Effects of Crop Resistance on the Tritrophic Interactions between Wheat Lines, Schizaphis graminum (Hemitera: Aphididae), and Propylaea japonica (Coleoptera: Coccinellidae)
Abstract
1. Introduction
2. Results
2.1. The Life-History traits of Four Wheat-Acclimated Aphid Populations
2.2. The Prey Consumption and Proportion of Prey Consumed of Ladybird Feeding with Four Prey Wheat-Acclimated Aphid Populations
2.3. The Functional Responses of Ladybeetle Feeding with Four Prey Acclimated Populations
2.4. The Attack Rate, Handling Times, and Predatory Efficiency of Ladybird Feeding with Four Prey Acclimated Populations
3. Discussion
4. Materials and Methods
4.1. Wheat, Aphid, and Ladybird
4.2. Aphid Population Acclimated on 4 Wheat Lines
4.3. The Life-History Traits of Four Wheat-Acclimated Aphid Populations
4.4. Functional Response of Ladybeetle Preying on Aphid
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dara, S.K. The new integrated pest management paradigm for the modern age. J. Integr. Pest Manag. 2019, 10, 12. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, S.M.; Wang, W.; Zhao, J.H.; Chen, X.W.; Guo, H.S.; He, G.C.; He, Z.H.; Kang, Z.S.; Li, Y.; et al. Plant immunity and sustainable control of pests in China: Advances, opportunities and challenges. Sci. China 2019, 49, 1479–1507. [Google Scholar]
- Stout, M.; Davis, J. Keys to the increased use of host plant resistance in integrated pest management. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 163–181. [Google Scholar]
- Nderitu, P.W.; Jonsson, M.; Arunga, E.; Otieno, M.; Muturi, J.J.; Wafula, G.O. Combining host plant resistance, selective insecticides, and biological control agents for integrated management of Tuta absoluta. Adv. Agric. 2020, 2, 6239491. [Google Scholar]
- Smith, C.M. Conventional breeding of insect-resistant crop plants: Still the best way to feed the world population. Curr. Opin. Insect Sci. 2021, 45, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Teetes, G.L. Plant Resistance to Insects: A fundamental component of IPM. In Radcliffe’s IPM World Textbook; Radcliffe, E.B., Hutchison, W.D., Cancelado, R.E., Eds.; University of Minnesota: St. Paul, MN, USA, 2006; Available online: https://ipmworld.umn.edu (accessed on 14 August 2022).
- Pati, P.; Behera, S.K.; Raghu, S.; Annamalai, M. Biological control of insect pests in vegetable crops: An eco-friendly approach. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1358–1373. [Google Scholar] [CrossRef]
- Michaud, J.P. Challenges to conservation biological control on the High Plains: 150 years of evolutionary rescue. Biol. Control 2018, 125, 65–73. [Google Scholar] [CrossRef]
- Gurr, G.M.; Kvedaras, O.L. Synergizing biological control: Scope for sterile insect technique, induced plant defences and cultural techniques to enhance natural enemy impact. Biol. Control 2010, 52, 198–207. [Google Scholar] [CrossRef]
- Kersch-Becker, M.F.; Thaler, J.S. Plant resistance reduces the strength of consumptive and non-consumptive effects of predators on aphids. J. Anim. Ecol. 2015, 84, 1222–1232. [Google Scholar] [CrossRef]
- Shannag, H.K.; Obeidat, W.M. Interaction between plant resistance and predation of Aphis fabae (Homoptera: Aphididae) by Coccinella septempunctata (Coleoptera: Coccinellidae). Ann. Appl. Biol. 2008, 152, 331–337. [Google Scholar] [CrossRef]
- Zanganeh, L.; Madadi, H.; Allahyari, H. Demographic parameters of Diuraphis noxia (Hemiptera: Aphididae) and Hippodamia variegata (Coleoptera: Coccinellidae) recorded in the context of D. noxia infesting resistant and susceptible cultivars of wheat. EJE 2015, 112, 453–459. [Google Scholar] [CrossRef]
- Kersch-Becker, M.F.; Kessler, A.; Thaler, J.S. Plant defences limit herbivore population growth by changing predator–prey interactions. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171120. [Google Scholar] [CrossRef]
- Bottrell, D.G.; Barbosa, P.; Gould, F. Manipulating natural enemies by plant variety selection and modification: A realistic strategy? Annu. Rev. Entomol. 1998, 43, 347–367. [Google Scholar] [CrossRef]
- Cortesero, A.M.; Stapel, J.O.; Lewis, W.J. Underestanding and manipulating plant attributes to enhance biological control. Biol. Control 2000, 17, 35–49. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Liu, X.F.; Hu, X.S.; Keller, M.A.; Zhao, H.Y.; Wu, Y.F.; Liu, T.X. Tripartite interactions of Barley yellow dwarf virus, Sitobion avenae and wheat varieties. PLoS ONE 2014, 9, e106639. [Google Scholar] [CrossRef]
- Li, D.; Zhang, C.; Tong, Z.; Su, D.; Zhang, G.; Zhang, S.; Zhao, H.; Hu, Z. Transcriptome response comparison between vector and non-vector aphids after feeding on virus-infected wheat plants. BMC Genom. 2020, 21, 638. [Google Scholar] [CrossRef]
- Aradottir, G.I.; Crespo-Herrera, L. Host plant resistance in wheat to Barley yellow dwarf viruses and their aphid vectors: A review. Curr. Opin. Insect Sci. 2021, 45, 59–68. [Google Scholar] [CrossRef]
- Michels, G.J. Graminaceous North American host plants of the greenbug with notes on biotypes. Southwest Entomol. 1986, 11, 55–66. [Google Scholar]
- Xu, X.; Li, G.; Bai, G.; Bernardo, A.; Carver, B.F.; St. Amand, P.; Armstrong, J.S. Development of KASP markers for wheat greenbug resistance gene Gb5. Crop. Sci. 2021, 61, 490–499. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, W.; Li, N.; Zhang, F.; Liu, T.-X. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, S.; Luo, J.Y.; Du, P.; Wu, L.K.; Li, Y.R.; Zhu, X.Z.; Wang, L.; Zhang, S.; Cui, J.J. Chromosome-level genome assembly of the predator “Propylea japonica” to understand its tolerance to insecticides and high temperatures. Mol. Ecol. Resour. 2020, 20, 292–307. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhang, H.R.; Xi, Y.Q.; Wang, G.P.; Zhao, M.; Zhang, L.J.; Guo, X.R. Population genetic variation and historical dynamics of the natural enemy insect Propylea japonica (Coleoptera: Coccinellidae) in China. J. Integr. Agric. 2022; In Press. [Google Scholar] [CrossRef]
- Simon, J.C.; Rispe, C.; Sunnucks, P. Ecology and evolution of sex in aphids. Trends Ecol. Evol. 2002, 17, 34–39. [Google Scholar] [CrossRef]
- Hu, X.S.; Liu, X.F.; Ridsdill-Smith, T.J.; Thieme, T.; Zhao, H.Y.; Liu, T.X. Effects of maternal diet on offspring fitness in the bird cherry-oat aphid. Ecol. Entomol. 2016, 14, 147–156. [Google Scholar] [CrossRef]
- Hu, X.S.; Zhang, Z.F.; Zhu, T.Y.; Song, Y.; Wu, L.J.; Liu, X.F.; Zhao, H.Y.; Liu, T.X. Maternal effects of the English grain aphids feeding on the wheat varieties with different resistance traits. Sci. Rep. 2018, 8, 7344. [Google Scholar] [CrossRef]
- Hu, X.S.; Luo, C.; Li, G.K.; Zhang, Z.F.; Wang, C.P.; Hu, Z.Q.; Zhao, H.Y.; Liu, T.X. Multi-generational effects of different resistant wheat varieties on fitness of Sitobion avenae (Hemiptera: Aphididae). J. Insect. Sci. 2021, 21, 14. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Lan, H.; Cao, H.H.; Hu, X.S.; Fan, Y.L.; Song, Y.; Wu, L.J.; Liu, T.-X. Impacts of constitutive and induced Benzoxazinoids levels on wheat resistance to the grain aphid (Sitobion avenae). Metabolites 2021, 11, 783. [Google Scholar] [CrossRef]
- Hu, X.S.; Keller, M.A.; Liu, X.F.; Hu, Z.Q.; Zhao, H.Y.; Liu, T.X. The resistance and correlation analysis to 3 species of cereal aphids (Hemiptera: Aphididae) on 10 wheat varieties or lines. J. Econ. Entomol. 2013, 106, 1894–1901. [Google Scholar] [CrossRef]
- Wu, X.H.; Zhou, X.R.; Pang, B.P. Influence of five host plants of Aphis gossypii Glover on some population parameters of Hippodamia variegata (Goeze). J. Pest Sci. 2010, 83, 77–83. [Google Scholar] [CrossRef]
- Pervez, A.; de Holanda Nunes Maia, A.; Bozdoğan, H. Reproduction and demography of an Aphidophagous ladybird, Hippodamia variegata on six aphid species. Int. J. Trop. Insect Sci. 2020, 40, 541–548. [Google Scholar] [CrossRef]
- Ugine, T.A.; Gill, H.K.; Hernandez, N.; Grebenok, R.J.; Behmer, S.T.; Losey, J.E. Predator performance and fitness is dictated by herbivore prey type plus indirect effects of their host plant. J. Chem. Ecol. 2021, 47, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Giles, K.L.; Madden, R.D.; Stockland, R.; Payton, M.E.; Dillwith, J.W. Host plants affect predator fitness via the nutritional value of herbivore prey: Investigation of a plant-aphid-ladybeetle system. BioControl 2002, 47, 1–21. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, S.; Akutse, K.S.; Hussain, M.; Wang, L. Diaphorina citri induces Huanglongbing-infected Citrus plant volatiles to repel and reduce the performance of Propylaea japonica. Front. Plant Sci. 2016, 7, 1969. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tan, X.L.; Liu, F.H.; Wang, S.; Chen, J.L.; Yi, T.Y. A functional response evaluation of pre-infestation with Bemisia tabaci cryptic species MEAM1 on predation by Propylea japonica of Myzus persicae on host plant tomatoes. Arthropod-Plant Interact. 2017, 11, 825–832. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, Z.X.; An, M.R.; Li, Y.D.; Liu, Z.G.; Wang, L.L.; Ren, J.Z.; Liu, T.X. Conspecific and heterospecific interactions modify the functional response of Harmonia axyridis and Propylea japonica to Aphis citricola. Entomol. Exp. Appl. 2018, 166, 873–882. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, Z.X.; An, M.R.; Yu, X.L.; Liu, T.X. The effects of prey distribution and digestion on functional response of Harmonia axyridis (Coleoptera: Coccinellidae). Biol. Control 2018, 124, 74–81. [Google Scholar] [CrossRef]
- Yan, F.; Li, C.; Ye, X.; Lian, Y.; Wu, Y.; Wang, X. Antifungal activity of lipopeptides from Bacillus amyloliquefaciens MG3 against Colletotrichum gloeosporioides in loquat fruits. Biol. Control 2020, 146, 104281. [Google Scholar] [CrossRef]
- Zhou, J.H.; Li, P.L.; Naiwuzhati, Z.N.; Zheng, H.N.; Huang, J.; Wang, Z.H. Functional response and predation preference of ladybeetle Propylea japonica to Asian citrus psyllid Diaphorina citri. J. Plant Prot. 2020, 47, 1062–1070. [Google Scholar]
- Watson, D.; Du, T.Y.; Li, M.; Xiong, J.J.; Lui, D.G.; Huang, M.D.; Rae, D.; Beattie, G.A.C. Functional responses of, and mutual interference in Aleurodothrips fasciapennis (Franklin) (Thysanoptera: Phlaeothripidae) and implications for its use as a biocontrol agent. Gen. Appl. Entomol. 2000, 29, 31–37. [Google Scholar]
- Omkar, U.; Srivastava, S. Functional response of the sevenspotted lady beetle, Coccinella septempunctata linnaeus on the mustard aphid, Lipaphis Erysimi (Kaltenbach). Insect Sci. Its Appl. 2011, 23, 149–152. [Google Scholar] [CrossRef]
- Pervez, A. Functional and numerical responses of Propylea dissecta (Col., Coccinellidae). J. Appl. Entomol. 2004, 128, 140–146. [Google Scholar] [CrossRef]
- Gupta, R.; Pervez, A.; Guroo, M.A.; Srivastava, K. Stage-specific functional response of an aphidophagous ladybird, Coccinella septempunctata (Coleoptera: Coccinellidae), to two aphid species. Int. J. Trop. Insect Sci. 2012, 32, 136–141. [Google Scholar] [CrossRef]
- Mandour, N.; El-Basha, N.; Liu, T.X. Functional response of the ladybird, Cydonia vicina nilotica to cowpea aphid, Aphis craccivora in the laboratory. Insect Sci. 2006, 13, 49–54. [Google Scholar] [CrossRef]
- Chi, H.; Yang, T.C. Two-sex life table and predation rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ. Entomol. 2003, 32, 327–333. [Google Scholar] [CrossRef]
- Wyatt, I.J.; White, P.F. Simple estimation of intrinsic increase rates for aphids and tetranychid mites. J. Appl. Ecol. 1977, 14, 757–766. [Google Scholar] [CrossRef]
- Leather, S.R.; Dixon, A.F.G. Aphid growth and reproductive rates. Entomol. Exp. Et Appl. 1984, 35, 137–140. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 14 August 2022).
- Pritchard, D.; Barrios-O’Neill, D.; Bovy, H.; Paterson, R.; Pritchard, M.D. Frair: Functional response analysis in R. R Package Version 0.4. 2017. Available online: http://CRAN.R-project.org/package=frair (accessed on 26 March 2017).
- Juliano, S.A. Nonlinear Curve Fitting: Predation and Functional Response Curve, 2nd ed.; Oxford University Press: New York, NY, USA, 2001; pp. 178–196. [Google Scholar]
- Loko, L.; Djagoun, A.; Elie, D.; Datinon, B.; Dansi, A.; Thomas-Odjo, A.; Tamo, M. Functional response of the predators Alloeocranum biannulipes (Hemiptera: Reduviidae) and Teretrius nigrescens (Coleoptera: Histeridae) feeding on Dinoderus porcellus (Coleoptera: Bostrichidae) infesting yam chips. Environ. Entomol. 2016, 46, 84–91. [Google Scholar]
- Rogers, D. Random search and insect population models. J. Anim. Ecol. 1972, 41, 369–383. [Google Scholar] [CrossRef]
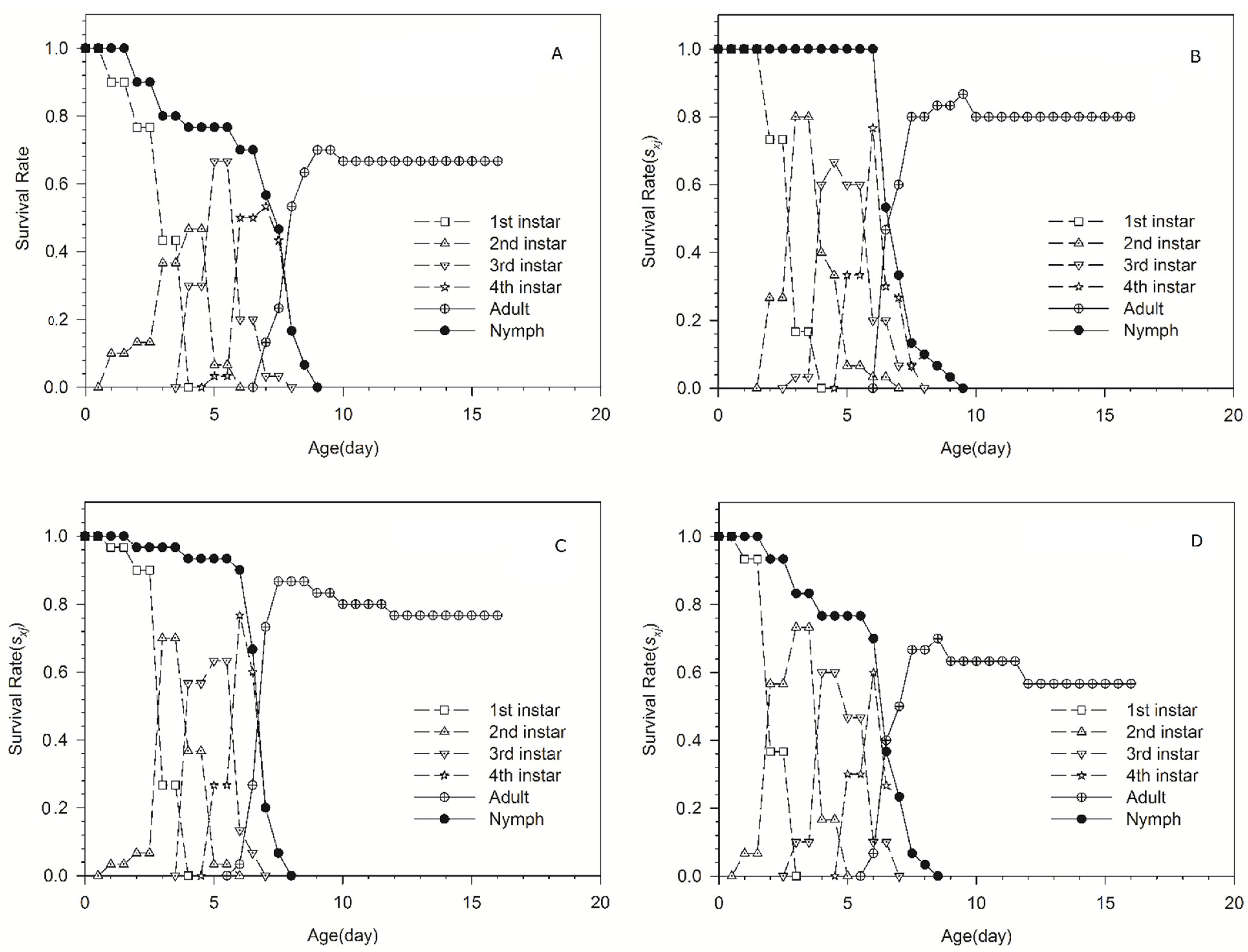
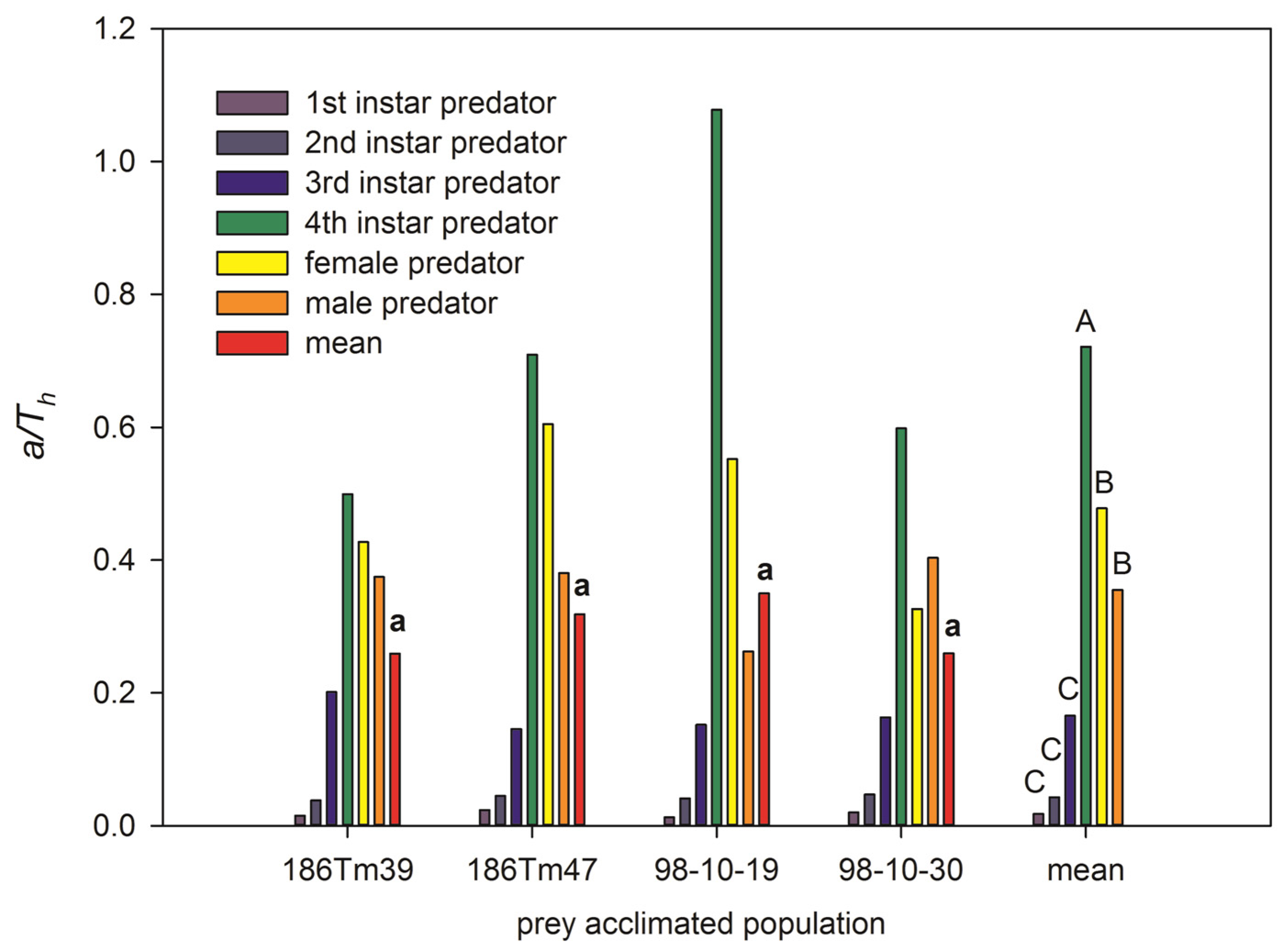
| Life-History Parameter | 186Tm39 | 186Tm47 | 98-10-19 | 98-10-30 | F | df | p |
|---|---|---|---|---|---|---|---|
| W1st (µg) | 23.63 ± 0.80 | 21.93 ± 0.75 | 22.87 ± 0.77 | 21.30 ± 0.59 | 1.97 | 3, 116 | 0.123 |
| Wa (µg) | 324.4 ± 15.3 B | 326.8 ± 12.0 B | 264.2 ± 20.3 C | 400.9 ± 16.8 A | 9.09 | 3, 91 | <0.001 |
| DT (d) | 6.12 ± 0.08 B | 6.24 ± 0.15 B | 7.05 ± 0.15 A | 6.41 ± 0.14 B | 10.80 | 3, 91 | <0.001 |
| MRGR | 0.431 ± 0.012 A | 0.437 ± 0.016 A | 0.352 ± 0.020 B | 0.459 ± 0.008 A | 7.33 | 3, 91 | <0.001 |
| Fecundity | 27.69 ± 1.95 BC | 33.52 ± 2.66 AB | 23.29 ± 2.59 C | 38.48 ± 2.64 A | 5.95 | 3, 91 | 0.001 |
| rm | 0.197 ± 0.007 A | 0.204 ± 0.007 A | 0.155 ± 0.012 B | 0.202 ± 0.009 A | 8.12 | 3, 91 | <0.001 |
| NRR | 2.30 ± 0.17 AB | 2.68 ± 0.19 A | 1.71 ± 0.20 B | 2.98 ± 0.19 A | 11.79 | 3, 91 | <0.001 |
| NSR | 0.867 ± 0.063 | 0.700 ± 0.085 | 0.700 ± 0.085 | 0.900 ± 0.056 | 2.11 | 3, 116 | 0.102 |
| WL | PD | EAS | WL × PD | WL × EAS | PD × EAS | WL × PD × EAS | ||
|---|---|---|---|---|---|---|---|---|
| Ne | df | 3 | 5 | 6 | 15 | 18 | 18 | 54 |
| F | 5.77 | 292.57 | 1816.27 | 3.13 | 2.48 | 81.18 | 1.46 | |
| p | 0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | 0.02 | |
| Ne/N0 | df | 3 | 5 | 6 | 15 | 18 | 18 | 54 |
| F | 4.47 | 278.27 | 193.88 | 1.95 | 3.20 | 7.18 | 0.74 | |
| p | 0.004 | <0.001 | <0.001 | 0.02 | <0.001 | <0.001 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.-S.; Li, J.-W.; Peng, J.-F.; Wang, H.; Yan, F.-Y.; Zhou, Z.-F.; Zhang, Z.-F.; Zhao, H.-Y.; Feng, Y.; Liu, T.-X. Effects of Crop Resistance on the Tritrophic Interactions between Wheat Lines, Schizaphis graminum (Hemitera: Aphididae), and Propylaea japonica (Coleoptera: Coccinellidae). Plants 2022, 11, 2754. https://doi.org/10.3390/plants11202754
Hu X-S, Li J-W, Peng J-F, Wang H, Yan F-Y, Zhou Z-F, Zhang Z-F, Zhao H-Y, Feng Y, Liu T-X. Effects of Crop Resistance on the Tritrophic Interactions between Wheat Lines, Schizaphis graminum (Hemitera: Aphididae), and Propylaea japonica (Coleoptera: Coccinellidae). Plants. 2022; 11(20):2754. https://doi.org/10.3390/plants11202754
Chicago/Turabian StyleHu, Xiang-Shun, Jing-Wen Li, Jing-Feng Peng, Han Wang, Fan-Ye Yan, Zi-Fang Zhou, Zhan-Feng Zhang, Hui-Yan Zhao, Yi Feng, and Tong-Xian Liu. 2022. "Effects of Crop Resistance on the Tritrophic Interactions between Wheat Lines, Schizaphis graminum (Hemitera: Aphididae), and Propylaea japonica (Coleoptera: Coccinellidae)" Plants 11, no. 20: 2754. https://doi.org/10.3390/plants11202754
APA StyleHu, X.-S., Li, J.-W., Peng, J.-F., Wang, H., Yan, F.-Y., Zhou, Z.-F., Zhang, Z.-F., Zhao, H.-Y., Feng, Y., & Liu, T.-X. (2022). Effects of Crop Resistance on the Tritrophic Interactions between Wheat Lines, Schizaphis graminum (Hemitera: Aphididae), and Propylaea japonica (Coleoptera: Coccinellidae). Plants, 11(20), 2754. https://doi.org/10.3390/plants11202754








