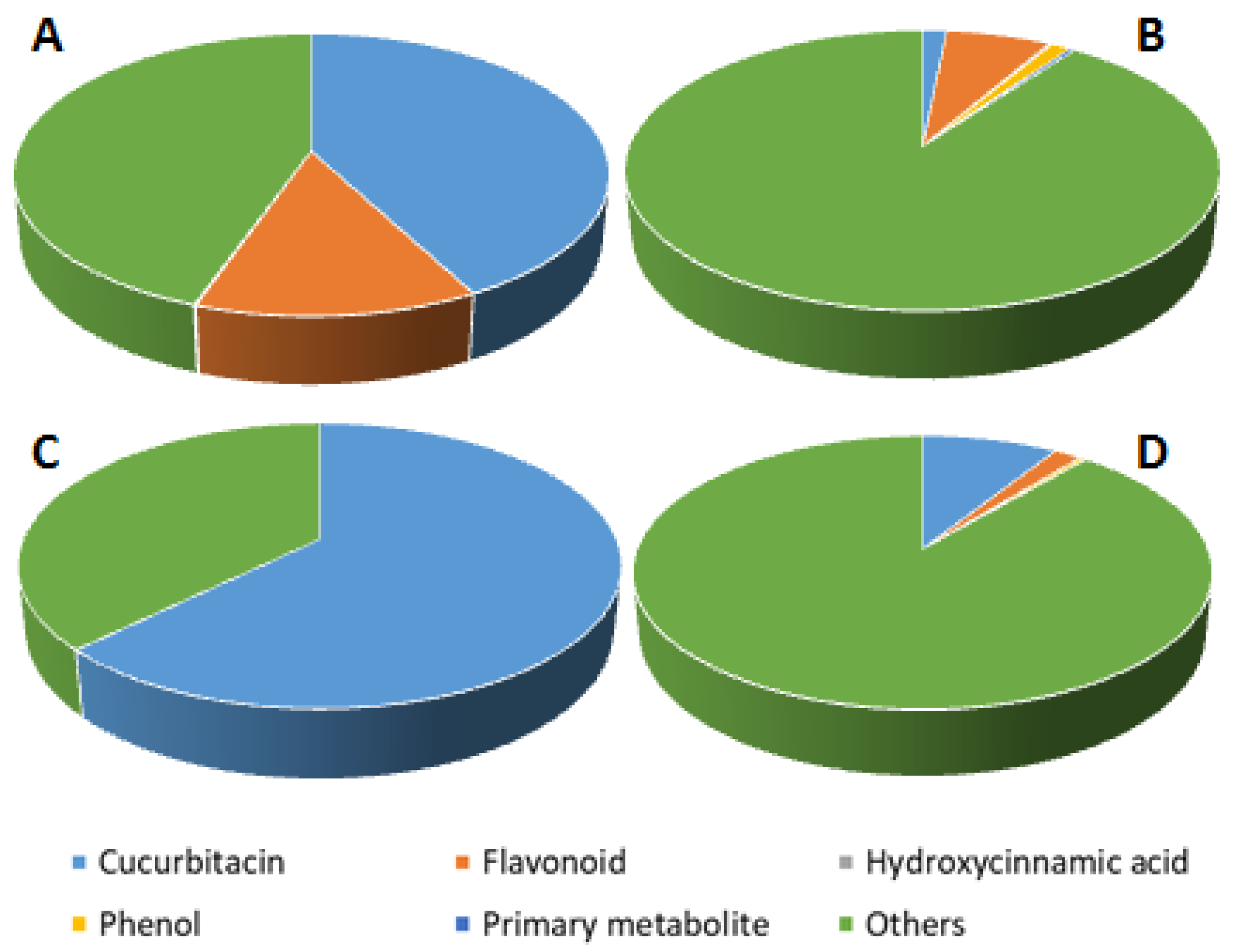
2.3.2. Phytochemical Characterization
2.4. Microorganisms and Biological Assays
2.4.1. Fungal Strains
2.4.2. Agar Well Diffusion Assay for Antifungal Activity Evaluation on Candida spp.
2.4.3. Radial Growth Assay for Antifungal Activity Evaluation on A. flavus Aflatoxigenic Strain
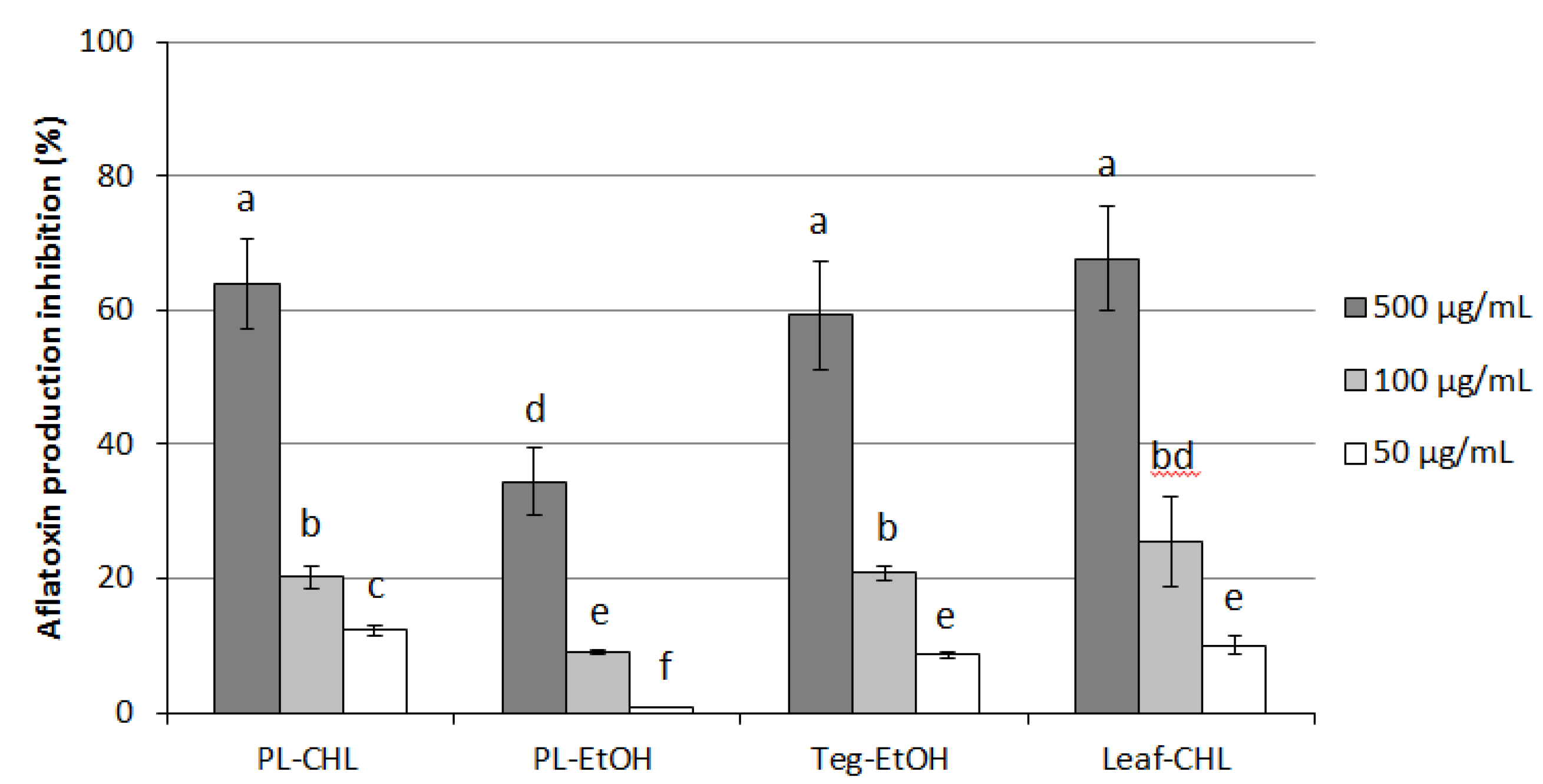
2.4.4. Multiwell Assay for Anti-Aflatoxigenic Activity Evaluation
2.5. Statistical Analysis
3. Results
3.1. Seed Germination In Vitro and Hydroponic Seedlings Cultivation for Extracts Production
3.2. Antioxidative and Phytochemical Characterization of C. colocynthis Extracts
3.3. Antifungal and Anti-Aflatoxigenic Activity of Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Centre for Traditional Medicine. Available online: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine (accessed on 5 May 2022).
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Kuzel, J.; Vydra, N.T.; Vrchotova, M.; Cigler, P. Elucidation of pharmacologically active substance in an intact medicinal plant. J. Agric. Food Chem. 2009, 57, 7909–7911. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defense mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Sudha, G.; Ravishankar, G.A. Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tissue Organ Cult. 2002, 71, 181–212. [Google Scholar] [CrossRef]
- Bairu, M.W.; Amoo, S.O.; van Staden, J. Comparative phytochemical analysis of wild and in vitro-derived greenhouse-grown tubers, in vitro shoots and callus-like basal tissues of Harpagophytum Procumbens. South Afr. J. Bot. 2011, 77, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.I.; Rathore, H.A.; Sattar, M.Z.A.; Chatha, S.A.S.; Sarker, S.D.; Gilani, A.H. Citrullus colocynthis (L.) Schrad (bitter apple fruit): A review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J. Ethnopharmacol. 2014, 155, 54–66. [Google Scholar]
- Saganuwan, A.S. Some medicinal plants of arabian peninsula. J. Med. Plant Res. 2010, 4, 766–788. [Google Scholar]
- Akbar, S. Citrullus colocynthis (L.) schrad. (cucurbitaceae). In Handbook of 200 Medicinal Plants; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Marzouk, B.; Marzouk, Z.; Décor, R.; Edziri, H.; Haloui, E.; Fenina, N.; Aouni, M. Antibacterial and anticandidal screening of Tunisian Citrullus colocynthis schrad. from Medenine. J. Ethnopharmacol. 2009, 125, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, I.; Peivastegan, B.; Kolahi, M. Antifungal activity of nettle (Urtica dioica L.), colocynth (Citrullus colocynthis L. Schrad), oleander (Nerium oleander L.) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pak. J. Biol. Sci. 2009, 12, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degola, F.; Marzouk, B.; Gori, A.; Brunetti, C.; Dramis, L.; Gelati, S.; Buschini, A.; Restivo, F.M. Aspergillus flavus as a Model System to Test the Biological Activity of Botanicals: An Example on Citrullus colocynthis L. Schrad. Organic Extracts. Toxins 2019, 11, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussi, F.; Montalbano, S.; Marzouk, B.; Arru, L.; Refifa, M.; Marzouk, Z.; Kraiem, J.; Degola, F.; Buschini, A. Potential of Citrullus colocynthis L. Schrad. Immature Seed Extracts as Food Preservative against a Fungal Mycotoxigenic Contaminant. J. Food Qual. 2021, 2021, 4470643. [Google Scholar] [CrossRef]
- Jayaraman, R.; Shivakumar, A.; Anitha, T.A.; Joshi, V.D.; Palei, N.N. Antidiabetic effect of petroleum ether extract of Citrullus colocynthis fruita against Streptozotocin-induced hyperglycemic rats. Plant Biol. 2009, 54, 127–134. [Google Scholar]
- Najafi, S.; Sanadgol, N.; Nejad, B.S.; Beiragi, M.A.; Sanadgol, E. Phytochemical screening and antibacterial activity of Citrullus colocynthis (Linn.) Schrad against Staphylococcus aureus. J. Med. Plants Res. 2010, 4, 2321–2325. [Google Scholar]
- Salama, H.M. Alkaloids and flavonoids from the air dried aerial parts of Citrullus colocynthis. J. Med. Plants Res. 2012, 6, 5150–5155. [Google Scholar]
- Delazar, A.; Gibbons, S.; Kosari, A.R.; Nazemiyeh, H.; Modarresi, M.; Nahar, L.; Sarker, S.D. Flavone C-glycosides and cucurbitacin glycosides from Citrullus colocynthis. DARU J. Pharm. Sci. 2006, 14, 109–114. [Google Scholar]
- Ogbuji, K.; McCutcheon, G.S.; Simmons, A.M.; Snook, M.E.; Harrison, H.F.; Levi, A. Partial Leaf Chemical Profiles of a Desert Watermelon Species (Citrullus colocynthis) and Heirloom Watermelon Cultivars (Citrullus lanatus var. lanatus). Hort. Sci. 2012, 47, 580–584. [Google Scholar] [CrossRef]
- Chawech, R.; Jarraya, R.; Girardi, C.; Vansteelandt, M.; Marti, G.; Nasri, I.; Racaud-Sultan, C.; Fabre, N. Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad. Molecules 2015, 20, 18001–18015. [Google Scholar] [CrossRef] [Green Version]
- Gacem, M.A.; Oul El Hadj, K.-A.; Gacemi, B. Evaluation of antifungal effect of organic extracts of Algerian Citrullus colocynthis seeds against four strains of Aspergillus isolate from wheat stored. J. Med. Plants Res. 2013, 7, 727–733. [Google Scholar]
- Devendra, N.K.; Everaldo, A.; Raghunandan, D.; Seetharam, Y.N. In vitro production of Cucurbitacins from Trichosanthes cucumerina L. var. cucumerina. Adv. Life Sci. 2012, 2, 108–111. [Google Scholar] [CrossRef]
- Dasari, R.; Gopu, C.; Vankudoth, S.; Dharavath, S.; Taduri, S. Enhancement of production of pharmaceutically important anti-cancerous compound; cucurbitacin E via elicitation and precursor feeding of in vitro culture of Citrullus colocynthis (L.) Schard. Vegetos 2020, 33, 323–334. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pottier-Alapetite, G. Flore De La Tunisie, Angiospermes-Dicotylédones: Gamopétales; Imprimerie Officielle de la République Tunisienne: Tunis, Tunisia, 1981; p. 930. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Commisso, M.; Negri, S.; Bianconi, M.; Gambini, S.; Avesani, S.; Ceoldo, S.; Avesano, L.; Guzzo, F. Untargeted and targeted metabolomics and tryptophan decarboxylase in vivo characterization provide novel insight on the development of kiwifruits (Actinidia deliciosa). Int. J. Mol. Sci. 2019, 20, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shawkey, A.M.; Rabeh, M.A.; Abdellatif, O.A. Biofunctional molecules from Citrullus colocynthis: An HPLC/MS analysis in correlation to antimicrobial and anticancer activities. Adv. Life Sci. Technol. 2014, 17, 51–61. [Google Scholar]
- Haq, F.U.; Ali, A.; Khan, M.N.; Shah, S.M.Z.; Kandel, R.C.; Aziz, N.; Adhikari, A.; Choudhary, M.I.; Ur-Rahman, A.; El-Seedi, H.R.; et al. Metabolite profiling and quantitation of cucurbitacins in Cucurbitaceae plants by liquid chromatography coupled to tandem mass spectrometry. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Bartoli, J.; Montalbano, S.; Spadola, G.; Rogolino, D.; Pelosi, G.; Bisceglie, F.; Restivo, F.M.; Degola, F.; Serra, O.; Buschini, A.; et al. Antiaflatoxigenic thiosemicarbazones as crop protective agents: A cytotoxic and genotoxic study. J. Agric. Food. Chem. 2019, 67, 10947–10953. [Google Scholar] [CrossRef]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Degola, F.; Dall’Asta, C.; Restivo, F.M. Development of a simple and high-throughput method for detecting aflatoxins production in culture media. Lett. Appl. Microbiol. 2012, 55, 82–89. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Paul, J.J.P.; Jeyachandran, R. Micropropagation of Citrullus colocynthis (L.) Schrad-an important medicinal plant. Ind. J. Bot. Res. 2008, 4, 183–188. [Google Scholar]
- Tariq, A.; Afrasiab, H.; Farhat, F. In vitro Micropropagation of Citrullus colocynthis (L.) Schrad: An endangered medicinal plant. Adv. Life Sci. 2020, 8, 52–56. [Google Scholar]
- Saberi, M.; Shahriari, A.; Tarnian, F.; Noori, S. Comparison the Effect of Different Treatments for Breaking Seed Dormancy of Citrullus colocynthis. J. Agricult. Sci. 2011, 3, 62. [Google Scholar] [CrossRef]
- Meena, M.C.; Meena, R.; Patni, V. High frequency plant regeneration from shoot tip explants of Citrullus colocynthis (Linn.) Schrad–An important medicinal herb. African J. Biotech. 2010, 9, 5037–5041. [Google Scholar]
- Satyavani, K.; Ramanathan, T.; Gurudeeban, S. Effect of plant growth regulators on callus induction and plantlet regeneration of Bitter Apple (Citrullus colocynthis) from stem explant. Asian J. Biotech. 2011, 3, 246–253. [Google Scholar] [CrossRef]
- Shrivastava, A.; Roy, S. Plant regeneration from the nodal part of Citrullus colocynthis (L.) Schrad. Int. J. Pharm. Sci. Res. 2011, 2, 2825–2827. [Google Scholar]
- Toker, G.; Memişoğlu, M.; Toker, M.C.; Yeşilada, E. Callus formation and cucurbitacin B accumulation in Ecballium elaterium callus cultures. Fitoterapia 2003, 74, 618–623. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Garg, V.K.; Buttar, H.S.; Setzer, W.N.; Sethi, G. Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. J. Funct. Foods 2018, 48, 457–471. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary luteolin: A narrative review focusing on its pharmacokinetic properties and effects on glycolipid metabolism. J. Agric. Food Chem. 2021, 69, 1441–1454. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Maja, D.; Mavengahama, S.; Mashilo, J. Cucurbitacin biosynthesis in cucurbit crops, their pharmaceutical value and agricultural application for management of biotic and abiotic stress: A review. South Afr. J. Bot. 2022, 145, 3–12. [Google Scholar] [CrossRef]
- Bartalis, J.; Halaweish, F.T. Relationship between cucurbitacins reversed-phase high-performance liquid chromatography hydrophobicity index and basal cytotoxicity on HepG2 cells. J. Chromatogr. B 2005, 818, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Raman, G.; Cho, M.; Brodbelt, J.S.; Patil, B.S. Isolation and purification of closely related Citrus limonoid glucosides by flash chromatography. Phytochemical Analysis: Int. J. Plant Chem. Biochem. Tech. 2005, 16, 155–160. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Grossman, S.; Dovrat, S.; Gottlieb, H.E.; Bergman, M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem. Pharmacol. 2007, 73, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.Z.; Mohamed, G.A.; Abdel-Naim, A.B.; Hasan, A.; Abdel-Lateff, A. Cucurbitacin E glucoside from Citrullus colocynthis inhibits testosterone-induced benign prostatic hyperplasia in mice. Drug Chem. Toxicol. 2021, 44, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Sajid, A.R.; Javeed, A.; Aslam, M.; Ahsan, T.; Hussain, D.; Mateen, A.; Li, X.; Qin, P.; Ji, M. Antioxidant, antifungal, and aphicidal activity of the triterpenoids spinasterol and 22,23-dihydrospinasterol from leaves of Citrullus colocynthis L. Sci. Rep. 2022, 12, 4910. [Google Scholar] [CrossRef]
- Degola, F.; Bisceglie, F.; Pioli, M.; Palmano, S.; Elviri, L.; Pelosi, G.; Lodi, T.; Restivo, F.M. Structural modification of cuminaldehyde thiosemicarbazone increases inhibition specificity toward aflatoxin biosynthesis and sclerotia development in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2017, 101, 6683–66961. [Google Scholar] [CrossRef] [PubMed]
- Orsoni, N.; Degola, F.; Nerva, L.; Bisceglie, F.; Spadola, G.; Chitarra, W.; Terzi, V.; Delbono, S.; Ghizzoni, R.; Morcia, C.; et al. Double Gamers-Can Modified Natural Regulators of Higher Plants Act as Antagonists against Phytopathogens? The Case of Jasmonic Acid Derivatives. Int. J. Mol. Sci. 2020, 21, 8681. [Google Scholar] [CrossRef]
- Montalbano, S.; Degola, F.; Bartoli, J.; Bisceglie, F.; Buschini, A.; Carcelli, M.; Feretti, D.; Galati, S.; Marchi, L.; Orsoni, N.; et al. The AFLATOX® Project: Approaching the Development of New Generation, Natural-Based Compounds for the Containment of the Mycotoxigenic Phytopathogen Aspergillus flavus and Aflatoxin Contamination. Int. J. Mol. Sci. 2021, 22, 4520. [Google Scholar] [CrossRef]
- Bhatnagar, D.; McCormick, S.P. The inhibitory effect of neem (Azadirachta indica) leaf extracts on aflatoxin synthesis in Aspergillus parasiticus. J. Am. Oil Chem. Soc. 1988, 65, 1166–1168. [Google Scholar] [CrossRef]
- Gacem, M.A.; Khelil, A.; Gacemi, B.; Halla, N.; Djerbaoui, A.N.; Bouderhem, A.; Hadef, S.; Benreguieg, M.; Adli, D.E.H. Antimycotoxigenic and antifungal activities of Citrullus colocynthis seeds against Aspergillus flavus and Aspergillus ochraceus contaminating wheat stored. Afr. J. Biotechnol. 2013, 12, 6222–6231. [Google Scholar] [CrossRef]
- Shafaei, H.; Rad, J.S.; Delazar, A.; Behjati, M. The effect of pulp and seed extract of Citrullus colocynthis, as an antidiabetic medicinal herb, on hepatocytes glycogen stores in diabetic rabbits. Adv. Biomed. Res. 2014, 3, 258. [Google Scholar] [CrossRef]
- Bourhia, M.; Messaoudi, M.; Bakrim, H.; Mothana, R.A.; Sddiqui, N.A.; Almarfadi, O.M.; el Mzibri, M.; Gmouh, S.; Laglaoui, A.; Benbacer, L. Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities. Open Chem. 2020, 18, 986–994. [Google Scholar] [CrossRef]

| Candida spp. Strain | PL-CHL | PL-EtOH | Amphotericyn B |
|---|---|---|---|
| C. krusei ATCC 6258 | 14.5 ± 0.5 b | 12.5 ± 0.6 c | 18.3 ± 1.7 a |
| C. albicans ATCC 90028 | 19.0 ± 1.0 a | 29.0 ± 1.0 d | 20.0 ± 0.5 a |
| C. parapsilosis ATCC 22019 | 19.7 ± 1.5 a | 12.3 ± 0.6 c | 15.2 ± 1.3 b |
| C. glabrata ATCC 90030 | 14.7 ± 0.6 b | 15.0 ± 1.0 b | 21.7 ± 1.0 a |
| PL-CHL | PL-EtOH | Teg-EtOH | Leaf-CHL | CNT |
|---|---|---|---|---|
| 50.3 ± 2.8 a | 49.4 ± 1.6 a | 47.0 ± 1.7 a | 46.5 ± 1.5 a | 44.9 ± 3.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzouk, B.; Refifà, M.; Montalbano, S.; Buschini, A.; Negri, S.; Commisso, M.; Degola, F. In Vitro Sprouted Plantlets of Citrullus colocynthis (L.) Schrad Shown to Possess Interesting Levels of Cucurbitacins and Other Bioactives against Pathogenic Fungi. Plants 2022, 11, 2711. https://doi.org/10.3390/plants11202711
Marzouk B, Refifà M, Montalbano S, Buschini A, Negri S, Commisso M, Degola F. In Vitro Sprouted Plantlets of Citrullus colocynthis (L.) Schrad Shown to Possess Interesting Levels of Cucurbitacins and Other Bioactives against Pathogenic Fungi. Plants. 2022; 11(20):2711. https://doi.org/10.3390/plants11202711
Chicago/Turabian StyleMarzouk, Belsem, Meher Refifà, Serena Montalbano, Annamaria Buschini, Stefano Negri, Mauro Commisso, and Francesca Degola. 2022. "In Vitro Sprouted Plantlets of Citrullus colocynthis (L.) Schrad Shown to Possess Interesting Levels of Cucurbitacins and Other Bioactives against Pathogenic Fungi" Plants 11, no. 20: 2711. https://doi.org/10.3390/plants11202711





