UAV Image-Based Crop Growth Analysis of 3D-Reconstructed Crop Canopies
Abstract
:1. Introduction
2. Results and Discussion
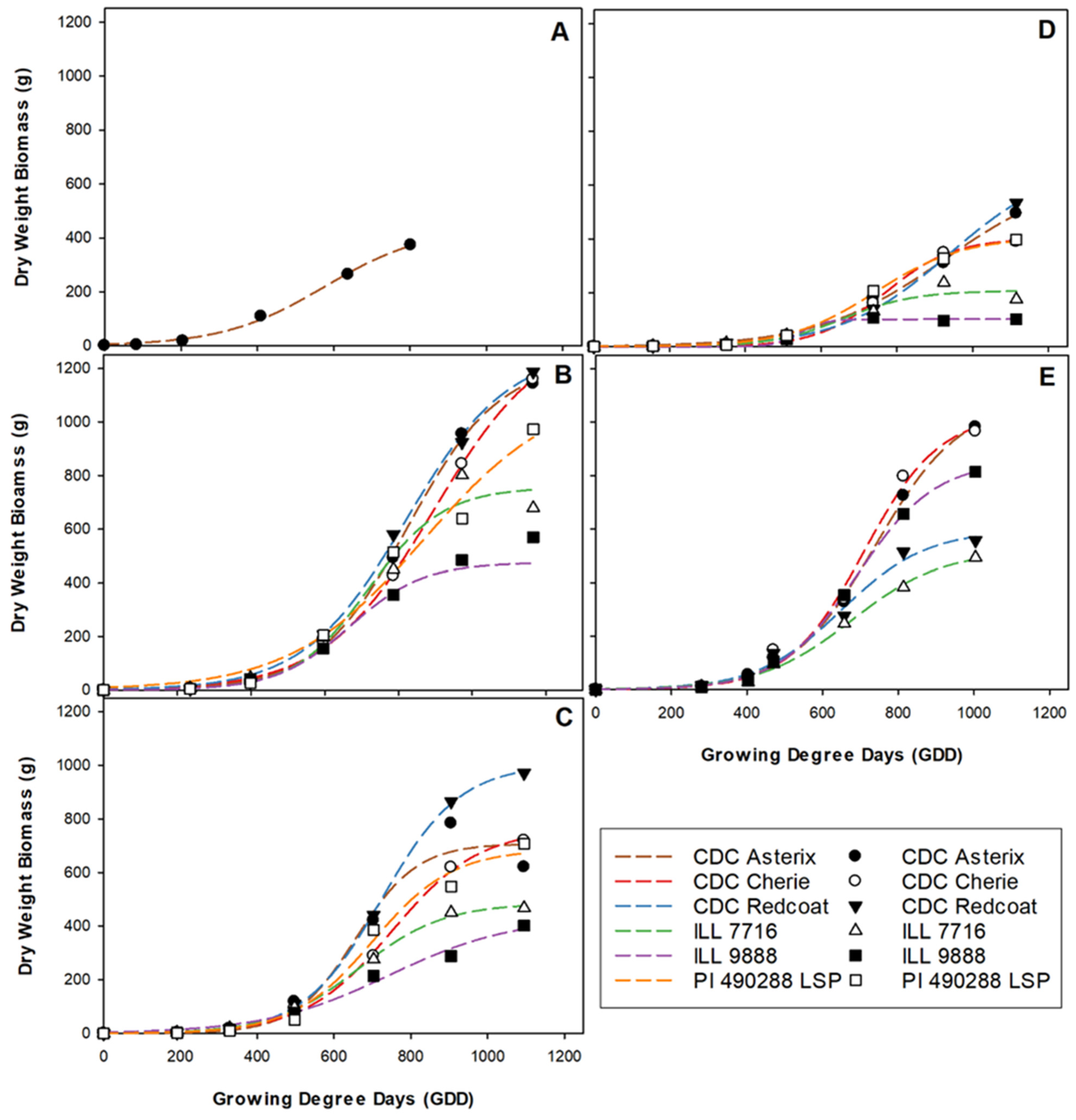
2.1. Ground-Measured Data
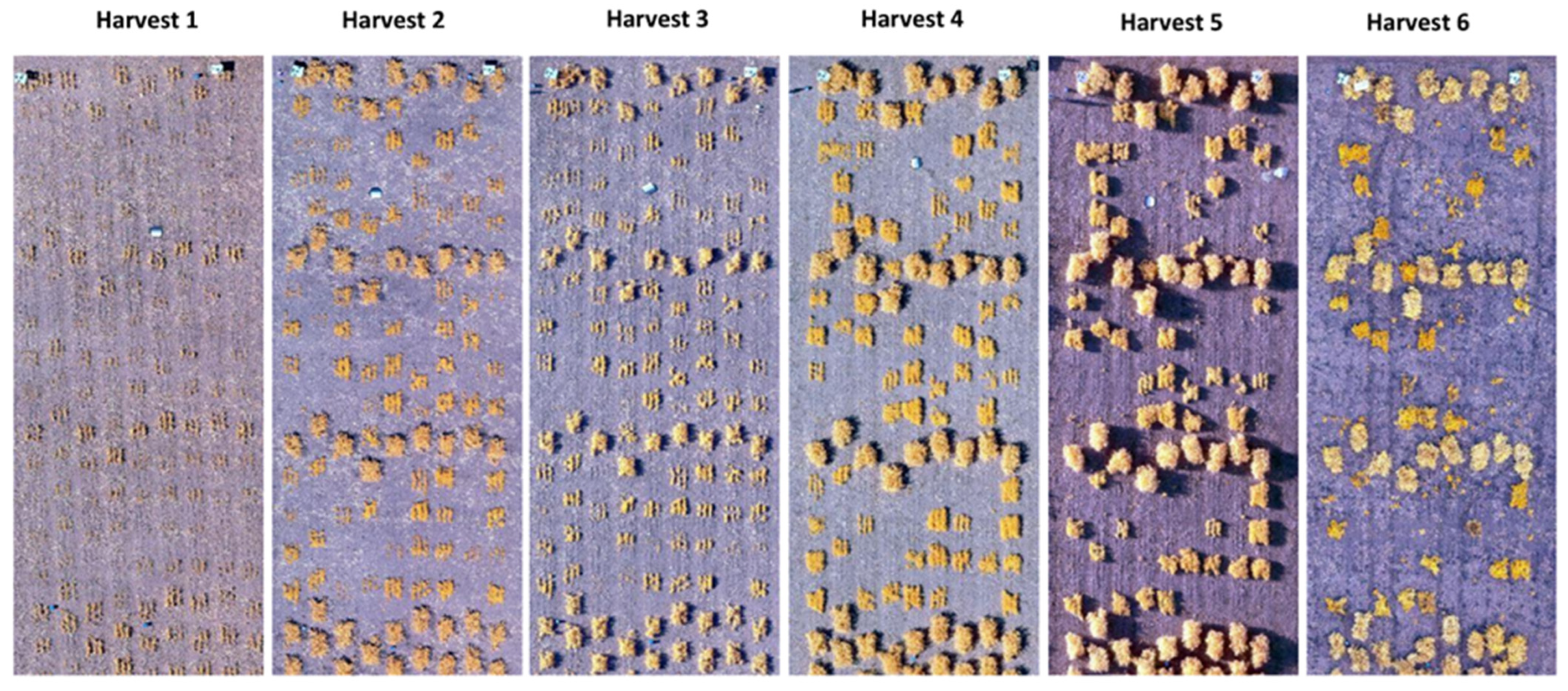
2.2. Two-Dimensional Analysis
2.3. Three-Dimensional Analysis
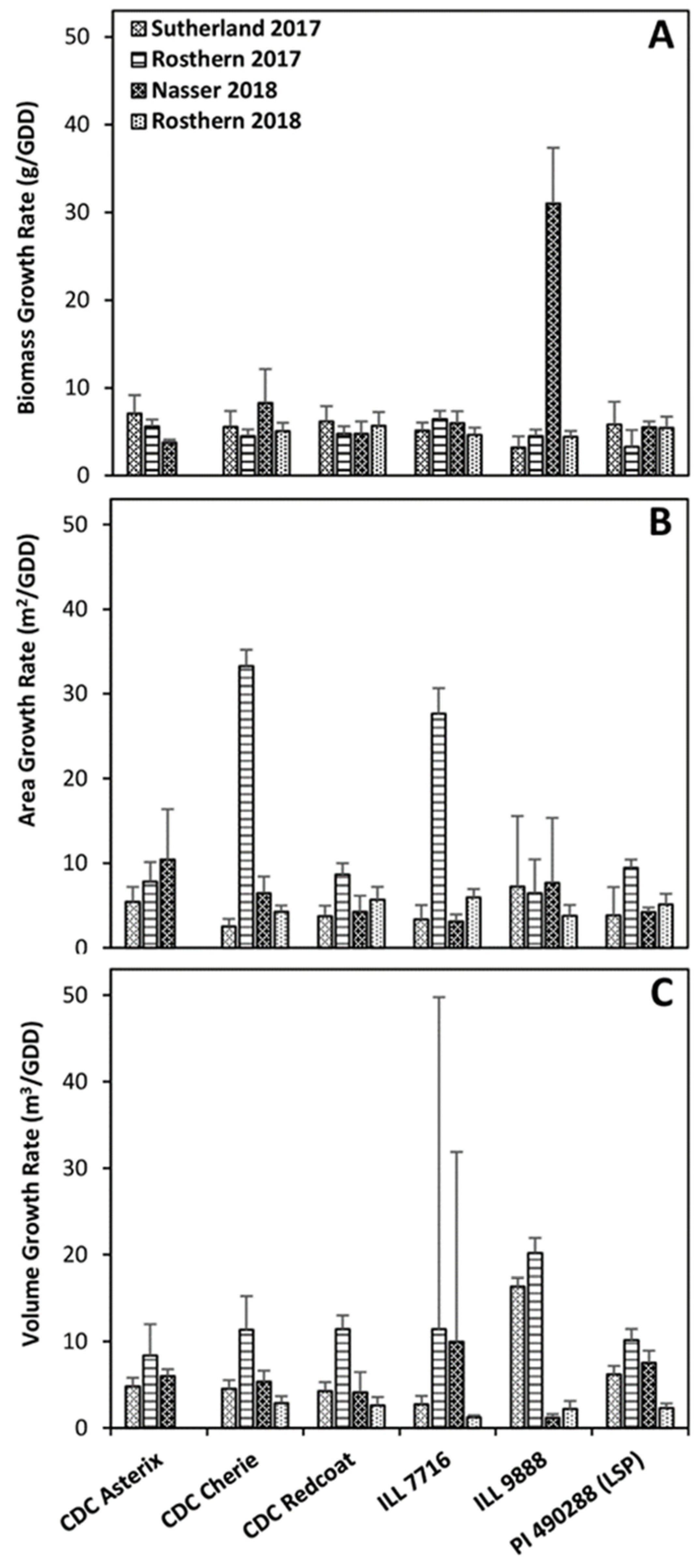
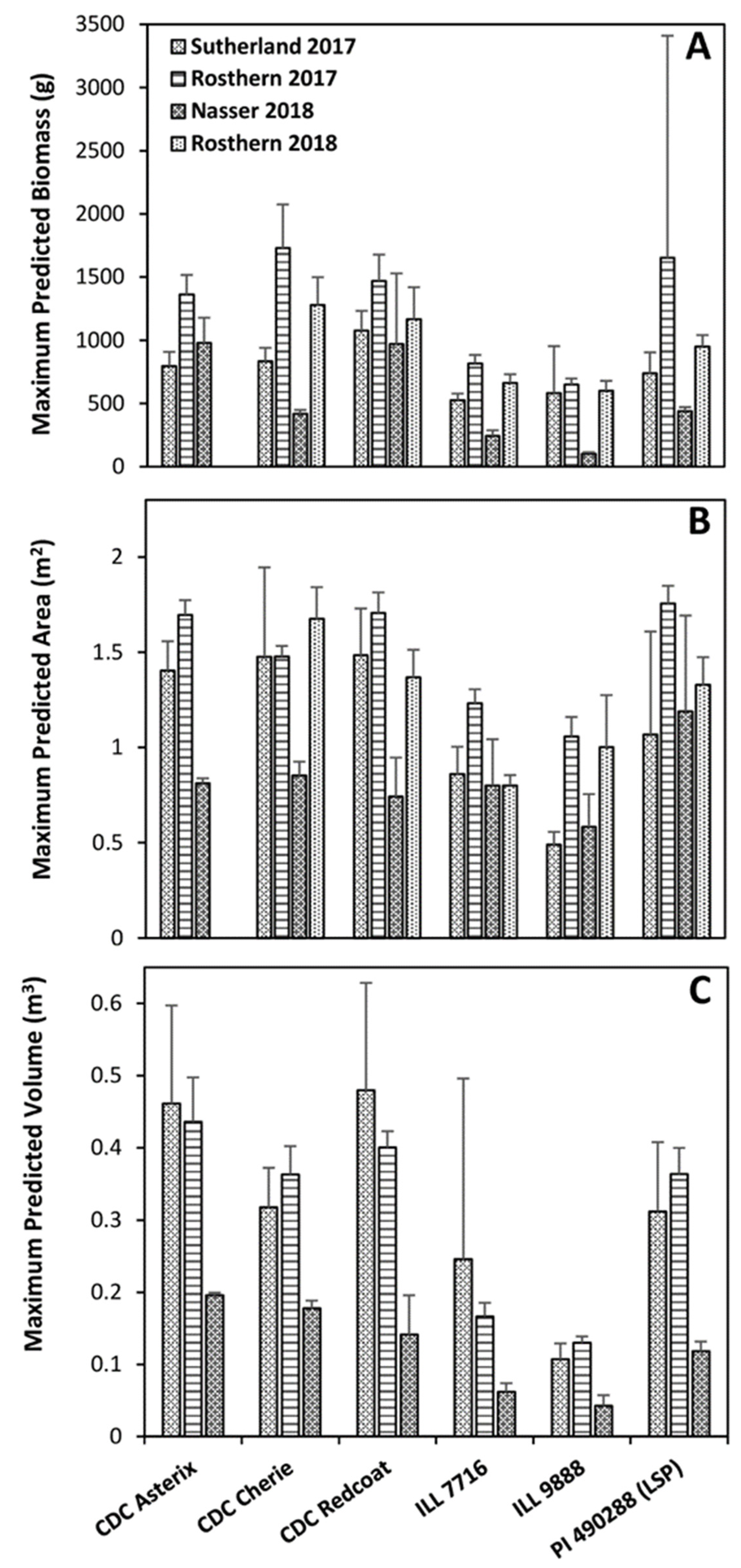
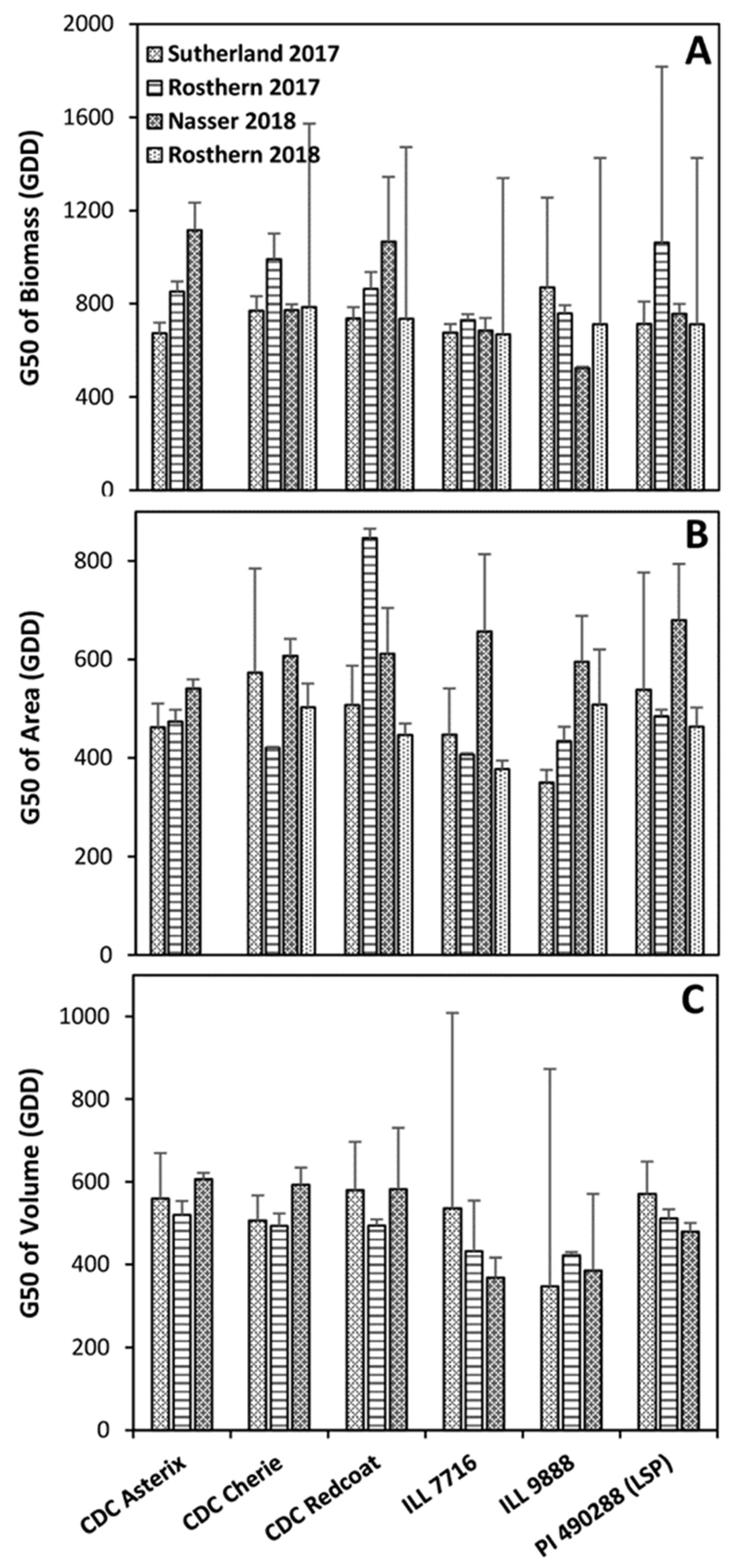
2.4. Vegetation Area as a Measurement of Plot Biomass
2.5. Vegetation Volume as a Measurement of Plot Biomass
3. Materials and Methods
3.1. Germplasm
3.2. Experimental Design
3.3. Field Data Collection
3.4. Aerial Image Acquisition
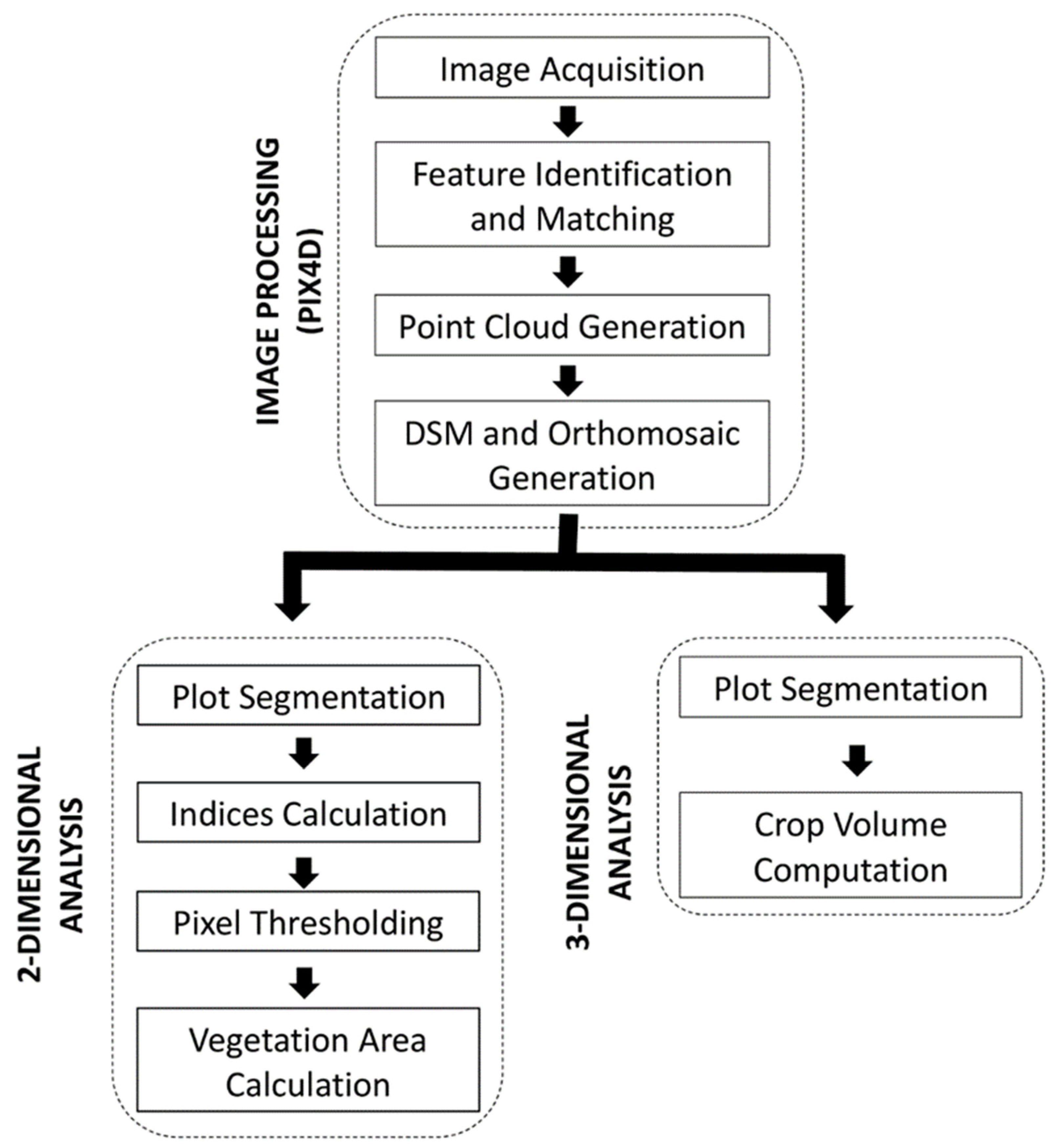
3.5. Image Processing
3.6. Image Analysis: Two-Dimensional (2-D)
3.7. Image Analysis: Three-Dimensional (3-D)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rees, M.; Osborne, C.P.; Woodward, F.I.; Hulme, S.P.; Turnbull, L.A.; Taylor, S.H. Partitioning the components of relative growth rate: How important is plant size variation? Am. Nat. 2010, 176, E152–E161. [Google Scholar] [CrossRef] [Green Version]
- Holman, F.H.; Riche, A.B.; Michalski, A.; Castle, M.; Wooster, M.J.; Hawkesford, M.J. High Throughput Field Phenotyping of Wheat Plant Height and Growth Rate in Field Plot Trials Using UAV Based Remote Sensing. Remote Sens. 2016, 8, 1031. [Google Scholar] [CrossRef]
- Tirado, S.B.; Hirsch, C.N.; Springer, N.M. UAV-Based Imaging Platform for Monitoring Maize Growth throughout Development. Plant Direct 2020, 4, e00230. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, R.; Song, W.; Han, L.; Liu, X.; Sun, X.; Luo, M.; Chen, K.; Zhang, Y.; Yang, H.; et al. Dynamic plant height QTL revealed in maize through remote sensing phenotyping using a high-throughput unmanned aerial vehicle (UAV). Sci. Rep. 2019, 9, 3458. [Google Scholar] [CrossRef] [Green Version]
- Pierre, C.C.; Crossa, J.J.; Bonnett, D.; Yamaguchi-Shinozaki, K.; Reynolds, M.P. Phenotyping transgenic wheat for drought resistance. J. Exp. Bot. 2012, 63, 1799–1808. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Vanderbeld, B.; Wan, J.; Huang, Y. Narrowing down the targets: Towards successful genetic engineering of droughttolerance crops. Mol. Plant 2010, 3, 469–490. [Google Scholar] [CrossRef]
- Fernandez, M.G.S.; Becraft, P.W.; Yin, Y.; Lübberstedt, T. From dwarves to giants? Plant height manipulation for biomass yield. Trends Plant Sci. 2009, 14, 454–461. [Google Scholar] [CrossRef]
- Thomson, E.; Mirza, S.N.; Afzal, J. Technical note: Predicting the components of aerial biomass of fourwing saltbush from shrub height and volume. J. Range Manag. 1998, 51, 323–325. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Kefauver, S.C.; Fernandez-Gallego, J.A.; Vergara-Díaz, O.; Nieto-Taladriz, M.T.; Araus, J.L. UAV and Ground Image-Based Phenotyping: A Proof of Concept with Durum Wheat. Remote Sens. 2019, 11, 1244. [Google Scholar] [CrossRef] [Green Version]
- Wijesingha, J.; Moeckel, T.; Hensgen, F.; Wachendorf, M. Evaluation of 3D point cloud-based models for the prediction of grassland biomass. Int. J. Appl. Earth Obs. Geoinf. 2019, 78, 352–359. [Google Scholar] [CrossRef]
- Alonzo, M.; Andersen, H.-E.; Morton, D.C.; Cook, B.D. Quantifying Boreal Forest Structure and Composition Using UAV Structure from Motion. Forests 2018, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Cole, J.; Ortiz-Malavassi, E.; Moya, R.; Murillo, O. Evaluation of Unmanned Aerial Vehicles (UAV) as a Tool to Predict Biomass and Carbon of Tectona grandis in Silvopastoral Systems (SPS) in Costa Rica. Drones 2021, 5, 47. [Google Scholar] [CrossRef]
- Jimenez-Brenes, F.M.; Lopez-Granados, F.; De Castro, A.I.; Torres-Sanchez, J.; Serrano, N.; Peña, J.M. Quantifying pruning impacts on olive tree architecture and annual canopy growth by using UAV-based 3D modelling. Plant Methods 2017, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Obanawa, H.; Yoshitoshi, R.; Watanabe, N.; Sakanoue, S. Portable LiDAR-Based Method for Improvement of Grass Height Measurement Accuracy: Comparison with SfM Methods. Sensors 2020, 20, 4809. [Google Scholar] [CrossRef]
- Seidel, D.; Beyer, F.; Hertel, D.; Fleck, S.; Leuschner, C. 3D-laser scanning: A non-destructive method for studying above- ground biomass and growth of juvenile trees. Agric. For. Meteorol. 2011, 151, 1305–1311. [Google Scholar] [CrossRef]
- Schirrmann, M.; Hamdorf, A.; Garz, A.; Ustyuzhanin, A.; Dammer, K.H. Estimating Wheat Biomass by Combining Image Clustering with Crop Height. Comput. Electron. Agric. 2016, 121, 374–384. [Google Scholar] [CrossRef]
- Walter, J.; Edwards, J.; McDonald, G.; Kuchel, H. Photogrammetry for the estimation of wheat biomass and harvest index. Field Crops Res. 2018, 216, 165–174. [Google Scholar] [CrossRef]
- Zhang, F.; Hassanzadeh, A.; Kikkert, J.; Pethybridge, S.J.; van Aardt, J. Comparison of UAS-Based Structure-from-Motion and LiDAR for Structural Characterization of Short Broadacre Crops. Remote Sens. 2021, 13, 3975. [Google Scholar] [CrossRef]
- Han, L.; Yang, G.; Yang, H.; Xu, B.; Li, Z.; Yang, X. Clustering field-based maize phenotyping of plant-height growth and canopy spectral dynamics using a UAV remote-sensing approach. Front. Plant Sci. 2018, 9, 1638. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Frelich, L.E. Flowering phenology and height growth pattern are associated with maximum plant height, relative growth rate and stem tissue mass density in herbaceous grassland species. J. Ecol. 2011, 99, 991–1000. [Google Scholar] [CrossRef]
- Marshall, M.T.; Husak, G.J.; Michaelsen, J.; Funk, C.; Pedreros, D.; Adoum, A. Testing a high-resolution satellite interpretation technique for crop area monitoring in developing countries. Int. J. Remote Sens. 2011, 32, 7997–8012. [Google Scholar] [CrossRef]
- Kumar, J.; Basu, P.S.; Srivastava, E.; Chaturvedi, S.K.; Nadarajan, N.; Kumar, S. Phenotyping of traits imparting drought tolerance in lentil. Crop. Pasture Sci. 2012, 63, 547–554. [Google Scholar] [CrossRef]
- Fedoruk, L.K.; Johnson, E.N.; Shirtliffe, S.J. The critical period of weed control for lentil in Western Canada. Weed Sci. 2011, 59, 517–526. [Google Scholar] [CrossRef]
- Revilla, P.; Butrón, A.; Malvar, R.A.; Ordás, A. Relationships among kernel weight, early vigor, and growth in maize. Crop Sci. 1999, 39, 654–658. [Google Scholar] [CrossRef]
- Tollenaar, M.; Wu, J. Yield improvement in temperate maize is attributable to greater stress tolerance. Crop Sci. 1999, 39, 1597–1604. [Google Scholar] [CrossRef]
- Montes, J.; Technow, F.; Dhillon, B.; Mauch, F.; Melchinger, A. High-throughput non-destructive biomass determination during early plant development in maize under field conditions. Field Crops Res. 2011, 121, 268–273. [Google Scholar] [CrossRef]
- Winterhalter, L.; Mistele, B.; Jampatong, S.; Schmidhalter, U. High-throughput sensing of aerial biomass and above-ground nitrogen uptake in the vegetative stage of well-watered and drought stressed tropical maize hybrids. Crop Sci. 2011, 51, 479–489. [Google Scholar] [CrossRef]
- Ritz, C.; Streibig, J. Dose Response Curves and Other Non-Linear Curves in Weed Science and Ecotoxicology with the Add-On Package drc in R. 2012. Available online: https://www.bioassaysys.com/ (accessed on 11 July 2022).
- El-Zeadani, H.; Puteh, A.B.; Mondal, M.M.A.; Selamat, A.; Ahmad, Z.A.; Shalgam, M.M. Seed growth rate, seed filling period and yield responses of soybean (Glycine max) to plant densities at specific reproductive growth stages. Int. J. Agric. Biol. 2014, 16, 923–928. [Google Scholar]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [Green Version]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef]
- Deng, J.; Zuo, W.; Wang, Z.; Fan, Z.; Ji, M.; Wang, G.; Ran, J.; Zhao, C.; Liu, J.; Niklas, K.; et al. Insights into plant size-density relationships from models and agricultural crops. Proc. Natl. Acad. Sci. USA 2012, 10, 8600–8605. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Sadras, V.; Vega, C.; Echarte, L. Physiological determinants of crop growth and yield in maize, sunflower, and soybean. J. Crop Improv. 2005, 14, 51–101. [Google Scholar] [CrossRef]
- Tomasel, F.; Paruelo, J.; Abras, G.; Ballarin, V.; Moler, E. A chromaticity-based technique for estimation of above-ground plant biomass. Appl. Veg. Sci. 2001, 4, 207–212. [Google Scholar] [CrossRef]
- Erskine, W.; Muehlbaur, F.; Sarker, A.; Sharma, B. The Lentil: Botany, Production, and Uses; CAB International: Wallingford, UK, 2009; pp. 4–12. [Google Scholar]
- Sankaran, S.; Zhou, J.; Khot, L.R.; Trapp, J.J.; Mndolwa, E.; Miklas, P.N. High-throughput field phenotyping in dry bean using small unmanned aerial vehicle based multispectral imagery. Comput. Electron. Agric. 2018, 151, 84–92. [Google Scholar] [CrossRef]
- Gil-Docampo, M.L.; Arza-García, M.; Ortiz-Sanz, J.; Martínez-Rodríguez, S.; Marcos-Robles, J.L.; Sánchez-Sastre, L.F. Above-ground biomass estimation of arable crops using UAV-based SfM photogrammetry. Geocarto Int. 2019, 35, 687–699. [Google Scholar] [CrossRef]
- Sun, S.; Li, C.; Paterson, A.H. In-Field High-Throughput Phenotyping of Cotton Plant Height Using LiDAR. Remote Sens. 2017, 9, 377. [Google Scholar] [CrossRef] [Green Version]
- Dixit, G.; Kumar, A.; Parihar, A. Variability for harvest index and biomass in lentil (Lens culinaris Medik) varieties. Legume Res. 2017, 40, 1093–1096. [Google Scholar] [CrossRef] [Green Version]
- Haile, T.A.; Heidecker, T.; Wright, D.; Neupane, S.; Ramsay, L.; Vandenberg, A.; Bett, K.E. Genomic selection for lentil breeding: Empirical evidence. Plant Genome 2019, 13, e20002. [Google Scholar] [CrossRef] [Green Version]
- Alba, O. Integrating the Organic Arsenal for Weed Control in Field Pea and Lentil. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2019. Available online: http://hdl.handle.net/10388/11874 (accessed on 11 July 2022).
- Neupane, S. Flowering Time Response of Diverse Lentil (Lens Culinaris Medik.) Germplasm Grown in Multiple Environments. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2019. Available online: http://hdl.handle.net/10388/11891 (accessed on 11 July 2022).
- McMaster, G.S.; Wilhelm, W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef] [Green Version]
- ArcGIS; Version 10.5.1; Environmental Systems Research Institute (ESRI): Redlands, CA, USA, 2015.
- Rasmussen, J.; Ntakos, G.; Nielsen, J.; Svensgaard, J.; Poulsen, R.N.; Christensen, S. Are vegetation indices derived from consumer-grade cameras mounted on UAVs sufficiently reliable for assessing experimental plots? Eur. J. Agron. 2016, 74, 75–92. [Google Scholar] [CrossRef]
- Ritz, C.; Strebig, J. Package “drc”. 2016. Available online: https://cran.r-project.org/web/packages/drc/drc.pdf (accessed on 11 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 11 July 2022).
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2016; Available online: http://www.rstudio.com/ (accessed on 11 July 2022).
- Paine, C.; Marthews, T.R.; Vogt, D.R.; Purves, D.; Rees, M.; Hector, A.; Turnbull, L.A. How to fit nonlinear plant growth models and calculate growth rates: An update for ecologists. Methods Ecol. Evol. 2012, 3, 245–256. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Box, G.; Cox, D. An analysis of transformations. J. R. Stat. Soc. Ser. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 11 July 2022).
- Ahmed, I.; Eramian, M.; Ovsyannikov, I.; van der Kamp, W.; Nielsen, K.; Duddu, H.S.; Rumali, A.; Shirtliffe, S.; Bett, K. Automatic Detection and Segmentation of Lentil Crop Breeding Plots from Multi-Spectral Images Captured by UAV-Mounted Camera. In Proceedings of the 2019 IEEE Winter Conference on Applications of Computer Vision (WACV), Waikoloa Village, HI, USA, 7–11 January 2018; pp. 1673–1681. [Google Scholar] [CrossRef]


| Data Collection 1 | Data Collection 2 | Data Collection 3 | Data Collection 4 | Data Collection 5 | Data Collection 6 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasser 2017 | DW | DW | DW | DW | DW | |||||||||||||
| 0.84 | Area | 0.97 | Area | 0.88 | Area | 0.82 | Area | 0.53 | Area | |||||||||
| 0.20 | 0.19 | Volume | 0.81 | 0.83 | Volume | 0.86 | 0.86 | Volume | 0.80 | 0.98 | Volume | 0.85 | 0.70 | Volume | ||||
| Sutherland 2017 | DW | DW | DW | DW | DW | DW | ||||||||||||
| 0.90 | Area | 0.96 | Area | 0.90 | Area | 0.76 | Area | 0.99 | Area | 0.91 | Area | |||||||
| 0.36 | 0.28 | Volume | 0.85 | 0.88 | Volume | 0.94 | 0.97 | Volume | 0.91 | 0.79 | Volume | 1.00 | 0.98 | Volume | 0.97 | 0.92 | Volume | |
| Nasser 2018 | DW | DW | DW | DW | DW | DW | ||||||||||||
| 0.87 | Area | 0.92 | Area | 0.90 | Area | 0.76 | Area | 0.96 | Area | 0.96 | Area | |||||||
| 0.17 | 0.40 | Volume | 0.42 | 0.43 | Volume | 0.69 | 0.68 | Volume | 0.83 | 0.81 | Volume | 0.77 | 0.75 | Volume | 0.95 | 0.91 | Volume | |
| Rosthern 2017 | DW | DW | DW | DW | DW | DW | ||||||||||||
| 0.84 | Area | 0.90 | Area | 0.93 | Area | 0.82 | Area | 0.77 | Area | 0.96 | Area | |||||||
| −0.03 | −0.26 | Volume | 0.85 | 0.74 | Volume | 0.77 | 0.88 | Volume | 0.57 | 0.74 | Volume | 0.34 | 0.62 | Volume | 0.62 | 0.60 | Volume | |
| Rosthern 2018 | DW | DW | DW | DW | DW | DW | ||||||||||||
| −0.30 | Area | 0.95 | Area | 0.89 | Area | 0.68 | Area | 0.89 | Area | 0.92 | Area | |||||||
| 0.02 | 0.08 | Volume | 0.43 | 0.44 | Volume | 0.63 | 0.78 | Volume | 0.64 | 0.95 | Volume | 0.68 | 0.79 | Volume | 0.78 | 0.77 | Volume | |
| Camera (Lens, Focal Length, F-Stop) | Altitude (m) | Ground Sample Distance (mm) |
|---|---|---|
| Sony RX100 Mark III 20.1 MP | 15 | 4.1 |
| (24–70 mm, 8.8 mm, f/1.8–2.8) | 20 | 5.5 |
| Sony QX1 20.1 MP | 15 | 5.3 |
| (16 mm, 24 mm, f/2.8) | 20 | 4 |
| Sony α5100 24.3 MP | 15 | 4.9 |
| (16 mm, 24 mm, f/2.8 mm) | 20 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, K.M.E.; Duddu, H.S.N.; Bett, K.E.; Shirtliffe, S.J. UAV Image-Based Crop Growth Analysis of 3D-Reconstructed Crop Canopies. Plants 2022, 11, 2691. https://doi.org/10.3390/plants11202691
Nielsen KME, Duddu HSN, Bett KE, Shirtliffe SJ. UAV Image-Based Crop Growth Analysis of 3D-Reconstructed Crop Canopies. Plants. 2022; 11(20):2691. https://doi.org/10.3390/plants11202691
Chicago/Turabian StyleNielsen, Karsten M. E., Hema S. N. Duddu, Kirstin E. Bett, and Steve J. Shirtliffe. 2022. "UAV Image-Based Crop Growth Analysis of 3D-Reconstructed Crop Canopies" Plants 11, no. 20: 2691. https://doi.org/10.3390/plants11202691
APA StyleNielsen, K. M. E., Duddu, H. S. N., Bett, K. E., & Shirtliffe, S. J. (2022). UAV Image-Based Crop Growth Analysis of 3D-Reconstructed Crop Canopies. Plants, 11(20), 2691. https://doi.org/10.3390/plants11202691






