Morphological and Physiological Responses of Hybrid Aspen (Populus tremuloides Michx. × Populus tremula L.) Clones to Light In Vitro
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Setup
4.2. Plant Material
4.3. Measurements
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konnert, M.; Fady, B.; Gömöry, D.; A’Hara, S.; Wolter, F.; Ducci, F.; Koskela, J.; Bozzano, M.; Maaten, T.; Kowalczyk, J. Use and Transfer of Forest Reproductive Material in Europe in the Context of Climate Change; European Forest Genetic Resources Programme (EUFORGEN); Bioversity International: Rome, Italy, 2015; 75p. [Google Scholar]
- Gömöry, D.; Himanen, K.; Tollefsrud, M.M.; Uggla, C.; Kraigher, H.; Bordács, S.; Alizoti, P.; A’Hara, S.; Frank, A.; Proschowsky, G.F.; et al. Genetic Aspects in Production and Use of Forest Reproductive Material: Collecting Scientific Evidence to Support the Developmentof Guide-Lines and Decision Support Tools; European Forest Genetic Resources Programme (EUFORGEN); European Forest Institute: Joensuu, Finland, 2021; 216p. [Google Scholar]
- Fahlvik, N.; Rytter, L.; Stener, L.G. Production of hybrid aspen on agricultural land during one rotation in southern Sweden. J. For. Res. 2021, 32, 181–189. [Google Scholar] [CrossRef]
- Niemczyk, M.; Przybysz, P.; Przybysz, K.; Karwański, M.; Kaliszewski, A.; Wojda, T.; Liesebach, M. Productivity, growth patterns, and cellulosic pulp properties of hybrid aspen clones. Forests 2019, 10, 450. [Google Scholar] [CrossRef] [Green Version]
- Sable, I.; Grinfelds, U.; Zeps, M.; Irbe, I.; Noldt, G.; Jansons, A.; Treimanis, A.; Koch, G. Chemistry and kraft pulping of seven hybrid aspen clones. Dimension measurements on the vessels and UMSP of the cell walls. Holzforschung 2013, 67, 505–510. [Google Scholar] [CrossRef]
- Jansons, Ā.; Zeps, M.; Rieksts-Riekstiņš, J.; Matisons, R.; Krišāns, O. Height increment of hybrid aspen Populus tremuloides × P. tremula as a function of weather conditions in central part of Latvia. Silva Fennica 2014, 48, 1124. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Thomas, B.R. Hormones and heterosis in hybrid Balsam poplar (Populus balsamifera L.). Forests 2019, 10, 143. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Howe, G.T.; Wu, R. Developmental factors responsible for heterosis in aspen hybrids (Populus tremuloides × P. tremula. Tree Physiol. 1998, 18, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Du, Y.; Warburton, M.L.; Xiao, Y.; Yan, J. Phenotypic plasticity contributes to maize adaptation and heterosis. Mol. Biol. Evol. 2021, 38, 1262–1275. [Google Scholar] [CrossRef]
- Birchler, J.A.; Yao, H.; Chudalayandi, S.; Vaiman, D.; Veitia, R.A. Heterosis. Plant Cell 2010, 22, 2105–2112. [Google Scholar] [CrossRef] [Green Version]
- Fridman, E. Consequences of hybridization and heterozygosity on plant vigor and phenotypic stability. Plant Sci. 2015, 232, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lippman, Z.B.; Zamir, D. Heterosis: Revisiting the magic. Trends Genet. 2007, 23, 60–66. [Google Scholar] [CrossRef]
- Kusmec, A.; de Leon, N.; Schnable, P.S. Harnessing phenotypic plasticity to improve maize yields. Front. Plant. Sci. 2018, 9, 1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanewich, K.P.; Pearce, D.W.; Rood, S.B. Heterosis in poplar involves phenotypic stability: Cottonwood hybrids outperform their parental species at suboptimal temperatures. Tree Physiol. 2018, 38, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Stanton, B.J.; Neale, D.; Li, S. Populus Breeding: From the Classical to the Genomic Approach. In Genetics and Genomics of Populus. Plant Genetics and Genomics: Crops and Models; Jansson, S., Bhalerao, R., Groover, A., Eds.; Springer: New York, NY, USA, 2010; Volume 8. [Google Scholar] [CrossRef]
- McKown, A.D.; Guy, R.D. Hybrid vigour–poplars play it cool. Tree Physiol. 2018, 38, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, R. Genetic causes of heterosis in juvenile aspen: A quantitative comparison across intra- and inter-specific hybrids. Theor. Appl. Genet. 1996, 93, 380–391. [Google Scholar] [CrossRef]
- Lawson, W. Tissue culture propagation of European aspen. For. Sci. 1971, 17, 348–350. [Google Scholar] [CrossRef]
- Zeps, M.; Kondratovičs, T.; Grigžde, E.; Jansons, Ā.; Zeltiņš, P.; Samsone, I.; Matisons, R. Plantlet anatomy of silver birch (Betula pendula Roth.) and hybrid aspen (Populus tremuloides Michx. × Populus tremula L.) shows intraspecific reactions to illumination In Vitro. Plants 2022, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; Motta de Castro, K.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.M.; Faria, D.V.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? In Vitro Cell Dev. Biol.-Plant 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Kwon, A.-R.; Cui, H.-Y.; Lee, H.; Shin, H. Light quality affects shoot regeneration, cell division, and wood formation in elite clones of Populus euramericana. Acta Physiol. Plant. 2015, 37, 65. [Google Scholar] [CrossRef]
- Lazzarin, M.; Meisenburg, M.; Meijer, D.; van Leperen, W.; Marcelis, L.F.M.; Kappers, I.F.; van der Krol, A.R.; van Loon, J.J.A.; Dicke, M. LEDs Make It Resilient: Effects on plant growth and defense. Trends Plant Sci. 2021, 26, 496–508. [Google Scholar] [CrossRef]
- Van Gelderen, K.; Kang, C.; Pierik, R. Light signaling, root development, and plasticity. Plant Physiol. 2018, 176, 1049–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballaré, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Mawphlang, O.I.L.; Kharshiing, E.V. Photoreceptor mediated plant growth responses: Implications for photoreceptor engineering toward improved performance in crops. Front. Plant. Sci. 2017, 11, 1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Van Lebeke, M.-C. Long-term effects of red- and blue light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant. Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Baroli, I.; Price, G.D.; Badger, M.R.; Von Caemmerer, S. The contribution of photosynthesis to the red light response of stomatal conductance. Plant Physiol. 2008, 146, 737–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lu, W.; Tong, Y.X.; Yang, Q.C. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.L.; Xue, X.Z.; Yang, Y.D.; Chen, F.; Zhao, J.; Wang, X.X.; Khan, A.T. Effects of red and blue LEDs on in vitro growth and microtuberization of potato single-node cuttings. Front. Agric. Sci. Eng. 2018, 5, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, A.; Kar, R.K. Integrated role of ROS and Ca+2 in blue light-induced chloroplast avoidance movement in leaves of Hydrilla verticillata (L.f.) Royle. Protoplasma 2016, 253, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Funkhauser, C.; Chory, J. Light control of plant development. Ann. Rev. Cell Develop. Biol. 1997, 13, 203–229. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Fu, Y.; Hu, D.; Yu, J.; Liu, H. Effect of green, yellow and purple radiation on biomass, photosynthesis, morphology and soluble sugar content of leafy lettuce via spectral wavebands “knock out”. Sci. Hortic. 2018, 236, 10–17. [Google Scholar] [CrossRef]
- Li, L.; Tong, Y.X.; Lu, J.L.; Li, Y.M.; Yang, Q.C. Lettuce growth, nutritional quality, and energy use efficiency as affected by red–blue light combined with different monochromatic wavelengths. HortScience 2020, 55, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB PLANTS 2018, 10, ply052. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.T.; Lefsrud, M.; Grave, V.; Hao, X. Different ratios of red and blue LED light effects on coriander productivity and antioxidant properties. Acta Hortic. 2016, 1134, 223–229. [Google Scholar] [CrossRef]
- Golovatskaya, I.F.; Karnachuk, R.A. Role of green light in physiological activity of plants. Rus. J. Plant Physiol. 2015, 62, 727–740. [Google Scholar] [CrossRef]
- Roig-Villanova, I.; Bou-Torrent, J.; Galstyan, A.; Carretero-Paulet, L.; Portolés, S.; Rodríguez-Concepción, M.; Martínez-García, J.F. Interaction of shade avoidance and auxin responses: A role for two novel atypical bHLH proteins. EMBO J. 2007, 26, 4756. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Maruhnich, S.A.; Folta, K.M. Green light induces shade avoidance symptoms. Plant Physiol. 2011, 157, 1528. [Google Scholar] [CrossRef] [Green Version]
- Pantazopoulou, C.K.; Bongers, F.J.; Pierik, R. Reducing shade avoidance can improve Arabidopsis canopy performance against competitors. Plant Cell Environ. 2021, 44, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Cioć, M.; Pawłowska, B. Leaf response to different light spectrum compositions during micropropagation of gerbera axillary Shoots. Agronomy 2020, 10, 1832. [Google Scholar] [CrossRef]
- Smith, H.L.; Mcausland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Hu, M.J.; Guo, Y.P. Regulation of photosynthesis by light quality and its mechanism in plants. J. Appl. Ecol. 2008, 19, 1619–1624. Available online: https://europepmc.org/article/med/18839928 (accessed on 3 October 2022).
- Urrestarazu, M.; Nájera, C.; del Mar Gea, M. Effect of the spectral quality and intensity of light-emitting diodes on several horticultural crops. HortScience 2016, 51, 268–271. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Fu, Y.; Liu, H.; Liu, H. Changes of the antioxidant capacity in Gynura bicolor DC under different light sources. Sci. Hortic. 2015, 184, 40–45. [Google Scholar] [CrossRef]
- Arena, C.; Tsonev, T.; Doneva, D.; de Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Env. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Xiang, N.; Hu, J.; Zhang, B.; Cheng, Y.; Wang, S.; Guo, X. Effect of light qualities on volatiles metabolism in maize (Zea mays L.) sprouts. Food Res. Internat. 2022, 156, 111340. [Google Scholar] [CrossRef]
- Yavari, N.; Tripathi, R.; Wu, B.S.; MacPherson, S.; Singh, J.; Lefsrud, M. The effect of light quality on plant physiology, photosynthetic, and stress response in Arabidopsis thaliana leaves. PLoS ONE 2021, 16, e0247380. [Google Scholar] [CrossRef] [PubMed]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. PCTOC 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Blum, A. Heterosis, stress, and the environment: A possible road map towards the general improvement of crop yield. J. Exp. Bot. 2013, 64, 4829–4837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Hartmann and Kester’s Plant Propagation: Principles and Practices, 7th ed.; Pearson: London, UK, 2002; 880p. [Google Scholar]
- Jiang, C.D.; Wang, X.; Gao, H.Y.; Shi, L.; Chow, W.S. Systemic regulation of leaf anatomical structure, photosynthetic performance, and high-light tolerance in sorghum. Plant Physiol. 2011, 155, 1416–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendrakumar, P.; Hariprasanna, K.; Seetharama, N. Prediction of heterosis in crop plants–status and prospects. J. Exp. Agric. 2015, 9, 1–16. [Google Scholar] [CrossRef]
- McCarthy, R.; Rytter, L. Productivity and thinning effects in hybrid aspen root sucker stands. For. Ecol. Manag. 2015, 354, 215–223. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Pinno, B.D.; Mock, K.E. Tamm Review: Seedling-based ecology, management, and restoration in aspen (Populus tremuloides). For. Ecol. Manag. 2019, 432, 231–245. [Google Scholar] [CrossRef]
- Devlin, P.F. Plants wait for the lights to change to red. Proc. Natl. Acad. Sci. USA 2016, 113, 7301–7303. [Google Scholar] [CrossRef] [Green Version]
- Macedo, A.F.; Leal-Costa, M.V.; Tavares, E.S.; Lage, C.L.S.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Li, S.; Zhou, L.; Wu, S.; Liu, L.; Huang, M.; Lin, S.; Ding, G. Effects of LED light on Acacia melanoxylon bud proliferation in vitro and root growth ex vitro. Open Life Sci. 2019, 14, 349–357. [Google Scholar] [CrossRef]
- Lerin, J.; Aragão, V.P.M.; Reis, R.S.; Silveira, V.; Santa-Catarina, C. Proteomic profile and polyamine contents are modulated by light source to promote in vitro shoot development in Cariniana legalis (Martius) O. Kuntze (Lecythidaceae). PCTOC 2019, 137, 329–342. [Google Scholar] [CrossRef]
- He, C.; Zeng, Y.; Fu, Y.; Wu, J.; Liang, Q. Light quality affects the proliferation of in vitro cultured plantlets of Camellia oleifera Huajin. PeerJ 2020, 8, e10016. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Zang, J.; Xu, Z.G.; Guo, S.R.; Jiao, X.L.; Liu, X.Y.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Yu, X.; Hyldgaard, B.; Rosenqvist, E.; Ottosen, C.-O.; Chen, J. Interspecific hybridization in Cucumis leads to the divergence of phenotypes in response to low light and extended photoperiods. Front. Plant Sci. 2015, 6, 802. [Google Scholar] [CrossRef] [Green Version]
- Saeki, N.; Kawanabe, T.; Ying, H.; Shimizu, M.; Kojima, M.; Abe, H.; Okazaki, K.; Kaji, M.; Taylor, J.M.; Sakakibara, H.; et al. Molecular and cellular characteristics of hybrid vigor in a commercial hybrid of Chinese cabbage. BMC Plant Biol. 2016, 16, 45. [Google Scholar] [CrossRef]
- Šēnhofa, S.; Zeps, M.; Matisons, R.; Smilga, J.; Lazdiņa, D.; Jansons, Ā. Effect of climatic factors on tree-ring width of Populus hybrids in Latvia. Silva Fen. 2016, 50, 1442. [Google Scholar] [CrossRef] [Green Version]
- Kirschbaum, M.U.F. Focus issue on enhancing photosynthesis: Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol. 2011, 155, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shengxin, C.; Chunxia, L.; Xuyang, Y.; Song, C.; Xuelei, J.; Xiaoying, L.; Zhigang, X.; Rongzhan, G. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the rosette stage. Front. Plant Sci. 2016, 7, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, R.; Taylor, J.M.; Shirasawa, S.; Peacock, W.J.; Dennis, E.S. Heterosis of Arabidopsis hybrids between C24 and Col is associated with increased photosynthesis capacity. Proc. Natl. Acad. Sci. USA 2012, 109, 7109–7114. [Google Scholar] [CrossRef] [Green Version]
- Forrester, D.I.; Bauhus, J. A Review of processes behind diversity—Productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef] [Green Version]
- Tazoe, Y.; Sazuka, T.; Yamaguchi, M.; Saito, C.; Ikeuchi, M.; Kanno, K.; Kojima, S.; Hirano, K.; Kitano, H.; Kasuga, S.; et al. Growth properties and biomass production in the hybrid C4 crop Sorghum bicolor. Plant Cell Physiol. 2016, 57, 944–952. [Google Scholar] [CrossRef] [Green Version]
- Alabadí, D.; Blázquez, M.A. Molecular interactions between light and hormone signaling to control plant growth. Plant Mol. Biol. 2008, 69, 409–417. [Google Scholar] [CrossRef]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hort. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Szüčová, L.; Doležal, K.; Finnie, J.F.; van Staden, J. Assessment of the role of meta-topolins on in vitro produced phenolics and acclimatization competence of micropropagated “Williams” banana. Acta Physiol. Plant. 2012, 34, 2265–2273. [Google Scholar] [CrossRef]
- Dobránszki, J.; Mendler-Drienyovszki, N. Cytokinin-induced changes in the chlorophyll content and fluorescence of in vitro apple leaves. J. Plant Physiol. 2014, 171, 1472–1478. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Dobránszki, J.; Hudák, I. Effect of cytokinin content of the regeneration media on in vitro rooting ability of adventitious apple shoots. Sci. Hort. 2011, 129, 910–913. [Google Scholar] [CrossRef]
- Yildiz, M. Evaluation of the effect of in vitro stress and competition on tissue culture response of flax. Biol. Plant. 2011, 55, 541–544. [Google Scholar] [CrossRef]
- Boccalandro, H.E.; Ploschuk, E.L.; Yanovsky, M.J.; Sánchez, R.A.; Gatz, C.; Casal, J.J. Increased phytochrome B alleviates density effects on tuber yield of field potato crops. Plant Physiol. 2003, 133, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhang, F.; Zhou, J.; Chen, F.; Wang, B.; Xie, X. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol. Biol. 2012, 78, 289–300. [Google Scholar] [CrossRef]
- Michelson, I.H.; Ingvarsson, P.K.; Robinson, K.M.; Edlund, E.; Eriksson, M.E.; Nilsson, O.; Jansson, S. Autumn senescence in aspen is not triggered by day length. Physiol. Plant. 2018, 162, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Smilga, J. Growth and morphology of aspen generative progeny. Jaun. Mežsaimniecībā 1970, 12, 3–16. (In Latvian) [Google Scholar]
- Smilga, J. Growth characteristics of aspen clones in juvenile age. Jaun. Mežsaimniecībā 1991, 33, 4–12. (In Latvian) [Google Scholar]
- Zeps, M.; Adamovics, A.; Smilga, J.; Sisenis, L. Productivity and quality of hybrid aspen at the age of 18 years. Res. Rur. Develop. 2016, 2, 55–61. [Google Scholar]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Tang, C. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. PCTOC 2010, 103, 155–163. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Chivkunova, O.B.; Merzlyak, M.N.; Reshetnikova, I.v.A.S.; Solovchenko, A.E.; Chivkunova, O.B.; Merzlyak, M.N.; Reshetnikova, I.V. A Spectrophotometric analysis of pigments in apples. Rus. J. Plant Physiol. 2001, 48, 693–700. [Google Scholar] [CrossRef]
- Hansatech. Operations Manual Setup, Installation & Maintenance: Handy PEA, Pocket PEA & PEA Plus Software, Version 1.0; Hansatech Instruments Ltd.: Norfolk, UK, 2006. [Google Scholar]
- Andersone, U.; Ievinsh, G. Changes of morphogenic competence in mature Pinus sylvestris L. buds in vitro. Ann. Bot. 2002, 90, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 3 October 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. _emmeans: Estimated Marginal Means, Aka Least-Squares Means_. R package version 1.7.5. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 3 October 2022).
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant. Biol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Yu, Q.; Tigerstedt, P.M.A.; Haapanen, M. Growth and phenology of hybrid aspen clones (Populus tremula L. × Populus tremuloides Michx.). Silva Fen. 2001, 35, 15–25. [Google Scholar] [CrossRef]
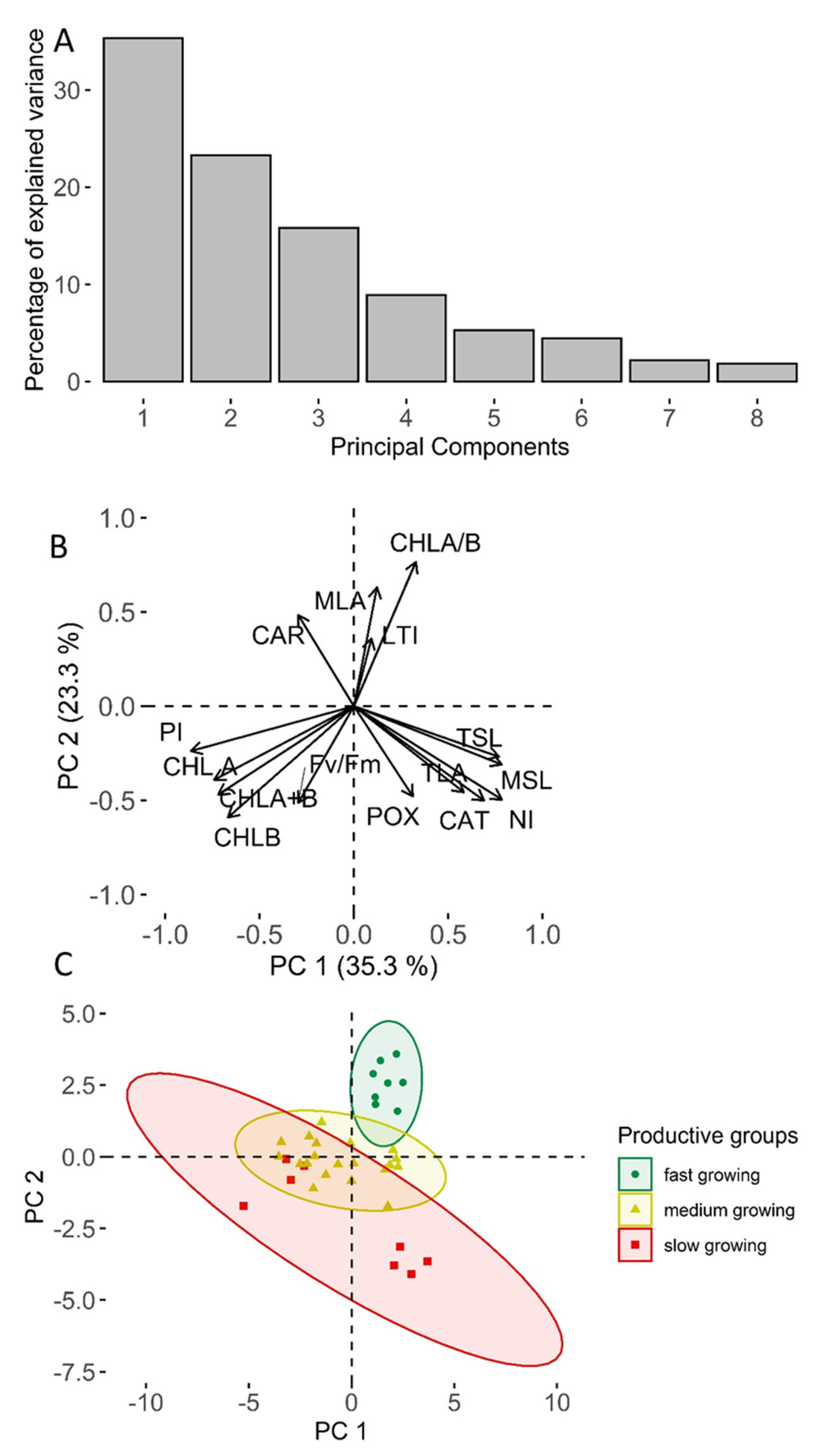
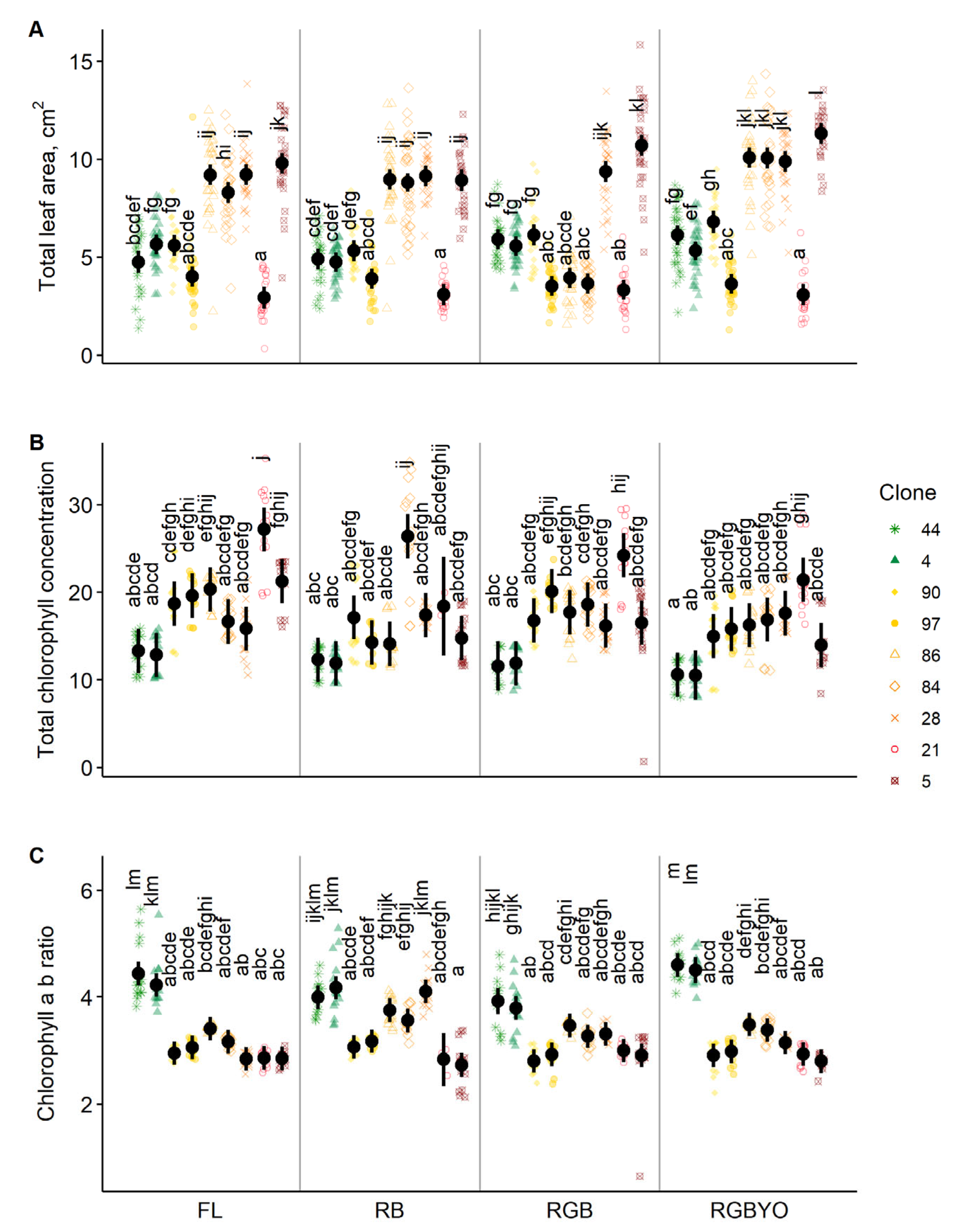

| Variable | Abbreviation | Range | Mean | Median | St. Dev. | Coef. of Variation |
|---|---|---|---|---|---|---|
| Number of internodes | NI | 4.25–10.48 | 6.37 | 5.90 | 1.80 | 0.28 |
| Length of third internode | LTI | 1.71–4.8 | 3.41 | 3.46 | 0.91 | 0.27 |
| Main shoot length | MSL | 1.34–3.41 | 2.34 | 2.24 | 0.67 | 0.28 |
| Total shoot length | TSL | 2.91–5.24 | 4.07 | 4.00 | 0.73 | 0.18 |
| Mean leaf area | MLA | 0.28–0.84 | 0.61 | 0.64 | 0.12 | 0.20 |
| Total leaf area | TLA | 2.93–11.32 | 6.55 | 5.78 | 2.64 | 0.40 |
| Concentration of carotenoids | CAR | 2.78–5.54 | 3.89 | 3.80 | 0.64 | 0.19 |
| Concentration of chlorophyll a | CHLA | 8.59–20.11 | 12.50 | 12.48 | 2.53 | 0.20 |
| Concentration of chlorophyll b | CHLB | 1.81–7.07 | 3.85 | 3.94 | 1.15 | 0.30 |
| Total chlorophyll concentration | CHLA+B | 10.5–27.2 | 16.66 | 16.55 | 3.96 | 0.24 |
| Chlorophyll a and b ratio | CHLA/B | 2.72–4.6 | 3.37 | 3.17 | 0.55 | 0.16 |
| Maximum quantum yield efficiency | Fv/Fm | 0.56–0.81 | 0.74 | 0.77 | 0.07 | 0.10 |
| Performance index | PI | 0.29–3.56 | 1.47 | 1.24 | 0.99 | 0.67 |
| Catalase activity | CAT | 1.08–4.06 | 1.95 | 1.87 | 0.67 | 0.34 |
| Peroxidase activity | POX | 0.34–5.12 | 1.40 | 1.19 | 1.07 | 0.76 |
| Concentration of Chlorophyll a and b | |
|---|---|
| Fixed Effects, χ2 | |
| Light treatment | 26.26 *** |
| Clone | 229.47 *** |
| Light by clone interaction | 83.33 *** |
| Random effects, variance, and ICC | |
| Jar | 7.50 (0.79) |
| Residual | 1.97 |
| Model fit | |
| R2 (marginal) | 0.63 |
| R2 (conditional) | 0.92 |
| Chlorophyll ratio (a/b) | |
| Fixed effects, χ2 | |
| Light treatment | 22.75 *** |
| Clone | 766.65 *** |
| Light by clone interaction | 107.43 *** |
| Random effects, variance | |
| Jar | 0.050 (0.56) |
| Residual | 0.039 |
| Model fit | |
| R2 (marginal) | 0.79 |
| R2 (conditional) | 0.91 |
| Total area of leaves | |
| Fixed effects, χ2 | |
| Light treatment | 104.12 *** |
| Clone | 2014.79 *** |
| Light by clone interaction | 368.41 *** |
| Random effects, variance | |
| Test tube holder | 0.15 (0.05) |
| Residual | 2.67 |
| Model fit | |
| R2 (marginal) | 0.69 |
| R2 (conditional) | 0.72 |
| Red and Blue (RB) | Red and Green and Blue (RGB) | Red and Green and Blue and Yellow and Orange (RGBYO) | Fluorescent Tubes (FL) | |
|---|---|---|---|---|
| Blue 400–500 nm | 23 | 18 | 17 | 17 |
| Green 500–570 nm | 0 | 22 | 17 | 25 |
| Yellow 570–590 nm | 0 | 0 | 3 | 7 |
| Orange 590–625 nm | 2 | 1 | 5 | 36 |
| Red 625–700 nm | 73 | 57 | 56 | 11 |
| Far-red 700–750 nm | 2 | 2 | 2 | 4 |
| Red:Blue (R:B) | 3.17 | 3.17 | 3.29 | 0.65 |
| Red:Far-red (R:FR) | 36.5 | 28.5 | 28 | 2.75 |
| Blue:Green (B:G) | n/a | 0.82 | 1.00 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondratovičs, T.; Zeps, M.; Rupeika, D.; Zeltiņš, P.; Gailis, A.; Matisons, R. Morphological and Physiological Responses of Hybrid Aspen (Populus tremuloides Michx. × Populus tremula L.) Clones to Light In Vitro. Plants 2022, 11, 2692. https://doi.org/10.3390/plants11202692
Kondratovičs T, Zeps M, Rupeika D, Zeltiņš P, Gailis A, Matisons R. Morphological and Physiological Responses of Hybrid Aspen (Populus tremuloides Michx. × Populus tremula L.) Clones to Light In Vitro. Plants. 2022; 11(20):2692. https://doi.org/10.3390/plants11202692
Chicago/Turabian StyleKondratovičs, Toms, Mārtiņš Zeps, Diāna Rupeika, Pauls Zeltiņš, Arnis Gailis, and Roberts Matisons. 2022. "Morphological and Physiological Responses of Hybrid Aspen (Populus tremuloides Michx. × Populus tremula L.) Clones to Light In Vitro" Plants 11, no. 20: 2692. https://doi.org/10.3390/plants11202692
APA StyleKondratovičs, T., Zeps, M., Rupeika, D., Zeltiņš, P., Gailis, A., & Matisons, R. (2022). Morphological and Physiological Responses of Hybrid Aspen (Populus tremuloides Michx. × Populus tremula L.) Clones to Light In Vitro. Plants, 11(20), 2692. https://doi.org/10.3390/plants11202692





