Maize Lodging Resistance with Plastic Film Removal, Increased Planting Density, and Cultivars with Different Maturity Periods
Abstract
:1. Introduction
2. Results
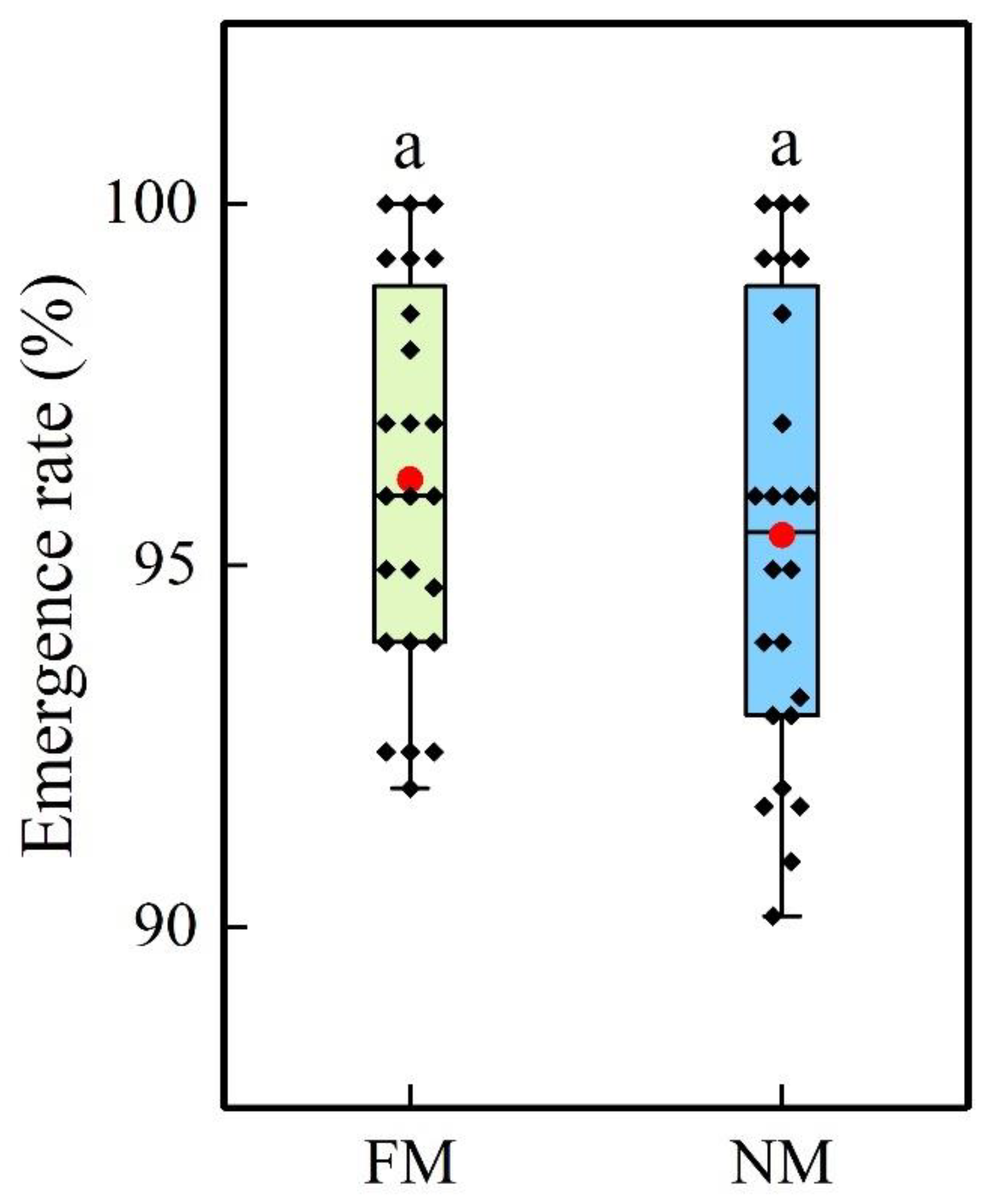
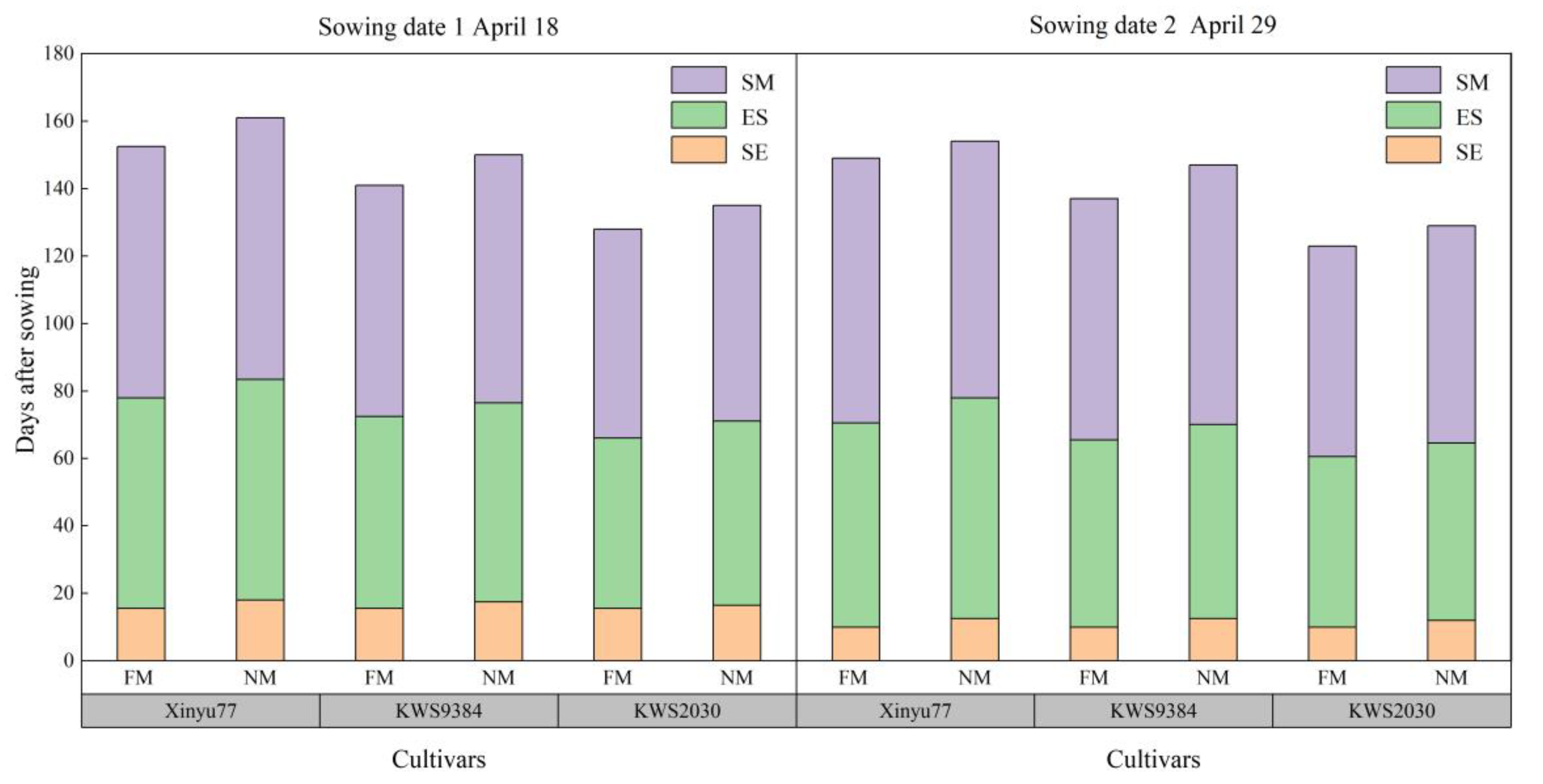
2.1. Emergence Rate and Growth Period
2.2. Stalk Breaking Force
2.3. Plant and Basal Internode Morphologies
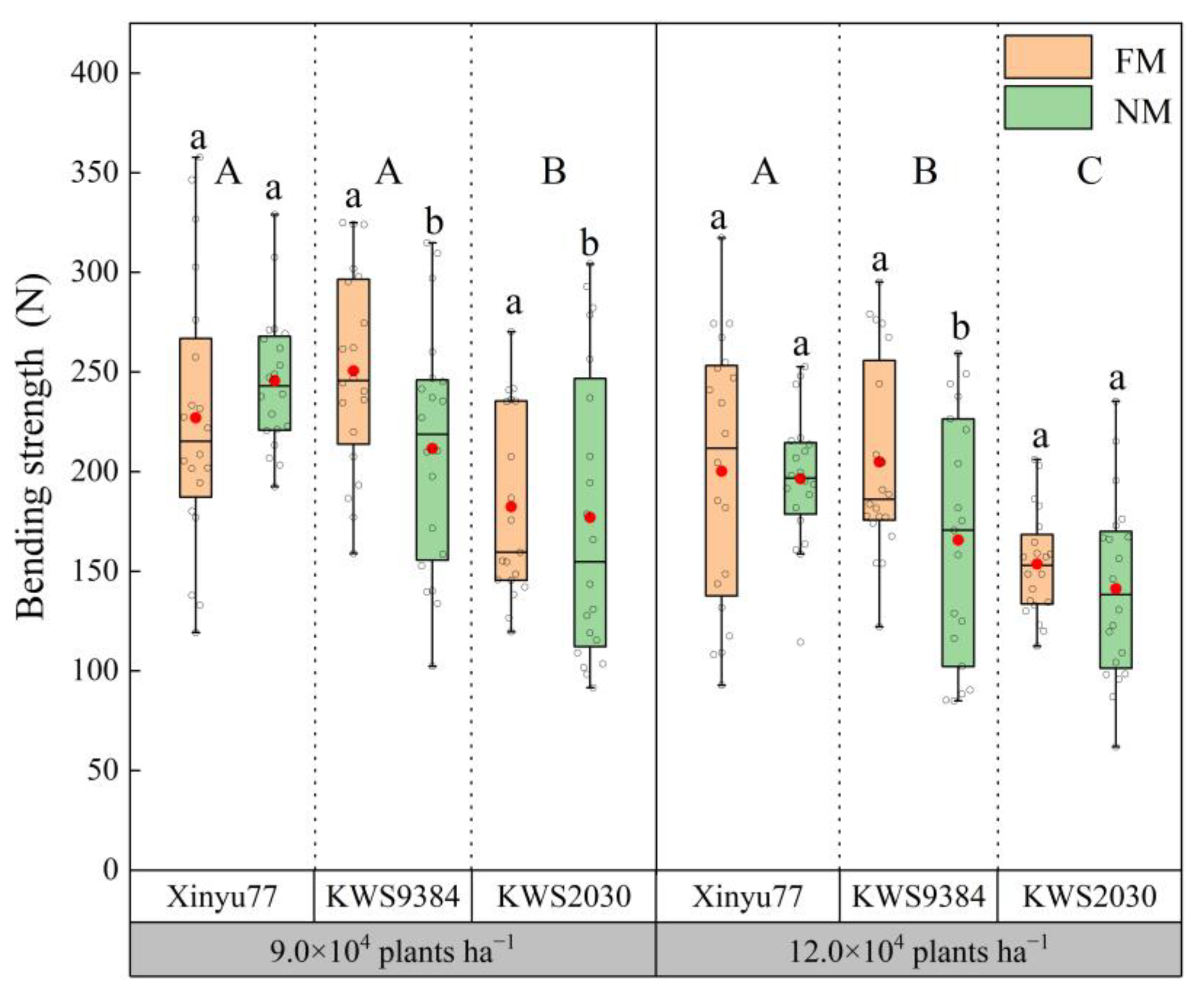
2.4. Basal Internode Bending Strength
2.5. Dry Weight per Unit length of the Basal Internode
2.6. Vertical Root-Pulling Force
2.7. Root Morphology
2.8. Factors That Influenced the Maize Stalk Breaking Force
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Management
4.2. Sampling and Measurements
4.2.1. Growth Period
4.2.2. Plant and Internode Measurements and Strengths
4.2.3. Vertical Root-Pulling Force
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enrique, G.S.; Braud, I.; Jean, L.T.; Michel, V.; Pierre, B.; Jean, C.C. Modelling heat and water exchanges of fallow land covered with plant-residue mulch. Agric. For. Meteorol. 1999, 97, 151–169. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, E.; Li, F.M.; Li, F.R. Runoff efficiency and the technique of micro-water harvesting with ridges and furrows, for potato production in semi-arid areas. Water Resour. Manag. 2008, 22, 1431–1443. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xie, Z.K.; Malhi, S.S.; Vera, C.L.; Zhang, Y.B.; Wang, J.N. Effects of rainfall harvesting and mulching technologies on water use efficiency and crop yield in the semi-arid loess plateau, China. Agric. Water Manag. 2009, 96, 374–382. [Google Scholar] [CrossRef]
- Zegada, L.W.; Berliner, P.R. Interrow mulch increase the water use efficiency of furrow-irrigated maize in an arid environment. J. Agron. Crop Sci. 2011, 197, 237–248. [Google Scholar] [CrossRef]
- Yang, Q.F.; Yue, Y.; Xiong, C.R.; Sun, D.X. Influence of different approaches of plastic film mulching on soil temperature of maize field in dry plateau of Longdong. Agric. Res. Arid. Areas 2008, 26, 29–33. [Google Scholar]
- Fang, Y.J.; Huang, G.B.; Li, L.L.; Wang, J. Yield and growth dynamics of rainfed maize in the system of completely mulched alternating narrow and wide ridges with furrow planting. Agric. Res. Arid. Areas 2010, 28, 128–134. [Google Scholar]
- Zhang, L.; Niu, F.J.; Li, X.Y.; Dou, G.L.; Li, D.R. Effects of planting in furrow and whole plastic-film mulching on double ridges in autumn on yield index of corn production and water use efficiency in dry lands. Chin. Agric. Sci. Bull. 2010, 26, 142–145. [Google Scholar]
- Ren, X.M.; Sun, D.B.; Wang, Q.S. Effects of plastic film mulching and plant density on yield and evapotranspiration of rainfed spring maize. Trans. Chin. Soc. Agric. Mach. 2017, 48, 206–211. [Google Scholar]
- Lv, W.; Dong, L.; Sun, Y.H.; Li, Y. Weed control methods at home and abroad. Chin. Agric. Sci. Bull. 2018, 34, 34–39. [Google Scholar]
- Du, T.; Song, L.; Luo, S.; Zhao, Y.H. Analysis on the status quo of waste mulching films recycling and related standards in China. Recycl. Resour. Circ. Econ. 2020, 13, 24–26. [Google Scholar]
- Yang, H.D. Farmland Plastic Film and Ecological Environment Protection; Chemical Industry Press: Beijing, China, 2000; pp. 110–113. [Google Scholar]
- Liu, E.K.; He, W.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’: Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.R.; He, W.Q.; Turner, N.C.; Liu, E.; Liu, Q.; Liu, S. Plastic-film mulch in Chinese agriculture: Importance and problems. World Agric. 2014, 4, 32–36. [Google Scholar]
- Xie, H.E.; Li, Y.S.; Yang, S.Q.; Wang, J.J.; Wu, X.F.; Wu, Z.X. Influence of residual plastic film on soil structure, crop growth and development in fields. J. Agro-Environ. Sci. 2007, 26, 153–156. [Google Scholar]
- Gao, Q.H.; Lu, X.M. Effects of plastic film residue on morphology and physiological characteristics of tomato seedlings. J. Trop. Subtrop. Bot. 2011, 19, 425–429. [Google Scholar]
- Dong, H.G.; Liu, T.; Li, Y.G.; Liu, H.F.; Wang, D. Effects of plastic film residue on cotton yield and soil physical and chemical properties in Xinjiang. Trans. Chin. Soc. Agric. Eng. 2013, 29, 91–99. [Google Scholar]
- Kim, Y.; Berger, S.; Kettering, J.; Tenhunen, J.; Haas, E.; Kiese, R. Simulation of N2O emissions and nitrate leaching from plastic mulch radish cultivation with Landscape DNDC. Ecol. Res. 2014, 29, 441–454. [Google Scholar] [CrossRef]
- Bai, J.; Wang, J.; Chen, X.; Luo, G.P.; Shi, H.; Li, L.H.; Li, J.L. Seasonal and interannual variations in carbon fluxes and evapotranspiration over cotton field under drip irrigation with plastic mulch in an arid region of Northwest China. J. Arid Land 2015, 7, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, L.J.; Li, Z.; Sun, F. Ecological thought of prevention and cure of agricultural tri-dimension pollution. Acta Ecol. Sin. 2005, 25, 904–909. [Google Scholar]
- Xu, Y.M.; Fang, S.J.; Ma, X.P.; Zhu, Q.Q. Prevention and control strategy for the pollution of agricultural plastic film. Strateg. Study Chin. Acad. Eng. 2018, 20, 96–102. [Google Scholar] [CrossRef]
- Li, J.; Xie, R.Z.; Wang, K.R.; Ming, B.; Gou, Y.Q.; Zhang, G.Q.; Li, S.K. Variations in maize dry matter, harvest index, and grain yield with plant density. Agron. J. 2015, 107, 829–834. [Google Scholar] [CrossRef]
- Assefa, Y.; Carter, P.; Hinds, M.; Bhalla, G.; Schon, R.; Jeschke, M.; Paszkiewicz, S.; Smith, S.; Ciampitti, I.A. Analysis of long term study indicates both agronomic optimal plant density and increase maize yield per plant contributed to yield gain. Sci. Rep. 2018, 8, 4937. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Huang, K.J.; Huang, Y.H.; Tan, H.; Wei, G.N. Main characteristics and Cultivation Techniques of semi compact long ear maize single cross variety GUI 22. J. Guangxi Agric. 1998, 1, 43–44. [Google Scholar]
- Chen, X.P.; Cui, Z.L.; Vitousek, P.M.; Cassman, K.G.; Matson, P.A.; Bai, J.S.; Meng, Q.F.; Hou, P.; Yue, S.C.; Romheld, V.; et al. Integrated soil crop system management for food security. Proc. Natl. Acad. Sci. USA 2011, 108, 6399–6404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.; Hou, P.; Wu, L.; Chen, X.; Cui, Z.; Zhang, F. Understanding production potentials and yield gaps in intensive maize production in China. Field Crops Res. 2013, 143, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.R.; Wang, R.H.; Zhao, J.R.; Yu, Y.; Hao, Y.B.; Xu, T.J.; Jiang, Y.B.; Gong, X.J.; Li, L.; Ge, X.L. Study on the grain filling characteristics and their relationship with temperature of maize hybrids differing in maturities. J. Agric. Sci. Technol. 2017, 19, 105–114. [Google Scholar]
- Assefa, Y.; Prasad, P.V.V.; Carter, P.; Hinds, M.; Bhalla, G.; Schon, R.; Jeschke, M.; Paszkiewicz, S.; Ciampitti, I.A. Yield responses to planting density for US modern corn hybrids: A synthesis-analysis. Crop Sci. 2016, 56, 2802–2817. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, A.J.; Thomison, P.R. Drought-tolerant corn hybrid and relative maturity yield response to plant population and planting date. Agron. J. 2016, 108, 229–242. [Google Scholar] [CrossRef]
- Huang, S.B.; Lv, L.H.; Zhu, J.C.; Li, Y.B.; Tao, H.B.; Wang, P. Extending growing period is limited to offsetting negative effects of climate changes on maize yield in the North China Plain. Field Crops Res. 2018, 215, 66–73. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, X.L.; Xu, C.C.; Tan, W.M.; Wang, P.; Meng, Q.F. Decreased Kernel Moisture in Medium-Maturing Maize Hybrids with High Yield for Mechanized Grain Harvest. Crop Sci. 2019, 59, 2794–2805. [Google Scholar] [CrossRef]
- Li, L.L.; Xue, J.; Xie, R.Z.; Wang, K.R.; Ming, B.; Hou, P.; Gao, S.; Li, S.K. Effects of grain moisture content on mechanical grain harvesting quality of summer maize. Acta Agron. Sin. 2018, 44, 1747–1754. [Google Scholar] [CrossRef]
- Li, S.K.; Wang, K.R.; Xie, R.Z.; Ming, B. Grain Mechanical Harvesting Technology Promotes the Transformation of Maize Production Mode. Sci. Agric. Sin. 2018, 51, 1842–1844. [Google Scholar]
- Yu, S.X. The significance of filmless cotton to promote the transformation and upgrading of China’s cotton industry. J. Agric. 2019, 9, 1–5. [Google Scholar]
- Tian, B.H. The methods of crop lodging resistance assessment and their application in foxtail millet. J. Plant Genet. Resour. 2013, 14, 265–269. [Google Scholar]
- Wang, Q.X.; Wang, P.; Yang, X.Y.; Zhai, Z.X.; Wang, X.L.; Shen, L.X. Effects of nitrogen application time on root distribution and its activity in maize (Zea mays L.). Sci. Agric. Sin. 2003, 12, 1469–1475. [Google Scholar]
- Bian, D.H.; Jia, G.P.; Cai, L.J.; Ma, Z.Y.; Eneji, A.E.; Cui, Y.H. Effects of tillage practices on root characteristics and root lodging resistance of maize. Field Crops Res. 2016, 185, 89–96. [Google Scholar] [CrossRef]
- Xue, J.; Gao, S.; Fan, Y.H.; Li, L.L.; Ming, B.; Wang, K.R.; Xie, R.Z.; Hou, P.; Li, S.K. Traits of plant morphology, stalk mechanical strength, and biomass accumulation in the selection of lodging-resistant maize cultivars. Eur. J. Agron. 2020, 117, 126073. [Google Scholar] [CrossRef]
- Berry, P.M.; Baker, C.J.; Hatley, D.; Dong, R.; Wang, X.; Blackburn, G.A.; Miao, Y.; Sterling, M.; Whyatt, J.D. Development and application of a model for calculating the risk of stem and root lodging in maize. Field Crops Res. 2021, 262, 108037. [Google Scholar] [CrossRef]
- Beck, D.L.; Darrah, L.L.; Zuber, M.S. An improved technique for measuring resistance to root pulling in maize. Crop Sci. 1987, 27, 356–358. [Google Scholar] [CrossRef]
- Li, D.X.; Kang, H.; Yuan, H.Y. Research methods of crop lodging resistance. Shanxi J. Agric. Sci. 2001, 7, 20–22. [Google Scholar]
- Xue, J.; Ming, B.; Xie, R.Z.; Wang, K.R.; Hou, P.; Li, S.K. Evaluation of maize lodging resistance based on the critical wind speed of stalk breaking during the late growth stage. Plant Methods 2020, 16, 148–160. [Google Scholar] [CrossRef]
- Ma, W.Y.; Ci, J.B.; Jiang, L.Y.; Yang, H.M.; Luan, Y.; Yang, W.G. Genetic improvement effect of lodging resistance related traits in maize. J. Jilin Agric. Univ. 2022, 44, 28–34. [Google Scholar]
- Xue, J.; Gou, L.; Zhao, Y.S.; Yao, M.N.; Yao, H.S.; Tian, J.S.; Zhang, W.F. Effects of light intensity within the canopy on maize lodging. Field Crops Res. 2016, 188, 133–141. [Google Scholar] [CrossRef]
- Xue, J.; Xie, R.Z.; Zhang, W.F.; Wang, K.R.; Hou, P.; Ming, B.; Gou, L.; Li, S.K. Research progress on reduced lodging of high-yield and density maize. J. Integr. Agric. 2017, 16, 2717–2725. [Google Scholar] [CrossRef]
- Wang, N.; Li, F.H.; Wang, Z.B.; Wang, H.W.; Lv, X.L.; Zhou, Y.F.; Shi, Z.S. Response to plant density of stem characters of maize hybrids and its relationship to lodging. Crops 2011, 3, 67–69. [Google Scholar]
- Liu, X.; Xie, R.Z.; Niu, X.K.; Xiu, W.W.; Li, S.K.; Gao, S.J.; Zhang, F.L. Effects of planting density on lodging resistance performance of maize varieties of different eras in north-east China. Crops 2012, 5, 126–130. [Google Scholar]
- Liang, R.Q.; Chen, X.G.; Zhang, B.C.; Meng, H.W.; Jiang, P.; Peng, X.B.; Kan, Z.; Li, W.M. Problems and countermeasures of recycling methods and resource reuse of residual film in cotton fields of Xinjiang. Trans. Chin. Soc. Agric. Eng. 2019, 35, 1–13. [Google Scholar]
- Xue, J.; Wang, Q.; Li, L.L.; Zhang, W.X.; Xie, R.Z.; Wang, K.R.; Ming, B.; Hou, P.; Li, S.K. Changes of maize lodging after physiological maturity and its influencing factors. Acta Agron. Sin. 2018, 44, 1782–1792. [Google Scholar] [CrossRef]
- Liu, X.M.; Gu, W.R.; Li, C.F.; Tong, T.; Wan, B.; Lv, Y.J.; Zhao, M.; Liu, Z.Y. Effects of nitrogen fertilization and chemical control on stalk traits and yield of spring maize under super high planting density in Heilongjiang province. Chin. J. Ecol. 2019, 38, 450–458. [Google Scholar]
- Esechie, H.A. Relationship of stalk morphology and chemical composition to lodging resistance in maize (Zea mays L.) in a rainforest zone. J. Agric. Sci. 1985, 104, 429–433. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wang, H.Q.; Wu, X.R.; Xu, J.; Liu, T.N.; Ding, R.X.; Han, Q.F. Mepiquat chloride application increases lodging resistance of maize by enhancing stem physical strength and lignin biosynthesis. Field Crops Res. 2018, 224, 148–159. [Google Scholar] [CrossRef]
- Robertson, D.J.; Julias, M.; Lee, S.Y.; Cook, D.D. Maize stalk lodging: Morphological determinants of stalk strength. Crop Sci. 2017, 57, 926–934. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.L.; Liu, P.; Zhang, X.X.; Zheng, Q.; Chen, M.; Ge, F.; Li, Z.L.; Sun, W.T.; Guan, Z.R.; Liang, T.H.; et al. Multi Locus genome-wide association study reveals the genetic architecture of stalk lodging resistance related traits in maize. Front. Plant Sci. 2019, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Ennos, A.R.; Crook, M.J.; Grimshaw, C. The anchorage mechanics of maize, Zea mays. Exp. Bot. 1993, 44, 147–153. [Google Scholar] [CrossRef]
- Loades, K.W.; Bengough, A.G.; Bransby, M.F.; Hallett, P.D. Biomechanics of nodal, seminal and lateral roots of barley: Effects of diameter, waterlogging and mechanical impedance. Plant Soil 2013, 370, 407–418. [Google Scholar] [CrossRef]
- Stamp, P.; Kiel, C. Root morphology of maize and its relationship to root lodging. J. Agron. Crop Sci. 1992, 168, 113–118. [Google Scholar] [CrossRef]
- Kamara, A.Y.; Kling, J.G.; Menkir, A.; Ibikunle, O. Association of vertical root-pulling resistance with root lodging and grain yield in selected S1 maize lines derived from a tropical low nitrogen population. J. Agron. Crop Sci. 2003, 189, 129–135. [Google Scholar] [CrossRef]
- Liu, S.Q.; Song, F.B.; Liu, F.L.; Zhu, X.C.; Xu, H.B. Effect of planting density on root lodging resistance and its relationship to nodal root growth characteristics in maize (Zea mays L.). J. Agric. Sci. 2012, 4, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.D.; Xue, J.Q.; Hao, Y.C.; Gao, J. Effects of black film mulching on soil environment and maize growth in dry land. Acta Ecol. Sin. 2016, 36, 1997–2004. [Google Scholar]
- Liu, W.M.; Liu, G.Z.; Yang, Y.S.; Guo, X.X.; Ming, B.; Xie, R.Z.; Liu, Y.E.; Wang, K.R.; Hou, P.; Li, S.K. Spatial variation of maize height morphological traits for the same cultivars at a large agroecological scale. Eur. J. Agron. 2021, 130, 126349. [Google Scholar] [CrossRef]
- Hou, P.; Liu, Y.E.; Liu, W.M.; Yang, H.S.; Xie, R.Z.; Wang, K.R.; Ming, B.; Liu, G.Z.; Xue, J.; Wang, Y.H.; et al. Quantifying maize grain yield losses caused by climate change based on extensive field data across China. Resour. Conserv. Recycl. 2021, 174, 105811. [Google Scholar] [CrossRef]
- Ren, B.C.; Li, L.L.; Dong, S.T.; Liu, P.; Zhao, B.; Yang, J.S.; Wang, D.B.; Zhang, J.W. Effects of plant density on stem traits and lodging resistance of summer maize hybrids with different plant heights. Acta Agron. Sin. 2016, 42, 1864–1872. [Google Scholar] [CrossRef]
- Stapper, M.; Fischer, R. Genotype, sowing date and plant spacing influence on high-yielding irrigated wheat in southern New South Wales. III. Potential yields and optimum flowering dates. Aust. J. Agric. Res. 1990, 41, 1021–1041. [Google Scholar] [CrossRef]
- Zhang, Z.C. Analysis on the causes of crop lodging and research progress on anti-lodging countermeasures. Tillage Cultiv. 2006, 26, 1–2. [Google Scholar]
- Han, L.J.; Li, Y.; Wang, S.F.; Yu, G.F. Effect of kalium fertilizer on growth of root system and accumulation of above-ground dry matter. J. Jilin Agric. Univ. 2004, 26, 10–12, 22. [Google Scholar] [CrossRef]
- Zhao, X.Q. Countermeasures and remedial measures for maize lodging prevention. Mod. Rural Sci. Technol. 2012, 14, 9. [Google Scholar]
- Zhang, J.Y.; Liu, S.; Song, C.Y.; Gao, J.L.; Sun, L.J. Cause analysis and preventive measures of maize lodging. Shandong Agric. Sci. 2009, 11, 119–121. [Google Scholar]
- Chen, S.Q.; Xu, H.T. Reasons of maize lodging and Countermeasures. Mod. Agric. Sci. Technol. 2011, 11, 121–122. [Google Scholar]
- Xue, J.; Gao, S.; Li, L.L.; Xu, H.G.; Ming, B.; Wang, K.R.; Hou, P.; Xie, R.Z.; Li, S.K. Synergistic development of maize stalk as a strategy to reduce lodging risk. Agron. J. 2020, 112, 4962–4975. [Google Scholar] [CrossRef]




| Planting Density (Plants ha−1) | Cultivar | Treatments | Plant Height (cm) | Ear Height (cm) | Coefficient of Ear Height (%) | Gravity Height (cm) | Coefficient of Gravity Height (%) | Internode Length (cm) | Internode Diameter (mm) |
|---|---|---|---|---|---|---|---|---|---|
| 9 × 104 | Xinyu77 | FM | 263.5 (0.2) b | 79.0 (1.4) b | 31.0 (0.7) ab | 86.4 (0.7) b | 33.1 (0.4) a | 45.7 (0.5) c | 21.3 (0.3) a |
| NM | 268.9 (0.6) a | 83.2 (0.8) a | 31.4 (0.5) ab | 90.0 (0.2) a | 33.5 (0) a | 45.3 (0.3) c | 20.7 (0.2) ab | ||
| KWS9384 | FM | 259.3 (0.7) c | 75.6 (0.3) c | 30.1 (0.5) b | 81.0 (0.6) d | 31.6 (0.3) b | 37.8 (0.8) e | 20.2 (0.3) bc | |
| NM | 257.3 (0.9) c | 76.6 (0.5) c | 30.6 (0.5) ab | 85.6 (0.9) bc | 33.0 (0.5) a | 41.9 (0.3) d | 19.2 (0.4) d | ||
| KWS2030 | FM | 257.5 (1.3) c | 79.7 (0.9) b | 31.3 (0.4) ab | 83.8 (0.6) c | 32.5 (0.2) a | 57.3 (0.5) a | 19.5 (0.2) cd | |
| NM | 258.0 (0.3) c | 82.4 (0.4) a | 32.2 (0.3) a | 84.7 (0.4) bc | 33.1 (0.2) a | 51.8 (0.7) b | 18.2 (0.3) e | ||
| 12 × 104 | Xinyu77 | FM | 254.2 (0.9) b | 81.5 (1.7) bc | 31.9 (0.7) b | 85.1 (0.8) b | 33.2 (0.2) b | 44.2 (0.5) c | 20.1 (0.2) a |
| NM | 263.4 (1.4) a | 84.9 (0.6) ab | 32.9 (0.7) ab | 87.4 (0.5) a | 32.9 (0.5) b | 45.5 (0.8) c | 19.8 (0.2) ab | ||
| KWS9384 | FM | 249.8 (0.4) c | 81.7 (0.8) bc | 32.4 (0.6) b | 82.5 (0.4) c | 32.9 (0.1) b | 39.3 (0.3) d | 19.2 (0.2) b | |
| NM | 247.6 (1.1) c | 78.9 (1.6) c | 32.2 (0.8) b | 82.2 (0.6) c | 32.7 (0.4) b | 39.5 (0.5) d | 18.3 (0.3) c | ||
| KWS2030 | FM | 248.6 (0.9) c | 83.5 (0.3) b | 33.4 (0.1) ab | 83.2 (0.4) c | 33.4 (0.1) b | 52.4 (0.6) b | 17.0 (0.2) d | |
| NM | 255.0 (0.9) b | 88.1 (0.4) a | 34.6 (0.1) a | 87.3 (0.3) a | 34.4 (0.2) a | 54.5 (0.9) a | 16.8 (0.3) d | ||
| Planting density (plants ha−1) | 9 × 104 | 260.8 | 79.4 | 31.1 | 85.3 | 32.8 | 46.6 | 19.9 | |
| 12 × 104 | 253.1 | 83.1 | 32.9 | 84.6 | 33.3 | 45.9 | 18.5 | ||
| Mulch treatment | FM | 255.5 | 80.2 | 31.7 | 83.7 | 32.8 | 46.1 | 19.55 | |
| NM | 258.4 | 82.4 | 32.3 | 86.2 | 33.3 | 46.4 | 18.83 | ||
| Cultivar | Xinyu77 | 262.5 | 82.2 | 31.8 | 87.2 | 33.2 | 45.2 | 20.48 | |
| KWS9384 | 253.5 | 78.2 | 31.3 | 82.8 | 32.6 | 39.6 | 19.23 | ||
| KWS2030 | 254.8 | 83.4 | 32.9 | 84.8 | 33.4 | 54.0 | 17.88 | ||
| Plant Density (Plants ha−1) | Cultivar | Treatments | Root Depth (cm) | Root Width (cm) | Total Dry Root Weight (g) | Total Root Length (cm) | Root Surface Area (cm2) | Root Diameter (mm) | Root Volume (cm3) |
|---|---|---|---|---|---|---|---|---|---|
| 9 × 104 | Xinyu77 | FM | 24.4 (0.2) a | 21.7 (0.4) a | 9.0 (0.4) a | 6772.8 (774.3) b | 1548.9 (190.0) a | 9.0 (0.5) a | 40.9 (2.6) a |
| NM | 22.6 (1.0) b | 16.8 (0.2) d | 6.8 (0.4) bc | 2491.3 (224.6) d | 642.2 (32.7) c | 5.0 (0.5) c | 13.6 (0.3) c | ||
| KWS9384 | FM | 22.3 (0.6) b | 17.8 (0.3) cd | 7.4 (0.2) b | 5710.0 (269.1) b | 1131.5 (48.8) b | 4.7 (0.3) c | 17.7 (0.3) c | |
| NM | 20.3 (0.3) c | 16.9 (0.5) d | 6.8 (0.3) bc | 8616.4 (637.0) a | 1693.4 (52.3) a | 7.3 (0.5) b | 27.1 (1.0) b | ||
| KWS2030 | FM | 19.4 (0.4) c | 20.2 (0.6) b | 6.1 (0.3) cd | 3934.4 (388.5) c | 766.5 (29.4) c | 4.1 (0.4) c | 16.7 (0.7) c | |
| NM | 20.4 (0.4) c | 18.7 (0.5) c | 5.7 (0.4) d | 3675.3 (199.8) cd | 867.4 (38.2) c | 5.4 (0.4) c | 16.7 (1.4) c | ||
| 12 × 104 | Xinyu77 | FM | 21.2 (0.4) ab | 18.3 (0.5) ab | 7.4 (0.3) a | 6773.8 (780.4) a | 1561.0 (143.9) a | 6.7 (0.4) a | 28.8 (0.4) a |
| NM | 20.0 (0.4) b | 16.3 (0.1) cd | 4.8 (0.3) b | 3356.8 (168.3) bc | 841.8 (51.7) b | 4.5 (0.4) b | 21.9 (1.8) b | ||
| KWS9384 | FM | 20.6 (0.9) ab | 16.6 (0.3) c | 4.8 (0.4) b | 4593.2 (679.5) b | 955.8 (72.4) b | 3.8 (0.5) b | 14.5 (0.7) c | |
| NM | 22.1 (0.5) a | 15.3 (0.6) d | 3.9 (0.1) c | 3689.5 (199.1) bc | 759.0 (36.1) b | 3.7 (0.3) b | 13.4 (0.1) c | ||
| KWS2030 | FM | 17.2 (0.3) c | 19.0 (0.4) a | 4.9 (0.3) b | 3581.8 (150.8) bc | 782.1 (38.4) b | 4.0 (0.3) b | 14.1 (0.3) c | |
| NM | 16.1 (0.6) c | 17.3 (0.1) bc | 4.5 (0.2) bc | 3078.9 (410.3) c | 712.5 (64.3) b | 3.5 (0.3) b | 11.8 (1) c | ||
| Plant density (plants ha−1) | 9 × 104 | 21.6 | 18.7 | 7.0 | 5200.0 | 1108.3 | 5.9 | 22.1 | |
| 12 × 104 | 19.5 | 17.1 | 5.1 | 4179.0 | 935.4 | 4.4 | 17.4 | ||
| Mulch treatment | FM | 20.9 | 18.9 | 6.6 | 5227.7 | 1124.3 | 5.4 | 22.1 | |
| NM | 20.3 | 16.9 | 5.4 | 4151.4 | 919.4 | 4.9 | 17.4 | ||
| Cultivar | Xinyu77 | 22.1 | 18.3 | 7.0 | 4848.7 | 1148.5 | 6.3 | 26.3 | |
| KWS9384 | 21.3 | 16.7 | 5.7 | 5652.3 | 1134.9 | 4.9 | 18.2 | ||
| KWS2030 | 18.3 | 18.8 | 5.3 | 3567.6 | 782.1 | 4.3 | 14.8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xue, J.; Tian, M.; Zhang, G.; Ming, B.; Wang, K.; Hou, P.; Xie, R.; Tang, Q.; Li, S. Maize Lodging Resistance with Plastic Film Removal, Increased Planting Density, and Cultivars with Different Maturity Periods. Plants 2022, 11, 2723. https://doi.org/10.3390/plants11202723
Zhang X, Xue J, Tian M, Zhang G, Ming B, Wang K, Hou P, Xie R, Tang Q, Li S. Maize Lodging Resistance with Plastic Film Removal, Increased Planting Density, and Cultivars with Different Maturity Periods. Plants. 2022; 11(20):2723. https://doi.org/10.3390/plants11202723
Chicago/Turabian StyleZhang, Xiyun, Jun Xue, Ming Tian, Guoqiang Zhang, Bo Ming, Keru Wang, Peng Hou, Ruizhi Xie, Qiuxiang Tang, and Shaokun Li. 2022. "Maize Lodging Resistance with Plastic Film Removal, Increased Planting Density, and Cultivars with Different Maturity Periods" Plants 11, no. 20: 2723. https://doi.org/10.3390/plants11202723






