Effect of Transgenic Cotton Expressing Bt Cry1Ac or Cry1Ab/Ac Toxins on Lacewing Larvae Mediated by Herbivorous Insect Pests
Abstract
1. Introduction
2. Results
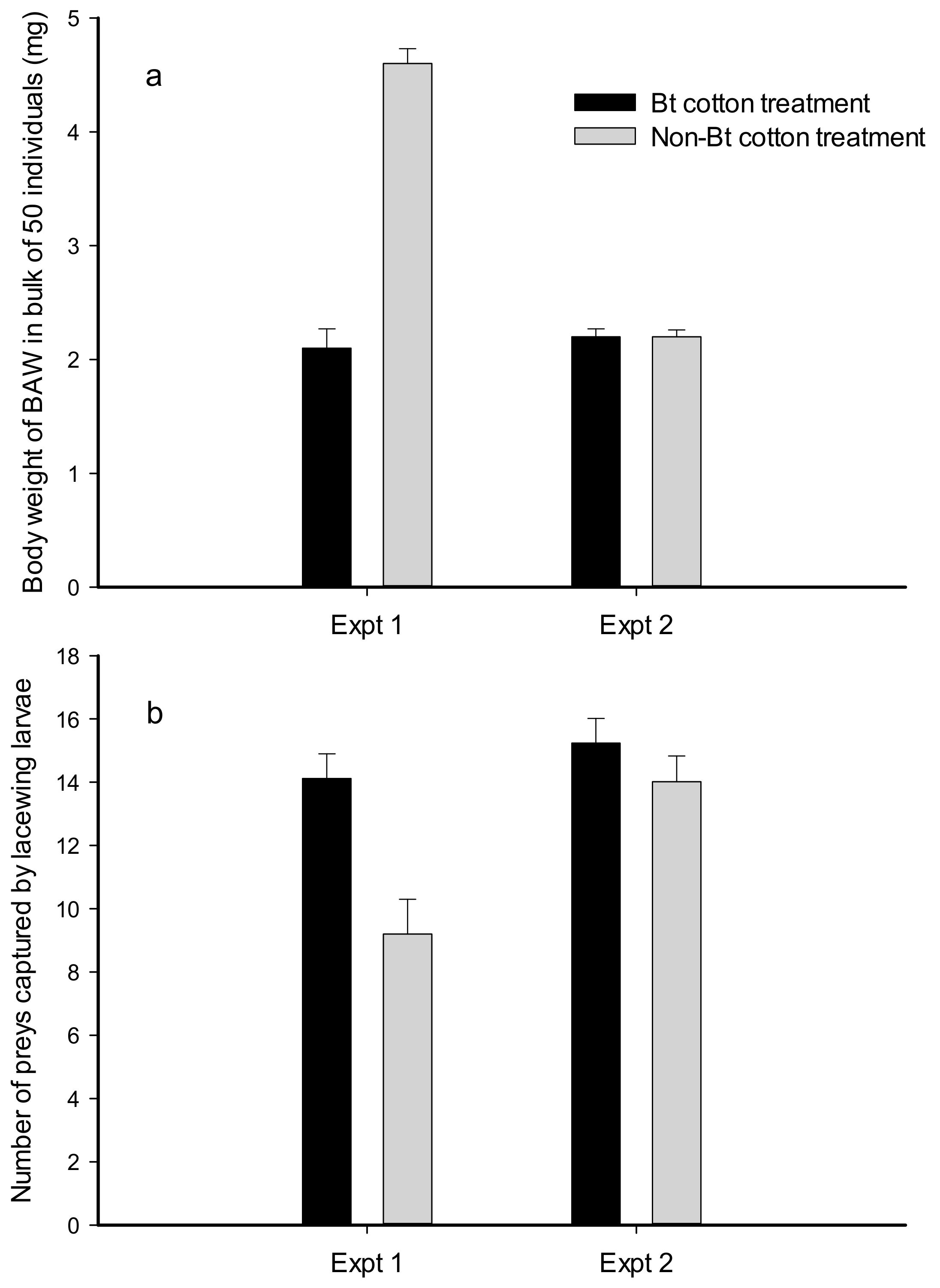
2.1. Number and Weight of Prey Consumed by Lacewing Larvae
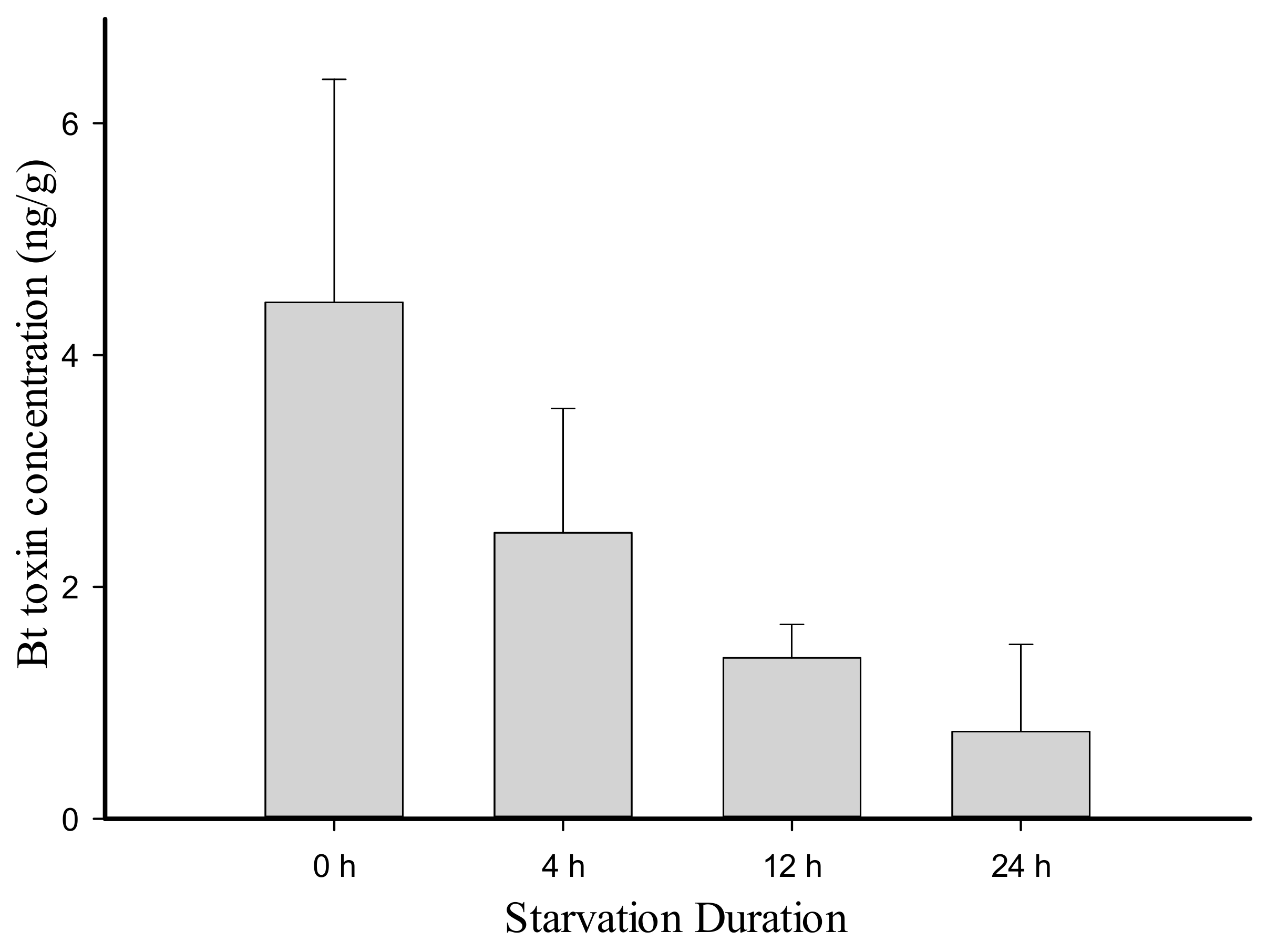
2.2. Persistence of Cry1Ac Toxin at Trophic Levels
2.3. Effect of Bt Toxin Residual in Larval Body on the Growth of Lacewing Larvae
3. Discussion
4. Materials and Methods
4.1. Plant and Insect Materials
4.2. Bioassays on Prey Consumption by Lacewing Larvae and Cry1Ac Toxin Transfer through Trophic Levels
4.2.1. Prey Consumption Bioassays
4.2.2. Detecting Cry1Ac Toxin Transfer through Trophic Levels
4.3. Bioassays on the Effect of Cry1Ab/1Ac Toxins Residual on Lacewing Larvae
4.4. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Expt. No. | Prey (Neonate Larvae) | Predator (3rd Instar Larva) | Prey: Predator Ratio | Predator Feeding Replicates | Preys Pre-Treated Hours | |||
|---|---|---|---|---|---|---|---|---|
| Tr. | Non-Tr. | Tr. (Zhong-30) | Non-Tr. (Zhong-16) | |||||
| Bioassay 1 | Expt. 1 | BAW | Chrysopa formosa | 10:1 | 8 | 9 | 24 | 24 |
| Expt. 2 | 20:1 | 7 | 4 | |||||
| Bioassay 2 | Expt. 1 | CBW | Chrysopa formosa | 20:1 | 5 | 5 | 24 | 24 |
| Expt. 2 | CLC+CBW | 6 | 5 | |||||
| Bioassay 3 | Expt. 1 | BAW | Chrysopa sinica | 20:1 | 5 | 5 | 24 | 24 |
| Expt. 2 | 24 | 12 | ||||||
References
- Guan, Z.-J.; Lu, S.-B.; Huo, Y.-L.; Guan, Z.-P.; Liu, B.; Wei, W. Do genetically modified plants affect adversely on soil microbial communities? Agric. Ecosys. Environ. 2016, 235, 289–305. [Google Scholar] [CrossRef]
- Wei, W.; Stewart, C.N., Jr. (Eds.) Gene Flow: Monitoring, Modeling and Mitigation; CABI: Wallingford, UK, 2021; p. 169. [Google Scholar]
- Sharmaa, P.; Singh, S.P.; Iqbal, H.M.N.; Parra-Saldivar, R.; Varjani, S.; Tong, Y.W. Genetic modifications associated with sustainability aspects for sustainable developments. Bioengineered 2022, 13, 9509–9521. [Google Scholar] [CrossRef] [PubMed]
- Belousova, M.E.; Malovichko, Y.V.; Shikov, A.E.; Nizhnikov, A.A.; Antonets, K.S. Dissecting the Environmental Consequences of Bacillus thuringiensis Application for Natural Ecosystems. Toxins 2021, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-H.; Wyckhuys, K.A.G.; Yang, L.; Liu, B.; Juan Zeng, J.; Jiang, Y.-Y.; Desneux, N.; Zhang, W.; Wu, K.M. Bt cotton area contraction drives regional pest resurgence, crop loss, and pesticide use. Plant Biotech. J. 2022, 20, 390–398. [Google Scholar] [CrossRef]
- Olsen, K.M.; Daly, J.C.; Holt, H.E.; Finnegan, E.J. Season-long variation in expression of Cry1Ac gene and efficacy of Bacillus thuringiensis toxin in transgenic cotton against Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 2005, 98, 1007–1017. [Google Scholar] [CrossRef]
- Liu, Z.; Eltayib, H.M.A.A.; Wu, H.; Zhou, M.-Y.; Zhang, X.; Chen, Y.; Chen, D.-H. Bt insecticidal efficacy variation and agronomic regulation in Bt cotton. J. Cotton Res. 2019, 2, 23. [Google Scholar] [CrossRef]
- Tang, F.-Y.; Gao, X.; Peng, J.-J. The dynamics of carbohydrate and associated gene expression in the stems and roots of upland cotton (Gossypium hirsutum L.) during carbon remobilization. Plant Physiol. Biochem. 2022, 178, 125–136. [Google Scholar] [CrossRef]
- Li, G.-P.; Wu, K.-M.; Gould, F.; Wang, J.-K.; Miao, J.; Gao, X.-W.; Guo, Y.-Y. Increasing tolerance to Cry1Ac cotton from cotton bollworm, Helicoverpa armigera, was confirmed in Bt cotton farming area of China. Ecol. Entomol. 2007, 32, 366–375. [Google Scholar] [CrossRef]
- Wu, K.-M.; Guo, Y.-Y.; Lv, N.; Greenplate, J.T.; Deaton, R. Efficacy of transgenic cotton containing a cry1Ac gene from Bacillus thuringiensis against Helicoverpa armigera (Lepidoptera: Noctuidae) in northern China. J. Econ. Entomol. 2003, 96, 1322–1328. [Google Scholar] [CrossRef]
- Wan, P.; Zhang, Y.; Wu, K.; Huang, M. Seasonal expression profiles of insecticidal protein and control efficacy against Helicoverpa armigera for Bt cotton in the Yangtze River valley of China. J. Econ. Entomol. 2005, 98, 195–201. [Google Scholar] [CrossRef]
- Wu, K.-M.; Lu, Y.-H.; Feng, H.-Q.; Jiang, Y.Y.; Zhao, J.Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin–containing cotton. Science 2008, 321, 1676–1678. [Google Scholar] [CrossRef]
- Zhang, H.-N.; Tian, W.; Zhao, J.; Jin, L.; Yang, J.; Liu, C.-H.; Yang, Y.-H.; Wu, S.-W.; Wu, K.-M.; Cui, J.-J.; et al. Diverse genetic basis of field-evolved resistance to Bt cotton in cotton bollworm from China. Proc. Nat. Acad. Sci. USA 2012, 109, 10275–10280. [Google Scholar] [CrossRef]
- Men, X.-Y.; Ge, F.; Liu, X.-H.; Yardim, E.-N. Diversity of arthropod communities in transgenic Bt cotton and nontransgenic cotton agroecosystems. Environ. Entomol. 2003, 32, 270–275. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, L.-F.; Hua, B.-Z.; Mi, X.-C.; Wei, W.; Zhang, Y.-J.; Ma, K.P. Insecticidal activity of residual Bt protein at the second trophic level. Chin. Sci. Bull. 2006, 51, 946–951. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carriere, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotech. 2008, 26, 199–202. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Wu, K.-M.; Guo, Y.Y. On the spatio-temporal expression of the contents of Bt insecticidal protein and the resistance of Bt transgenic cotton to cotton bollworm. Acta Phytophyl. Sin. 2001, 28, 1–6. [Google Scholar]
- Wu, K.-M.; Guo, Y.-Y.; Gao, S. Evaluation of the natural refuge function for Helicoverpa armigera (Lepidoptera: Noctuidae) within Bacillus thuringiensis transgenic cotton growing areas in north China. J. Econ. Entomol. 2002, 95, 832–837. [Google Scholar] [CrossRef]
- Lu, Y.-H.; Wu, K.-M.; Jiang, Y.-Y.; Xia, B.; Li, P.; Feng, H.-Q.; Wyckhuys, K.A.; Guo, Y.-Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef]
- Catarino, R.; Ceddia, G.; Areal, F.J.; Park, J. The impact of secondary pests on Bacillus thuringiensis (Bt) crops. Plant Biotech. J. 2015, 13, 601–612. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Evolution of resistance to Bacillus thuringiensis. Ann. Rev. Entomol. 1994, 39, 47–79. [Google Scholar] [CrossRef]
- Adamczyk, J.J., Jr.; Gore, J. Laboratory and field performance of cotton containing Cry1ac, Cry1f, and both Cry1ac and Cry1f (widestrike®) against beet armyworm and fall armyworm larvae (Lepidoptera: Noctuidae). Fla. Entomol. 2004, 87, 427–432. [Google Scholar] [CrossRef]
- Wu, G.; Harris, M.K.; Guo, J.-Y.; Wan, F.-H. Response of multiple generations of beet armyworm, Spodoptera exigua (Hübner), feeding on transgenic Bt cotton. J. Appl. Entomol. 2009, 133, 90–100. [Google Scholar] [CrossRef]
- Guo, J.-Y.; Wu, G.; Wan, F.-H. Activities of digestive and detoxification enzymes in multiple generations of beet armyworm, Spodoptera exigua (Hübner), in response to transgenic Bt cotton. J. Pest Sci. 2010, 83, 453–460. [Google Scholar] [CrossRef]
- Shu, Y.-H.; Du, Y.; Chen, J.; Wei, J.-X.; Wang, J.-W. Responses of the cutworm Spodoptera litura (Lepidoptera: Noctuidae) to two Bt corn hybrids expressing Cry1Ab. Sci. Rep. 2017, 7, 41577. [Google Scholar]
- Hilbeck, A.; Otto, M. Specificity and combinatorial effects of Bacillus thuringiensis Cry toxins in the context of GMO risk assessment. Front. Environ. Sci. 2015, 3, 71. [Google Scholar] [CrossRef]
- Dutton, A.; Klein, H.; Romeis, J.; Bigler, F. Uptake of Bt-toxin by herbivores feeding on transgenic maize and consequences for the predator Chrysoperla carnea. Ecol. Entomol. 2002, 27, 441–447. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Wan, F.-H.; Lövei, G.L.; Liu, W.-X.; Guo, J.-Y. Transmission of Bt toxin to the predator Propylaea japonica (Coleoptera: Coccinellidae) through its aphid prey feeding on transgenic Bt cotton. Environ. Entomol. 2006, 35, 143–150. [Google Scholar] [CrossRef]
- Tian, J.-C.; Wang, X.-P.; Long, L.-P.; Romeis, J.; Naranjo, S.E.; Hellmich, R.L.; Wang, P.; Earle, E.D.; Shelton, A.M. Bt crops producing Cry1Ac, Cry2Ab and Cry1F do not harm the green lacewing, Chrysoperla rufilabris. PLoS ONE 2012, 8, e60125. [Google Scholar] [CrossRef]
- Romeis, J.; Meissle, M.; Naranjo, S.E.; Li, Y.; Bigler, F. The end of a myth—Bt (Cry1Ab) maize does not harm green lacewings. Front. Plant Sci. 2014, 5, 391. [Google Scholar] [CrossRef]
- Yao, Y.-S.; Han, P.; Niu, C.-Y.; Dong, Y.-C.; Gao, X.-W.; Cui, J.J.; Desneux, N. Transgenic Bt cotton does not disrupt the top-down forces regulating the cotton aphid in central China. PLoS ONE 2016, 11, e0166771. [Google Scholar] [CrossRef]
- Van Den Berg, J.; Warren, J.F.; Du Plessis, H. The potential effect of Bt maize on Chrysoperla pudica (Neuroptera: Chrysopidae). Environ. Entomol. 2017, 46, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Zhang, S.; Muhammad, M.S.; Iqbal, M.; Cui, J.J. Bt proteins have no detrimental effects on larvae of the green lacewing, Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Neotrop. Entomol. 2018, 47, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Schuler, T.H.; Clark, S.J.; Stewart, C.N., Jr.; Poppy, G.M. Movement of transgenic plant-expressed Bt Cry1Ac proteins through high trophic levels. J. Appl. Entomol. 2008, 132, 1–11. [Google Scholar] [CrossRef]
- Meissle, M.; Romeis, J. Transfer of Cry1Ac and Cry2A proteins from genetically engineered Bt cotton to herbivores and predators. Insect Sci. 2017, 25, 823–832. [Google Scholar] [CrossRef]
- Lu, Y.-H.; Wu, K.-M.; Jiang, Y.-Y.; Guo, Y.-Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef]
- Hagenbucher, S.; Wäckers, F.L.; Wettstein, F.E.; Olson, D.M.; Ruberson, J.R.; Romeis, J. Pest trade-offs in technology: Reduced damage by caterpillars in Bt cotton benefits aphids. Proc. Roy. Soc. B Biol. Sci. 2013, 280, 20130042. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, M.; Wang, H.-Y.; Wang, Z.-M.; Wang, Q.; Dong, H.-Z. Comparative incidence of cotton spider mites on transgenic Bt versus conventional cotton in relation to contents of secondary metabolites. Arthropod. Plant Interact. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Svobodova, Z.; Shu, Y.-H.; Habustova, O.S.; Romeis, J.; Meissle, M. Stacked Bt maize and arthropod predators: Exposure to insecticidal Cry proteins and potential hazards. Proc. Roy. Soc. B Biol. Sci. 2017, 284, 20170440. [Google Scholar] [CrossRef]
- New, T.R. The biology of Chrysopidae and Hemerobiidae (Neuroptera), with reference to their usage as biocontrol agents: A review. Ecol. Entomol. 1975, 127, 115–140. [Google Scholar] [CrossRef]
- Icoz, I.; Stotzky, G. Fate and effects of insect-resistant Bt crops in soil ecosystems. Soil Biol. Biochem. 2008, 40, 559–586. [Google Scholar] [CrossRef]
- Romeis, J.; Bartsch, D.; Bigler, F.; Candofi, M.P.; Hartley, S.E.; Hellmich, R.L.; Huesing, J.E.; Jepson, P.C.; Layton, R.; Quemada, H.; et al. Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat. Biotech. 2008, 26, 203–208. [Google Scholar] [CrossRef]
- Meissle, M.; Zünd, J.; Waldburger, M.; Romeis, J. Development of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) on pollen from Bt-transgenic and conventional maize. Sci. Rep. 2015, 4, 5900. [Google Scholar] [CrossRef]
- Hassanpour, M.; Mohaghegh, J.; Iranipour, S.; Nouri-Ganbalani, G.; Enkegaard, A. Functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) to Helicoverpa armigera (Lepidoptera: Noctuidae): Effect of prey and predator stages. Insect Sci. 2011, 18, 217–224. [Google Scholar] [CrossRef]
- Obrycki, J.J.; Hamid, M.N.; Sajap, A.S.; Lewis, L.C. Suitability of corn insect pests for development and survival of Chrysoperla carnea and Chrysopa oculata (Neuroptera: Chrysopidae). Environ. Entomol. 1989, 18, 1126–1130. [Google Scholar] [CrossRef]
- Klingen, I.; Johansen, N.S.; Hofsvang, T. The predation of Chrysoperla carnea (Neurop., Chrysopidae) on eggs and larvae of Mamestra brassicae (Lep., Noctuidae). J. Appl. Entomol. 1996, 120, 363–367. [Google Scholar] [CrossRef]
- Meier, M.S.; Hilbeck, A. Influence of transgenic Bacillus thuringiensis corn-fed prey on prey preference of immature Chrysoperla carnea (Neuroptera: Chrysopidae). Basic Appl. Ecol. 2001, 2, 35–44. [Google Scholar] [CrossRef]
- Lawo, N.C.; Wäckers, F.L.; Romeis, J. Characterizing indirect prey-quality mediated effects of a Bt crop on predatory larvae of the green lacewing, Chrysoperla carnea. J. Insect Physiol. 2010, 56, 1702–1710. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Hellmich, R.L.; Romeis, J.; Shelton, A.M.; Velez, A.M. The role and use of genetically engineered insect-resistant crops in integrated pest management systems. In Integrated Management of Insect Pests: Current and Future Developments; Kogan, M., Higley, L., Eds.; Burleigh Dodds Science Publishing: London, UK, 2019; pp. 283–340. [Google Scholar] [CrossRef]
- Kos, M.; van Loon, J.J.A.; Dicke, M.; Louise, E.M.; Vet, L.E.M. Transgenic plants as vital components of integrated pest management. Trends Biotechnol. 2009, 27, 621–627. [Google Scholar] [CrossRef]
- Anderson, J.A.; Ellsworth, P.C.; Faria, J.C.; Head, G.P.; Owen, M.D.K.; Pilcher, C.D.; Shelton, A.M.; Meissle, M. Genetically Engineered Crops: Importance of Diversified Integrated Pest Management for Agricultural Sustainability. Front. Bioeng. Biotechnol. 2019, 7, 24. [Google Scholar] [CrossRef]
- Jia, S.-R.; Guo, S.-D.; An, D.-C. Transgenic Bt Cotton; Science Press: Beijing, China, 2001. (In Chinese) [Google Scholar]
- Zhang, F.; Wang, S.-Q.; Luo, C.; Chen, Y.-H.; Li, F. Effects of the artificial diets and breeding means on growth and development of Chrysopa septempunctata Wesmael. Plant Prot. 2004, 30, 36–40, (In Chinese with English abstract). [Google Scholar]
- Romeis, J.; Dutton, A.; Bigler, F. Bacillus thuringiensis toxin (Cry1Ab) has no direct effect on larvae of the green lacewing Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). J. Insect Physiol. 2004, 50, 175–183. [Google Scholar] [CrossRef]


| Bt Toxin Content in GK-19 Leaves (n = 12) (ng/g Fresh Weight) | Bt Toxin Content in the Powder of Larvae Fed on GK-19 Cotton Leaves (ng/g Fresh Weight) | Bt Toxin Content in Diet Mixture (ng/g Diet Weight) | |
|---|---|---|---|
| 1 | 77.4 ± 10.47 | 0.77 | 0.004 |
| 2 | 77.8 ± 18.84 | 1.48 | 0.016 |
| 3 | 155.5 ± 25.86 | 19.48 | 0.418 |
| 4 | 61.1 ± 8.32 | 11.72 | 0.251 |
| Treatment | No. of Treated Larvae | No. of Pupae | Eclosion Rate (%) | No. of Adult Males | No. of Adult Females |
|---|---|---|---|---|---|
| A | 30 | 17 | 88.2 | 10 | 5 |
| B | 30 | 22 | 100 | 10 | 12 |
| C | 20 | 11 | 100 | 7 | 4 |
| D | 20 | 15 | 100 | 7 | 8 |
| E | 20 | 13 | 92.3 | 5 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Z.-J.; Zhou, Q.-J.; Shi, H.; Tang, Z.-X.; Liu, B.; Wei, W. Effect of Transgenic Cotton Expressing Bt Cry1Ac or Cry1Ab/Ac Toxins on Lacewing Larvae Mediated by Herbivorous Insect Pests. Plants 2022, 11, 2755. https://doi.org/10.3390/plants11202755
Guan Z-J, Zhou Q-J, Shi H, Tang Z-X, Liu B, Wei W. Effect of Transgenic Cotton Expressing Bt Cry1Ac or Cry1Ab/Ac Toxins on Lacewing Larvae Mediated by Herbivorous Insect Pests. Plants. 2022; 11(20):2755. https://doi.org/10.3390/plants11202755
Chicago/Turabian StyleGuan, Zheng-Jun, Qiu-Ju Zhou, Hong Shi, Zhi-Xi Tang, Biao Liu, and Wei Wei. 2022. "Effect of Transgenic Cotton Expressing Bt Cry1Ac or Cry1Ab/Ac Toxins on Lacewing Larvae Mediated by Herbivorous Insect Pests" Plants 11, no. 20: 2755. https://doi.org/10.3390/plants11202755
APA StyleGuan, Z.-J., Zhou, Q.-J., Shi, H., Tang, Z.-X., Liu, B., & Wei, W. (2022). Effect of Transgenic Cotton Expressing Bt Cry1Ac or Cry1Ab/Ac Toxins on Lacewing Larvae Mediated by Herbivorous Insect Pests. Plants, 11(20), 2755. https://doi.org/10.3390/plants11202755







