Ocimum basilicum-Mediated Synthesis of Silver Nanoparticles Induces Innate Immune Responses against Cucumber Mosaic Virus in Squash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and CMV Isolate
2.2. Green Synthesis of Silver Nanoparticles (Ag-NPs)
2.3. Characterization of the Green Synthesized Ag-NPs
2.4. The Experimental Greenhouse Design
2.5. Evaluation of the Free Radical Scavenging Activity
2.6. Total Phenolic and Flavonoid Contents Evaluation
2.7. Total Soluble Protein and Carbohydrate Determination
2.8. Impact of Ag-NPs on Antioxidant Enzyme Activity
2.8.1. Polyphenol Oxidase (PPO) Activity Evaluation
2.8.2. Superoxide Dismutase (SOD)
2.8.3. Peroxidase Activity (POX)
2.9. Impact of Ag-NPs on Defense-Related Genes Expression and CMV Accumulation Level
2.10. Statistical Analysis
3. Results and Discussion
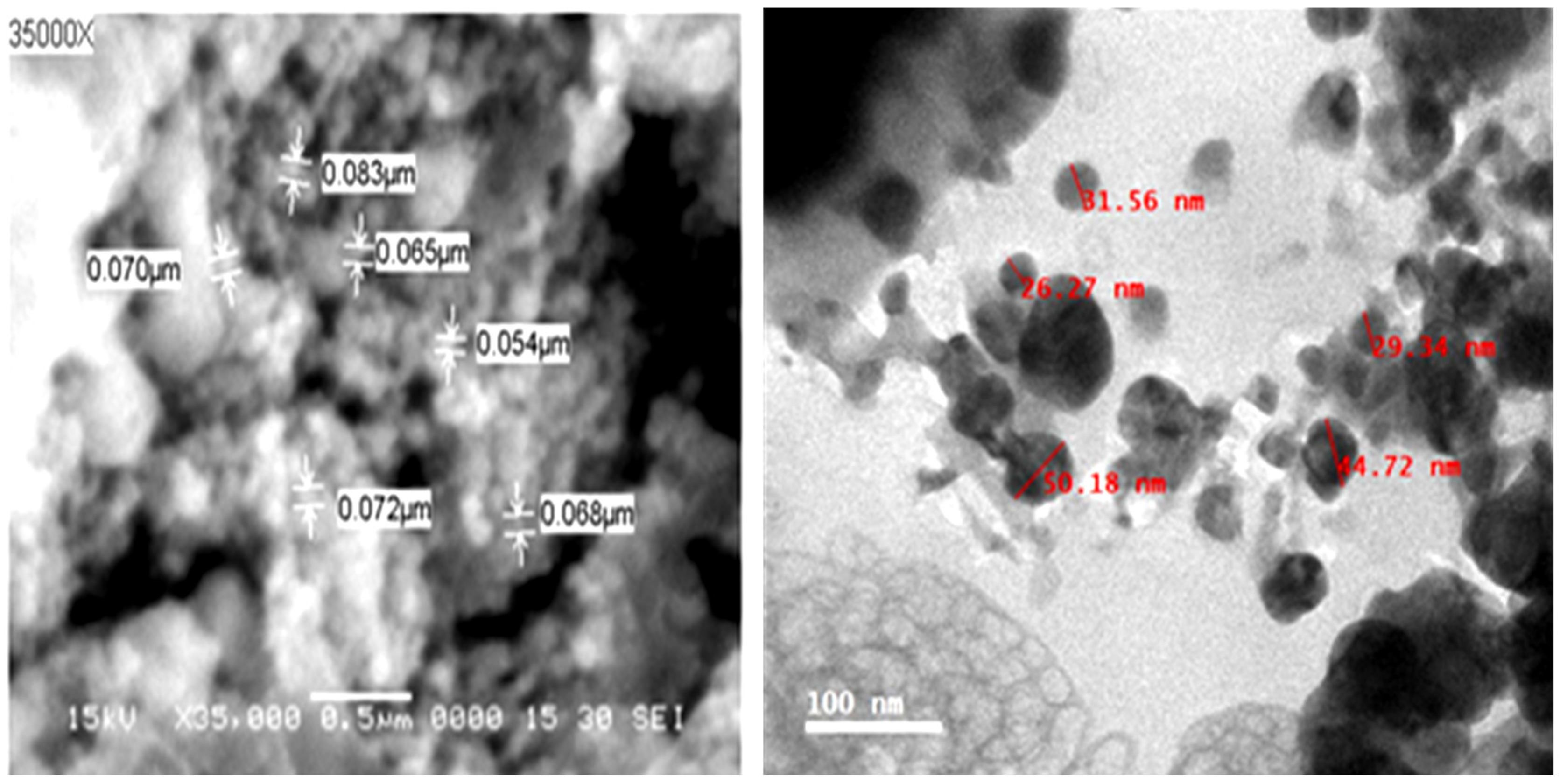
3.1. Morphological Characterization of the Biosynthesized Ag-NPs
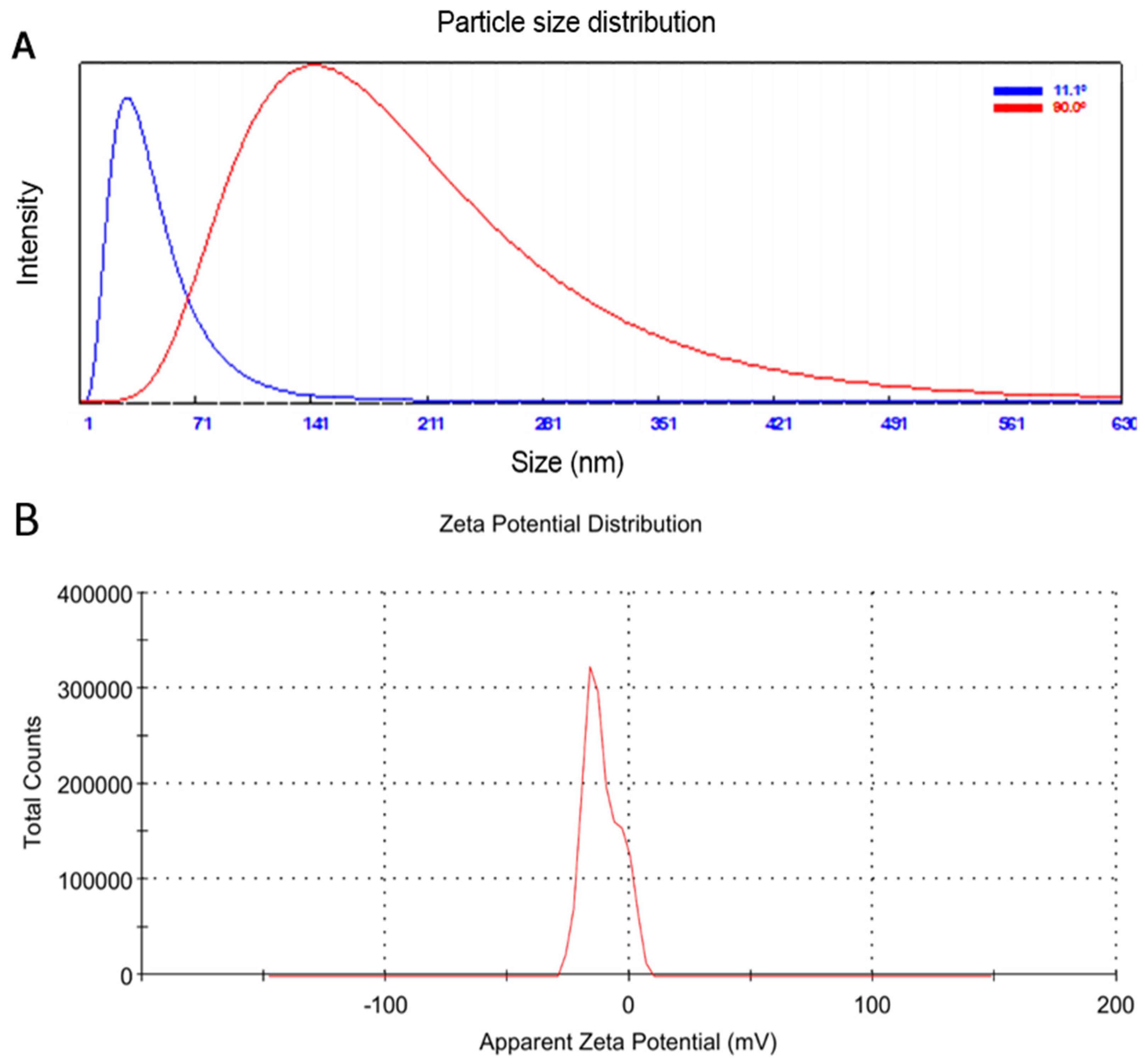
3.2. Particle Size Distribution and Zeta Potential Analysis
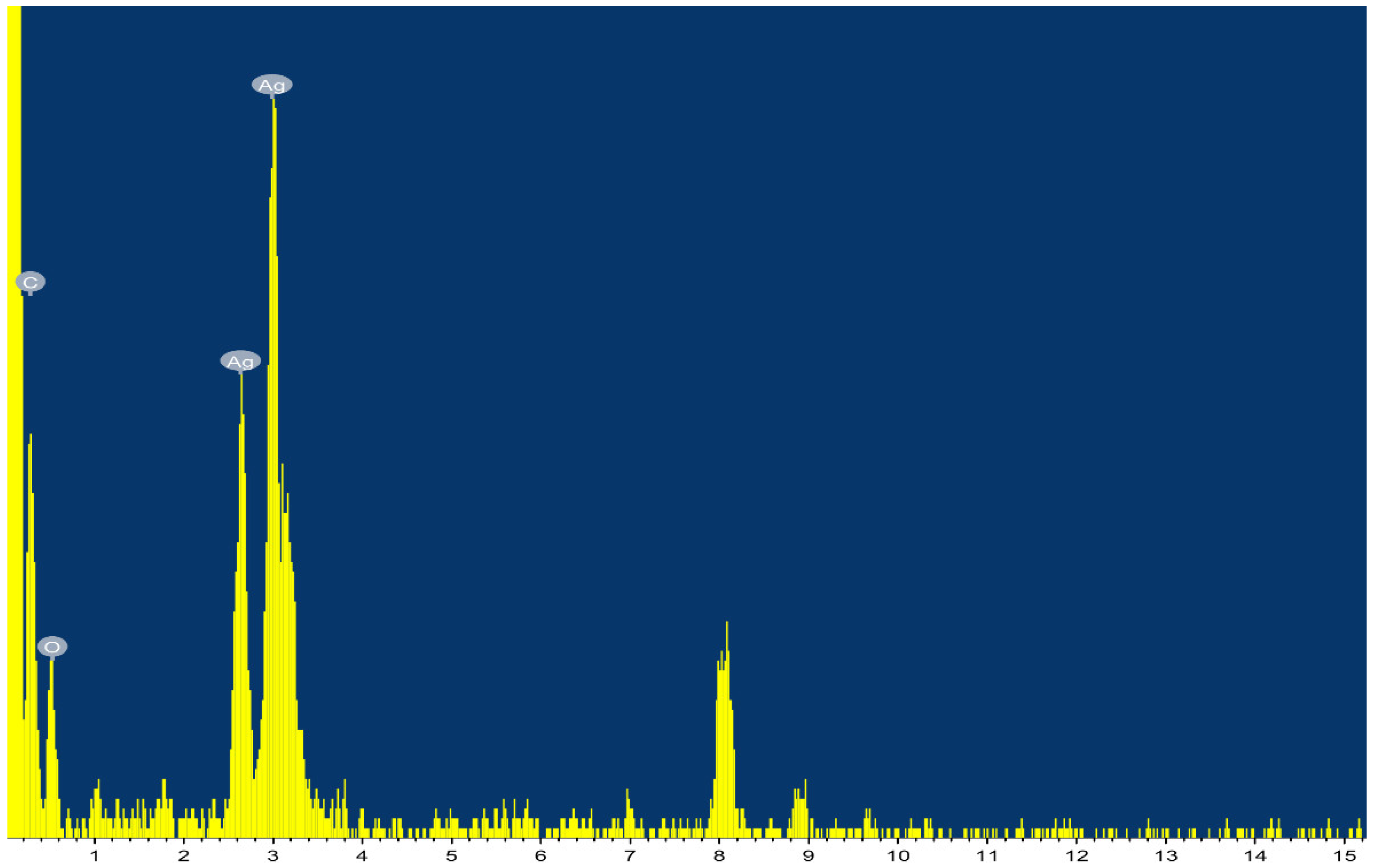
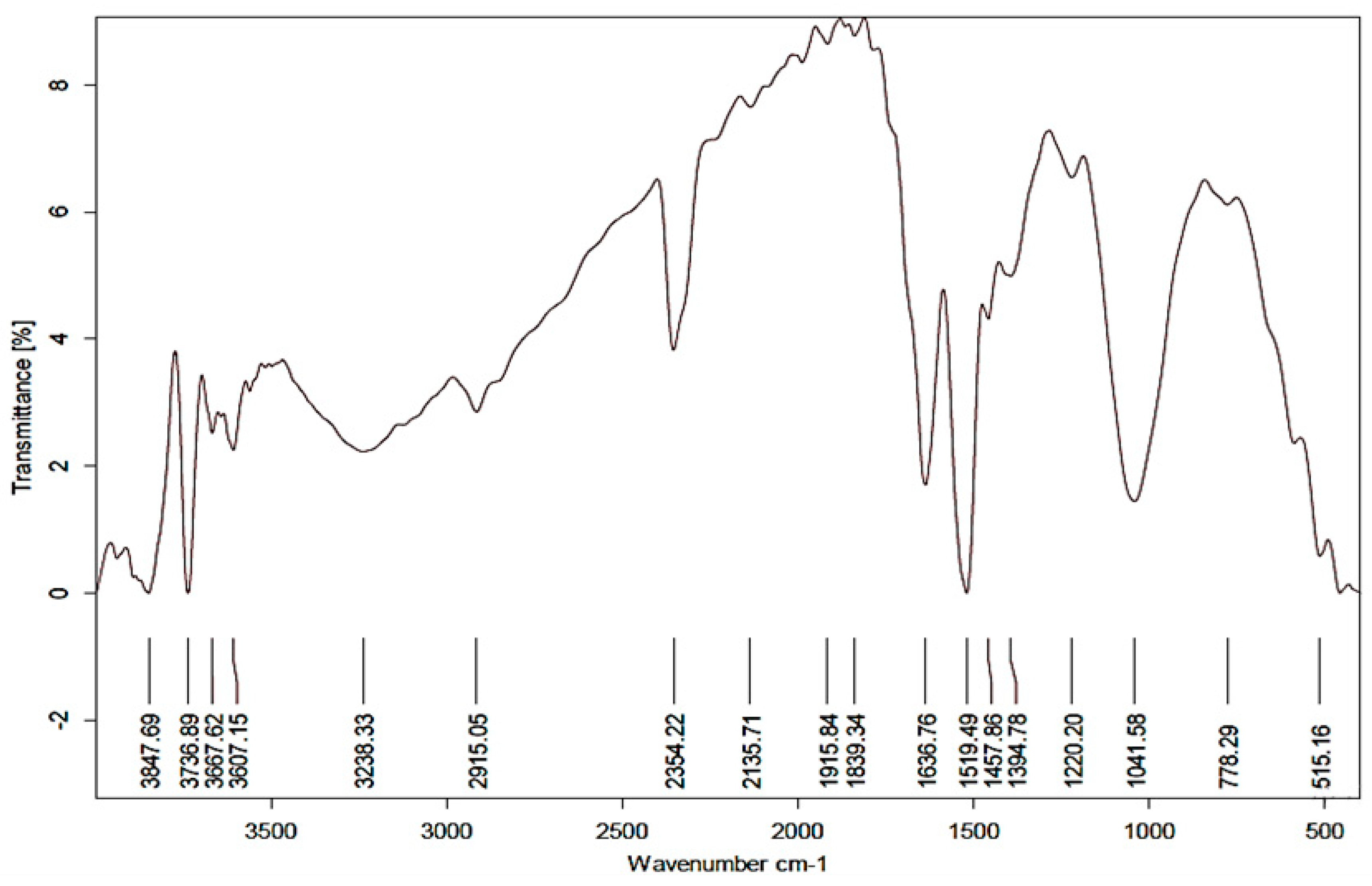
3.3. EDS Analysis and FTIR Spectroscopy
3.4. Effect of Ag-NPs on Growth Parameters and Viral Accumulation Level
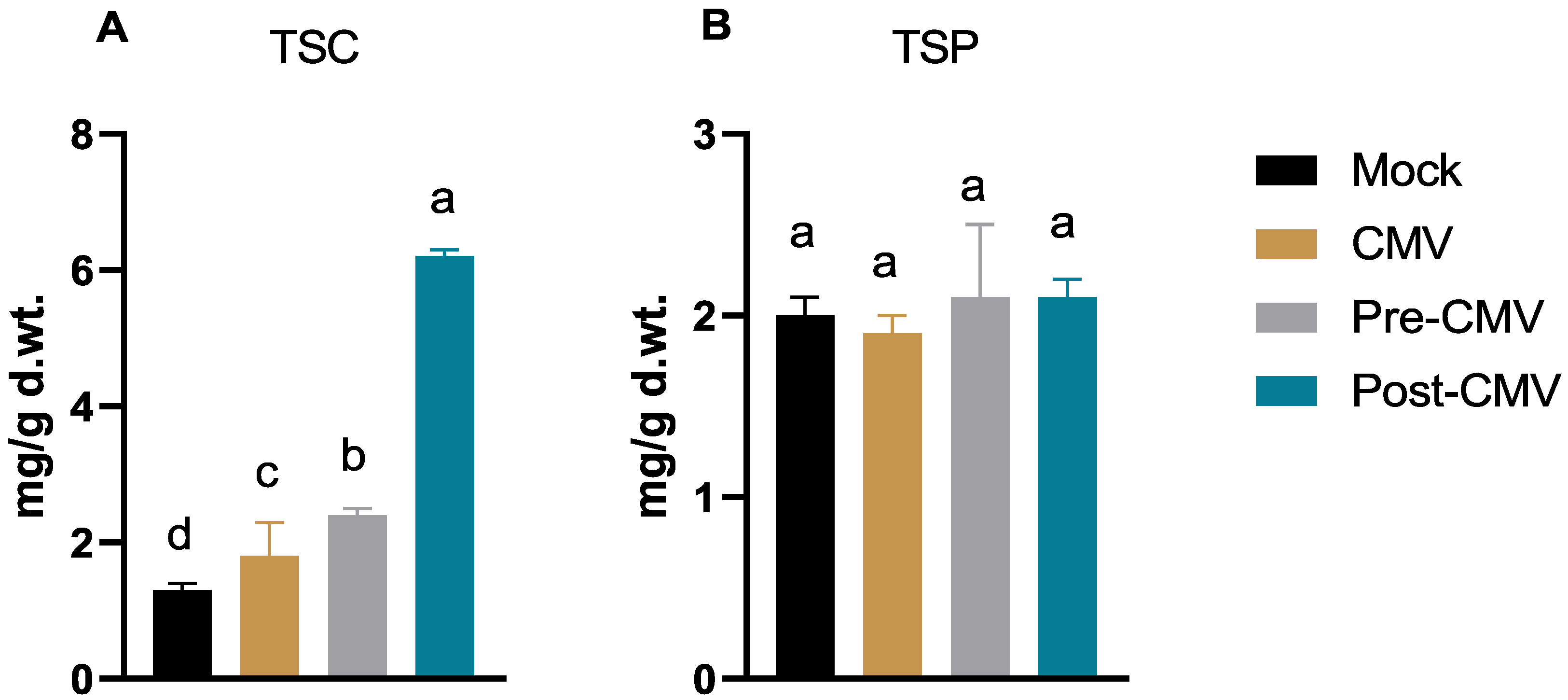
3.5. Total Soluble Carbohydrate and Protein Determination
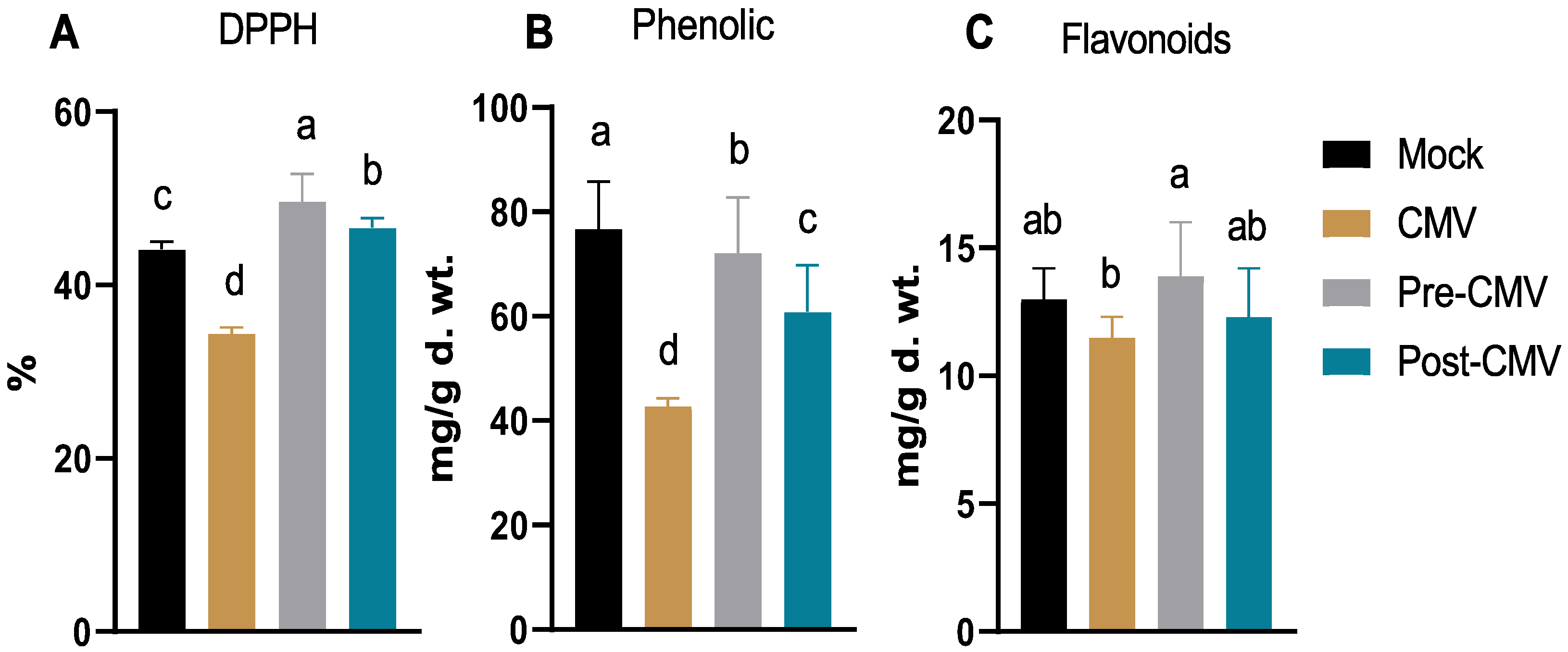
3.6. Evaluation of the Free Radical Scavenging Activity, Total Phenolic, and Flavonoid Contents
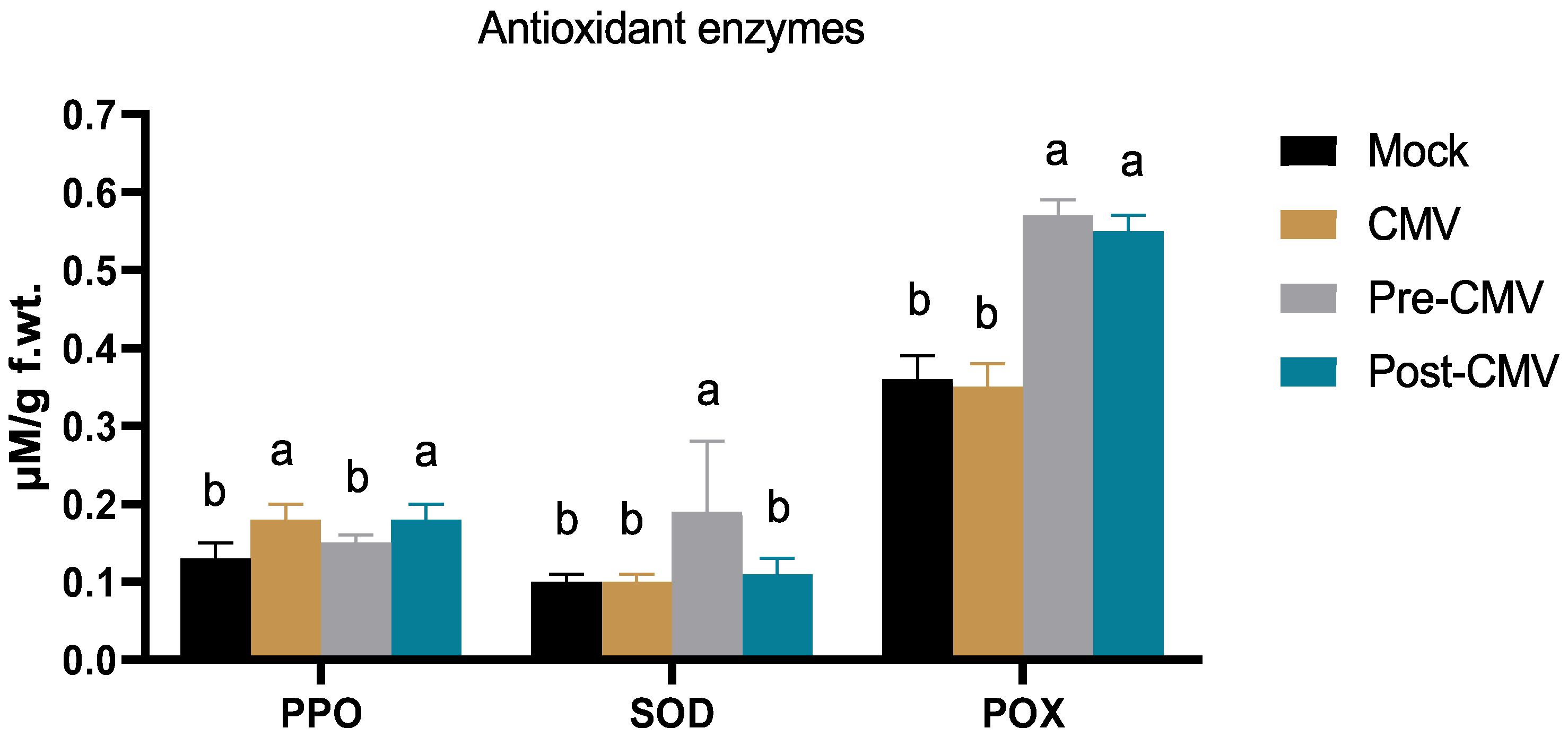
3.7. Impact of Ag-NPs on Antioxidant Enzymes Activity under CMV Challenge
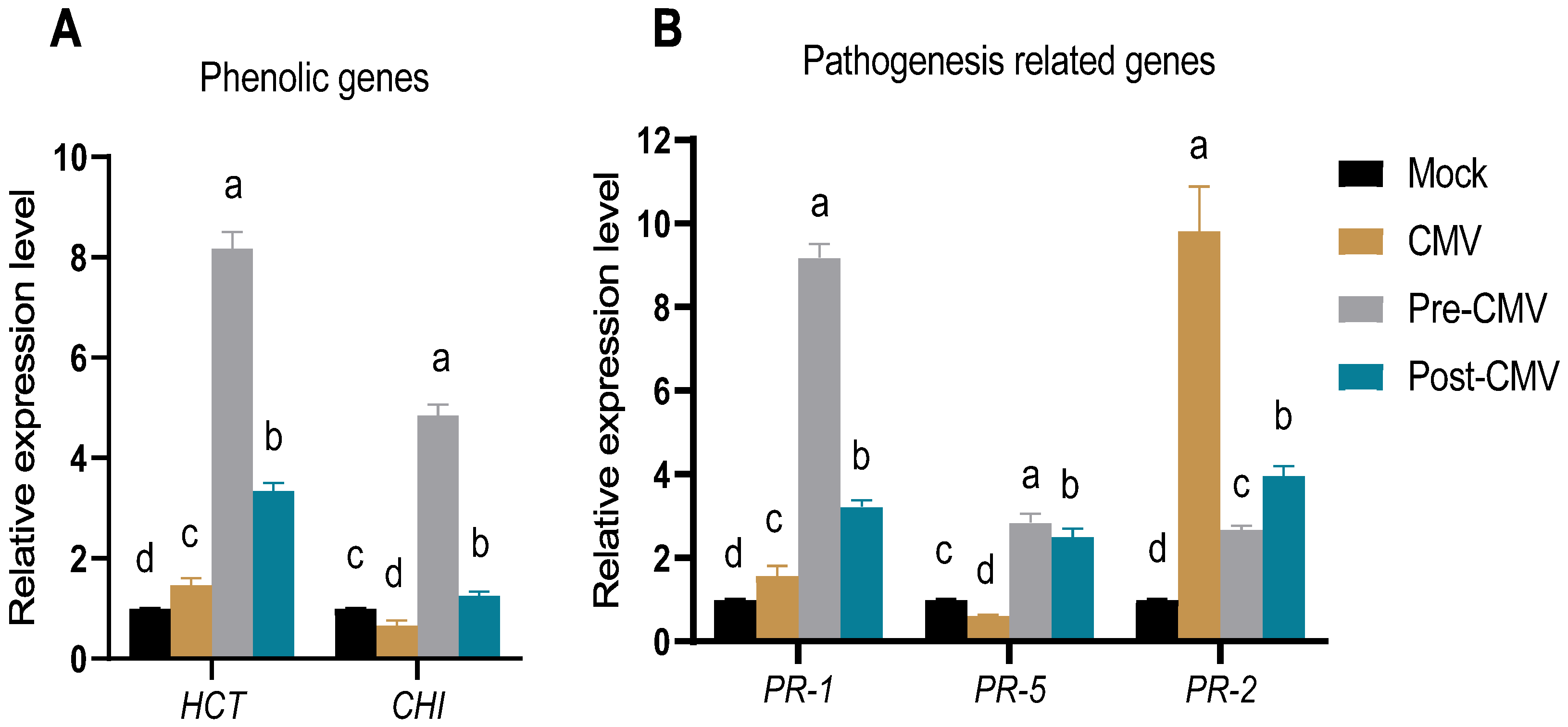
3.8. The Impact of Ag-NPs Application on Gene Expression under CMV Challenge
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novello, G.; Cesaro, P.; Bona, E.; Massa, N.; Gosetti, F.; Scarafoni, A.; Todeschini, V.; Berta, G.; Lingua, G.; Gamalero, E. The effects of plant growth-promoting bacteria with biostimulant features on the growth of a local onion cultivar and a commercial zucchini variety. Agronomy 2021, 11, 888. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Naidu, R.A. Global dimensions of plant virus diseases: Current status and future perspectives. Annu. Rev. Virol. 2019, 6, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.P.; Reddy, M.G. Overview of yield losses due to plant viruses. In Applied Plant Virology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 531–562. [Google Scholar]
- Karimi, K.; Sadeghi, A.; Maroufpoor, M.; Azizi, A. Induction of resistance to Myzus persicae-nicotianae in Cucumber mosaic virus infected tobacco plants using silencing of CMV-2b gene. Sci. Rep. 2022, 12, 4096. [Google Scholar] [CrossRef] [PubMed]
- Montes, N.; Alonso-Blanco, C.; García-Arenal, F. Cucumber mosaic virus infection as a potential selective pressure on Arabidopsis thaliana populations. PLoS Pathog. 2019, 15, e1007810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-K.; Jeong, R.-D.; Kwak, H.-R.; Lee, S.-H.; Kim, J.-S.; Kim, K.-H.; Cha, B.; Choi, H.-S. First Report of Cucumber mosaic virus Isolated from Wild Vigna angularis var. nipponensis in Korea. Plant Pathol. J. 2014, 30, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Gildow, F.E.; Shah, D.A.; Sackett, W.M.; Butzler, T.; Nault, B.A.; Fleischer, S.J. Transmission efficiency of Cucumber mosaic virus by aphids associated with virus epidemics in snap bean. Phytopathology 2008, 98, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Kim, K.S.; Anderson, E.J. Seed transmission of cucumber mosaic virus in spinach. Phytopathology 1997, 87, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Abdelkhalek, A.; Király, L.; Al-Mansori, A.-N.A.; Younes, H.A.; Zeid, A.; Elsharkawy, M.M.; Behiry, S.I. Defense Responses and Metabolic Changes Involving Phenylpropanoid Pathway and PR Genes in Squash (Cucurbita pepo L.) following Cucumber mosaic virus Infection. Plants 2022, 11, 1908. [Google Scholar] [CrossRef]
- Shongwe, L.T.; Masarirambi, M.T.; Oseni, T.O.; Wahome, P.K.; Nxumalo, K.A.; Gule, P.I. Effects of hydroponics systems on growth, yield and quality of zucchini (Cucurbita pepo L.). J. Plant Stud. 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Thanh, N.C.; Eed, E.M.; Elfasakhany, A.; Brindhadevi, K. Antioxidant, anti-inflammatory and anti-proliferative activities of green and yellow zucchini (Courgette). Appl. Nanosci. 2022, 3, 1–10. [Google Scholar] [CrossRef]
- Bhan, C.; Kannaujia, P.K.; Shrivastava, A.K.; Duhan, S.K.B.S.; Prasad, H.; Prasad, D. Antidiabetic potential of bioactive extracts from fruits and vegetables: A review. J. Progress. Agric. 2015, 6, 1–4. [Google Scholar]
- Hu, Q.; Niu, Y.; Zhang, K.; Liu, Y.; Zhou, X. Virus-derived transgenes expressing hairpin RNA give immunity to Tobacco mosaic virus and Cucumber mosaic virus. Virol. J. 2011, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revathy, K.A.; Jiby, M.V.; Bhat, A.I. Coat protein–mediated resistance to cucumber mosaic virus subgroup IB in black pepper (Piper nigrum L.). Vitr. Cell. Dev. Biol.—Plant 2022, 58, 351–360. [Google Scholar] [CrossRef]
- Lee, G.H.; Ryu, C.-M. Spraying of leaf-colonizing Bacillus amyloliquefaciens protects pepper from Cucumber mosaic virus. Plant Dis. 2016, 100, 2099–2105. [Google Scholar] [CrossRef] [Green Version]
- Mo, Q.; Lv, B.; Sun, Y.; Wu, X.; Song, L.; Cai, R.; Tang, X. Screening and production of dsRNA molecules for protecting Cucumis sativus against Cucumber mosaic virus through foliar application. Plant Biotechnol. Rep. 2022, 16, 409–418. [Google Scholar] [CrossRef]
- El Gamal, A.Y.; Tohamy, M.R.; Abou-Zaid, M.I.; Atia, M.M.; El Sayed, T.; Farroh, K.Y. Silver nanoparticles as a viricidal agent to inhibit plant-infecting viruses and disrupt their acquisition and transmission by their aphid vector. Arch. Virol. 2022, 167, 85–97. [Google Scholar] [CrossRef]
- Bapat, M.S.; Singh, H.; Shukla, S.K.; Singh, P.P.; Vo, D.V.N.; Yadav, A.; Goyal, A.; Sharma, A.; Kumar, D. Evaluating green silver nanoparticles as prospective biopesticides: An environmental standpoint. Chemosphere 2022, 286, 131761. [Google Scholar] [CrossRef]
- Heflish, A.A.; Hanfy, A.E.; Ansari, M.J.; Dessoky, E.S.; Attia, A.O.; Elshaer, M.M.; Gaber, M.K.; Kordy, A.; Doma, A.S.; Abdelkhalek, A. Green biosynthesized silver nanoparticles using Acalypha wilkesiana extract control root-knot nematode. J. King Saud Univ. 2021, 33, 101516. [Google Scholar]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize Under Drought Stress Conditions. J. Plant Growth Regul. 2022, 41, 364–375. [Google Scholar] [CrossRef]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of Silica Nanoparticles in Abiotic and Biotic Stress Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A. Green Synthesized ZnO Nanoparticles Mediated by Mentha Spicata Extract Induce Plant Systemic Resistance against Tobacco Mosaic Virus. Appl. Sci. 2020, 10, 5054. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Qari, S.H.; Abu-Saied, M.A.A.-R.; Khalil, A.M.; Younes, H.A.; Nehela, Y.; Behiry, S.I. Chitosan Nanoparticles Inactivate Alfalfa Mosaic Virus Replication and Boost Innate Immunity in Nicotiana glutinosa Plants. Plants 2021, 10, 2701. [Google Scholar] [CrossRef] [PubMed]
- Ouda, S.M. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 2014, 9, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Akpinar, I.; Unal, M.; Sar, T. Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 2021, 3, 506. [Google Scholar] [CrossRef]
- Sadeghi, B.; Rostami, A.; Momeni, S.S. Facile green synthesis of silver nanoparticles using seed aqueous extract of Pistacia atlantica and its antibacterial activity. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2015, 134, 326–332. [Google Scholar] [CrossRef]
- Tuncsoy, B. Nematicidal activity of silver nanomaterials against plant-parasitic nematodes. In Silver Nanomaterials for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 527–548. [Google Scholar] [CrossRef]
- Nassar, A.M.K. Effectiveness of Silver Nano-particles of Extracts of Urtica urens (Urticaceae) Against Root-knot Nematode Meloidogyne incognita. Asian J. Nematol. 2016, 5, 14–19. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Mackled, M.I.; Abdelsalam, N.R.; Behiry, S.I.; Al-Askar, A.A.; Basile, A.; Abdelkhalek, A.; Elsharkawy, M.M.; Salem, M.Z.M. Impact of Silver Nanoparticles on Lemon Growth Performance: Insecticidal and Antifungal Activities of Essential Oils From Peels and Leaves. Front. Plant Sci. 2022, 13, 898846. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.A.; Koul, A.M.; Jiang, L. Fabrication of Silver Nanoparticles Against Fungal Pathogens. Front. Nanotechnol. 2021, 3, 679358. [Google Scholar] [CrossRef]
- Noha, K.; Bondok, A.M.; El-Dougdoug, K.A. Evaluation of silver nanoparticles as antiviral agent against ToMV and PVY in tomato plants. Sciences 2018, 8, 100–111. [Google Scholar]
- Hafez, E.E.; El-Morsi, A.A.; El-Shahaby, O.A.; Abdelkhalek, A.A. Occurrence of iris yellow spot virus from onion crops in Egypt. Virus Disease 2014, 25, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelkhalek, A.; Al-Askar, A.A.; Elbeaino, T.; Moawad, H.; El-Gendi, H. Protective and Curative Activities of Paenibacillus polymyxa against Zucchini yellow mosaic virus Infestation in Squash Plants. Biology 2022, 11, 1150. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Ghosh, S.; Derle, A.; Ahire, M.; More, P.; Jagtap, S.; Phadatare, S.D.; Patil, A.B.; Jabgunde, A.M.; Sharma, G.K.; Shinde, V.S.; et al. Phytochemical analysis and free radical scavenging activity of medicinal plants Gnidia glauca and Dioscorea bulbifera. PLoS ONE 2013, 8, e82529. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Islam, M.J.; Kim, J.W.; Begum, M.K.; Sohel, M.A.; Lim, Y.-S. Physiological and Biochemical Changes in Sugar Beet Seedlings to Confer Stress Adaptability under Drought Condition. Plants 2020, 9, 1511. [Google Scholar] [CrossRef]
- Cho, Y.K.; Ahn, H.K. Purification and characterization of polyphenol oxidase from potato: II. Inhibition and catalytic mechanism. J. Food Biochem. 1999, 23, 593–605. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Angelini, R.; Manes, F.; Federico, R. Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 1990, 182, 89–96. [Google Scholar] [CrossRef]
- AbdEl-Rahim, W.M.; Khalil, W.K.B.; Eshak, M.G. Evaluation of the gene expression changes in Nile tilapia (Oreochromis niloticus) as affected by the bio-removal of toxic textile dyes from aqueous solution in small-scale bioreactor. Environmentalist 2010, 30, 242–253. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real- Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar]
- Habeeb Rahuman, H.B.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 2022, 16, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Ragab, G.A.; Saad-Allah, K.M. Green synthesis of sulfur nanoparticles using Ocimum basilicum leaves and its prospective effect on manganese-stressed Helianthus annuus (L.) seedlings. Ecotoxicol. Environ. Saf. 2020, 191, 110242. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Saroja, M.; Venkatachalam, M.; Evanjelene, V.K. Characterization and antibacterial activity of green synthesized ZnO nanoparticles from Ocimum basilicum leaf extract. Adv. Biores 2017, 8, 29–35. [Google Scholar] [CrossRef]
- Hawar, S.N.; Al-Shmgani, H.S.; Al-Kubaisi, Z.A.; Sulaiman, G.M.; Dewir, Y.H.; Rikisahedew, J.J. Green Synthesis of Silver Nanoparticles from Alhagi graecorum Leaf Extract and Evaluation of Their Cytotoxicity and Antifungal Activity. J. Nanomater. 2022, 2022, 1058119. [Google Scholar] [CrossRef]
- Elbeshehy, E.K.F.; Elazzazy, A.M.; Aggelis, G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against Bean Yellow Mosaic Virus and human pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef] [Green Version]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Gade, A.; Ingle, A.; Whiteley, C.; Rai, M. Mycogenic metal nanoparticles: Progress and applications. Biotechnol. Lett. 2010, 32, 593–600. [Google Scholar] [CrossRef]
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef] [Green Version]
- El-Gioushy, S.F.; Ding, Z.; Bahloul, A.M.E.; Gawish, M.S.; Abou El Ghit, H.M.; Abdelaziz, A.M.R.A.; El-Desouky, H.S.; Sami, R.; Khojah, E.; Hashim, T.A. Foliar Application of Nano, Chelated, and Conventional Iron Forms Enhanced Growth, Nutritional Status, Fruiting Aspects, and Fruit Quality of Washington Navel Orange Trees (Citrus sinensis L. Osbeck). Plants 2021, 10, 2577. [Google Scholar] [CrossRef] [PubMed]
- Sukhwal, A.; Jain, D.; Joshi, A.; Rawal, P.; Kushwaha, H.S. Biosynthesised silver nanoparticles using aqueous leaf extract of Tagetes patula L. and evaluation of their antifungal activity against phytopathogenic fungi. IET Nanobiotechnol. 2017, 11, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wan Mat Khalir, W.K.A.; Shameli, K.; Jazayeri, S.D.; Othman, N.A.; Che Jusoh, N.W.; Hassan, N.M. Biosynthesized Silver Nanoparticles by Aqueous Stem Extract of Entada spiralis and Screening of Their Biomedical Activity. Front. Chem. 2020, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Z.; Liu, H.; Zhang, L.; Gao, P.; Li, D. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf. B Biointerfaces 2011, 88, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Ramesh, V.; Thivaharan, V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab. J. Chem. 2017, 10, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Oves, M.; Rauf, M.A.; Hussain, A.; Qari, H.A.; Khan, A.A.P.; Muhammad, P.; Rehman, M.T.; Alajmi, M.F.; Ismail, I.I.M. Antibacterial silver nanomaterial synthesis from Mesoflavibacter zeaxanthinifaciens and targeting biofilm formation. Front. Pharmacol. 2019, 10, 801. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Zhang, Y.; Wang, C.; Lei, R.; Wu, Y.; Li, X.; Zhu, S. Cucumber mosaic virus coat protein induces the development of chlorotic symptoms through interacting with the chloroplast ferredoxin I protein. Sci. Rep. 2018, 8, 1205. [Google Scholar] [CrossRef] [Green Version]
- Mahfouze, H.A.; El-Dougdoug, N.K.; Mahfouze, S.A. Virucidal activity of silver nanoparticles against Banana bunchy top virus (BBTV) in banana plants. Bull. Natl. Res. Cent. 2020, 44, 199. [Google Scholar] [CrossRef]
- Khandelwal, N.; Kaur, G.; Kumar, N.; Tiwari, A. Application of Silver Nanoparticles in Viral Inhibition: A New Hope for Antivirals. Dig. J. Nanomater. Biostructures 2014, 9, 175–186. [Google Scholar]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Shalitin, D.; Wolf, S. Cucumber mosaic virus infection affects sugar transport in melon plants. Plant Physiol. 2000, 123, 597–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeel, M.T.; Amer, M.A.; Al-Saleh, M.A.; Ashfaq, M.; Haq, M.I. Changes in chlorophyll, phenols, sugars and mineral contents of cucumber plants infected with cucumber mosaic virus. J. Phytopathol. Pest Manag. 2016, 3, 1–11. [Google Scholar]
- Gonçalves, M.C.; Vega, J.; Oliveira, J.G.; Gomes, M. Sugarcane yellow leaf virus infection leads to alterations in photosynthetic efficiency and carbohydrate accumulation in sugarcane leaves. Fitopatol. Bras. 2005, 30, 10–16. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Reddy, N.V.; Satyanarayana, B.M.; Sivasankar, S.; Pragathi, D.; Subbaiah, K.V.; Vijaya, T. Eco-friendly synthesis of silver nanoparticles using leaf extract of Flemingia wightiana: Spectral characterization, antioxidant and anticancer activity studies. SN Appl. Sci. 2020, 2, 884. [Google Scholar] [CrossRef] [Green Version]
- Varadavenkatesan, T.; Vinayagam, R.; Selvaraj, R. Structural characterization of silver nanoparticles phyto-mediated by a plant waste, seed hull of Vigna mungo and their biological applications. J. Mol. Struct. 2017, 1147, 629–635. [Google Scholar] [CrossRef]
- Johnson, P.; Krishnan, V.; Loganathan, C.; Govindhan, K.; Raji, V.; Sakayanathan, P.; Vijayan, S.; Sathishkumar, P.; Palvannan, T. Rapid biosynthesis of Bauhinia variegata flower extract-mediated silver nanoparticles: An effective antioxidant scavenger and α-amylase inhibitor. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.H.; Soshnikova, V.; Markus, J.; Kim, Y.J.; Lee, S.C.; Singh, P.; Castro-Aceituno, V.; Ahn, S.; Kim, D.H.; Shim, Y.J. Biosynthesized gold and silver nanoparticles by aqueous fruit extract of Chaenomeles sinensis and screening of their biomedical activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco Mosaic Virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Rendina, N.; Nuzzaci, M.; Sofo, A.; Campiglia, P.; Scopa, A.; Sommella, E.; Pepe, G.; De Nisco, M.; Basilicata, M.G.; Manfra, M. Yield parameters and antioxidant compounds of tomato fruit: The role of plant defence inducers with or without Cucumber mosaic virus infection. J. Sci. Food Agric. 2019, 99, 5541–5549. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 2022, 8, 301. [Google Scholar] [CrossRef]
- Nagai, A.; Duarte, L.M.L.; Chaves, A.L.R.; dos Santos, D.Y.A.C. Potato virus Y infection affects flavonoid profiles of Physalis angulata L. Brazilian J. Bot. 2015, 38, 729–735. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Ilyas, N.; Naz, F.; Amjad, M.S.; Malik, N.Z.; Chaudhari, S.K. Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis. Green Process. Synth. 2022, 11, 29–43. [Google Scholar] [CrossRef]
- El-Waseif, A.A.; Attia, M.S.; El-Ghwas, D.E. Potential effects of silver nanoparticles, synthesized from Streptomyces clavuligerus, for controlling of wilt disease caused by Fusarium oxysporum. Egypt. Pharm. J. 2019, 18, 228. [Google Scholar] [CrossRef]
- Hernández, J.A.; Gullner, G.; Clemente-Moreno, M.J.; Künstler, A.; Juhász, C.; Díaz-Vivancos, P.; Király, L. Oxidative stress and antioxidative responses in plant–virus interactions. Physiol. Mol. Plant Pathol. 2016, 94, 134–148. [Google Scholar] [CrossRef]
- Lan, H.; Lai, B.; Zhao, P.; Dong, X.; Wei, W.; Ye, Y.; Wu, Z. Cucumber mosaic virus infection modulated the phytochemical contents of Passiflora edulis. Microb. Pathog. 2020, 138, 103828. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Arishi, A.A.; Behiry, S.I. Trichoderma hamatum Strain Th23 Promotes Tomato Growth and Induces Systemic Resistance against Tobacco Mosaic Virus. J. Fungi 2022, 8, 228. [Google Scholar] [CrossRef]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Li, B.; Hong, Q.; Yan, Z.; Yang, X.; Lu, K.; Chen, G.; Wang, L.; Chen, Y. A Glutathione Peroxidase Gene from Litopenaeus vannamei Is Involved in Oxidative Stress Responses and Pathogen Infection Resistance. Int. J. Mol. Sci. 2022, 23, 567. [Google Scholar] [CrossRef] [PubMed]
- Smit, F.; Dubery, I.A. Cell wall reinforcement in cotton hypocotyls in response to a Verticillium dahliae elicitor. Phytochemistry 1997, 44, 811–815. [Google Scholar] [CrossRef]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Padmakshan, D.; Timokhin, V.I.; Lu, F.; Schatz, P.F.; Vanholme, R. Synthesis of hydroxycinnamoyl shikimates and their role in monolignol biosynthesis. Holzforschung 2022, 76, 133–144. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef] [PubMed]
- André, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.-F.; Larondelle, Y.; Evers, D. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 2009, 70, 1107–1116. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Dessoky, E.S.E.S.; Hafez, E. Polyphenolic genes expression pattern and their role in viral resistance in tomato plant infected with Tobacco mosaic virus. Biosci. Res. 2018, 15, 3349–3356. [Google Scholar]
- Chung, I.-M.; Rajakumar, G.; Thiruvengadam, M. Effect of silver nanoparticles on phenolic compounds production and biological activities in hairy root cultures of Cucumis anguria. Acta Biol. Hung. 2018, 69, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Heflish, A.A.; Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Protective and Curative Effects of Trichoderma asperelloides Ta41 on Tomato Root Rot Caused by Rhizoctonia solani Rs33. Agronomy 2021, 11, 1162. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Wei, X.; Zhang, J.; Wang, H.; Liu, D. Expression analysis and functional characterization of a pathogen-induced thaumatin-like gene in wheat conferring enhanced resistance to Puccinia triticina. J. Plant Interact. 2017, 12, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Abdelkhalek, A.; El-Gendi, H.; Al-Askar, A.A.; Maresca, V.; Moawad, H.; Elsharkawy, M.M.; Younes, H.A.; Behiry, S.I. Enhancing systemic resistance in faba bean (Vicia faba L.) to Bean yellow mosaic virus via soil application and foliar spray of nitrogen-fixing Rhizobium leguminosarum bv. viciae strain 33504-Alex1. Front. Plant Sci. 2022, 13, 933498. [Google Scholar] [CrossRef] [PubMed]
- Beffa, R.S.; Hofer, R.M.; Thomas, M.; Meins, F.J. Decreased Susceptibility to Viral Disease of [beta]-1,3-Glucanase-Deficient Plants Generated by Antisense Transformation. Plant Cell 1996, 8, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Bucher, G.L.; Tarina, C.; Heinlein, M.; Di Serio, F.; Meins, F., Jr.; Iglesias, V.A. Local expression of enzymatically active class I β-1, 3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001, 28, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR 2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Rezzonico, E.; Flury, N.; Meins, F.; Beffa, R. Transcriptional down-regulation by abscisic acid of pathogenesis-related β-1, 3-glucanase genes in tobacco cell cultures. Plant Physiol. 1998, 117, 585–592. [Google Scholar] [CrossRef] [Green Version]
- ElMorsi, A.; Abdelkhalek, A.; Alshehaby, O.; Hafez, E.E.E.E. Pathogenesis-related genes as tools for discovering the response of onion defence system against iris yellow spot virus infection. Botany 2015, 93, 735–744. [Google Scholar] [CrossRef]

| Primer Name | Abbreviation | Direction | Nucleotide Sequence |
|---|---|---|---|
| Cucumber mosaic virus-coat protein | CMV-CP | Forward | GGATGCTTCTCCACGAG |
| Reverse | AGTGACTTCAGGCAGT | ||
| Hydroxycinnamoyl transferase | HCT | Forward | TCTCCAACCCCT TTTAACGAACC |
| Reverse | CAACTTGTCCTTCTACCACAGGGAA | ||
| Pathogenesis related protein-1 | PR-1 | Forward | CCAAGACTATCTTGCGGTTC |
| Reverse | GAACCTAAGCCACGATACCA | ||
| Chalcone isomerase | CHI | Forward | GGCAGGCCATTGAAAAGTTCC |
| Reverse | CTAATCGTCAATGATCCAAGCGG | ||
| Endoglucanase | PR-2 | Forward | TCAATTATCAAAACTTGTTC |
| Reverse | AACCGGTCTCGGATACAAC | ||
| Thaumatin-like protein | PR-5 | Forward | CCGAGGTAATTGTGAGACTGGAG |
| Reverse | CCTGATTGGGTTGATTAAGTGCA | ||
| Elongation factor 1-alpha | EF1a | Forward | ATTCGAGAAGGAAGCTGCTG |
| Reverse | TTGGTGGTCTAAACTTCCAC |
| Treatment Groups | Growth Parameters | The Relative Expression Level of CMV-CP | |
|---|---|---|---|
| Fresh Weight | Dry Weight | ||
| Mock | 9.71 ± 1.21 a | 0.96 ± 0.12 a | 00.00 ± 0.01 d |
| CMV | 7.29 ± 1.02 d | 0.72 ± 0.22 d | 82.71 ± 1.94 a |
| Pre-CMV | 8.83 ± 0.92 b | 0.93 ± 0.23 b | 06.88 ± 0.94 c |
| Post-CMV | 8.37 ± 1.02 c | 0.88 ± 0.25 c | 11.66 ± 0.98 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkhalek, A.; El-Gendi, H.; Alotibi, F.O.; Al-Askar, A.A.; Elbeaino, T.; Behiry, S.I.; Abd-Elsalam, K.A.; Moawad, H. Ocimum basilicum-Mediated Synthesis of Silver Nanoparticles Induces Innate Immune Responses against Cucumber Mosaic Virus in Squash. Plants 2022, 11, 2707. https://doi.org/10.3390/plants11202707
Abdelkhalek A, El-Gendi H, Alotibi FO, Al-Askar AA, Elbeaino T, Behiry SI, Abd-Elsalam KA, Moawad H. Ocimum basilicum-Mediated Synthesis of Silver Nanoparticles Induces Innate Immune Responses against Cucumber Mosaic Virus in Squash. Plants. 2022; 11(20):2707. https://doi.org/10.3390/plants11202707
Chicago/Turabian StyleAbdelkhalek, Ahmed, Hamada El-Gendi, Fatimah O. Alotibi, Abdulaziz A. Al-Askar, Toufic Elbeaino, Said I. Behiry, Kamel A. Abd-Elsalam, and Hassan Moawad. 2022. "Ocimum basilicum-Mediated Synthesis of Silver Nanoparticles Induces Innate Immune Responses against Cucumber Mosaic Virus in Squash" Plants 11, no. 20: 2707. https://doi.org/10.3390/plants11202707
APA StyleAbdelkhalek, A., El-Gendi, H., Alotibi, F. O., Al-Askar, A. A., Elbeaino, T., Behiry, S. I., Abd-Elsalam, K. A., & Moawad, H. (2022). Ocimum basilicum-Mediated Synthesis of Silver Nanoparticles Induces Innate Immune Responses against Cucumber Mosaic Virus in Squash. Plants, 11(20), 2707. https://doi.org/10.3390/plants11202707










