Potato (Solanum tuberosum L.) Leaf Extract Concentration Affects Performance and Oxidative Stress in Green Peach Aphids (Myzus persicae (Sulzer)
Abstract
:1. Introduction
2. Results
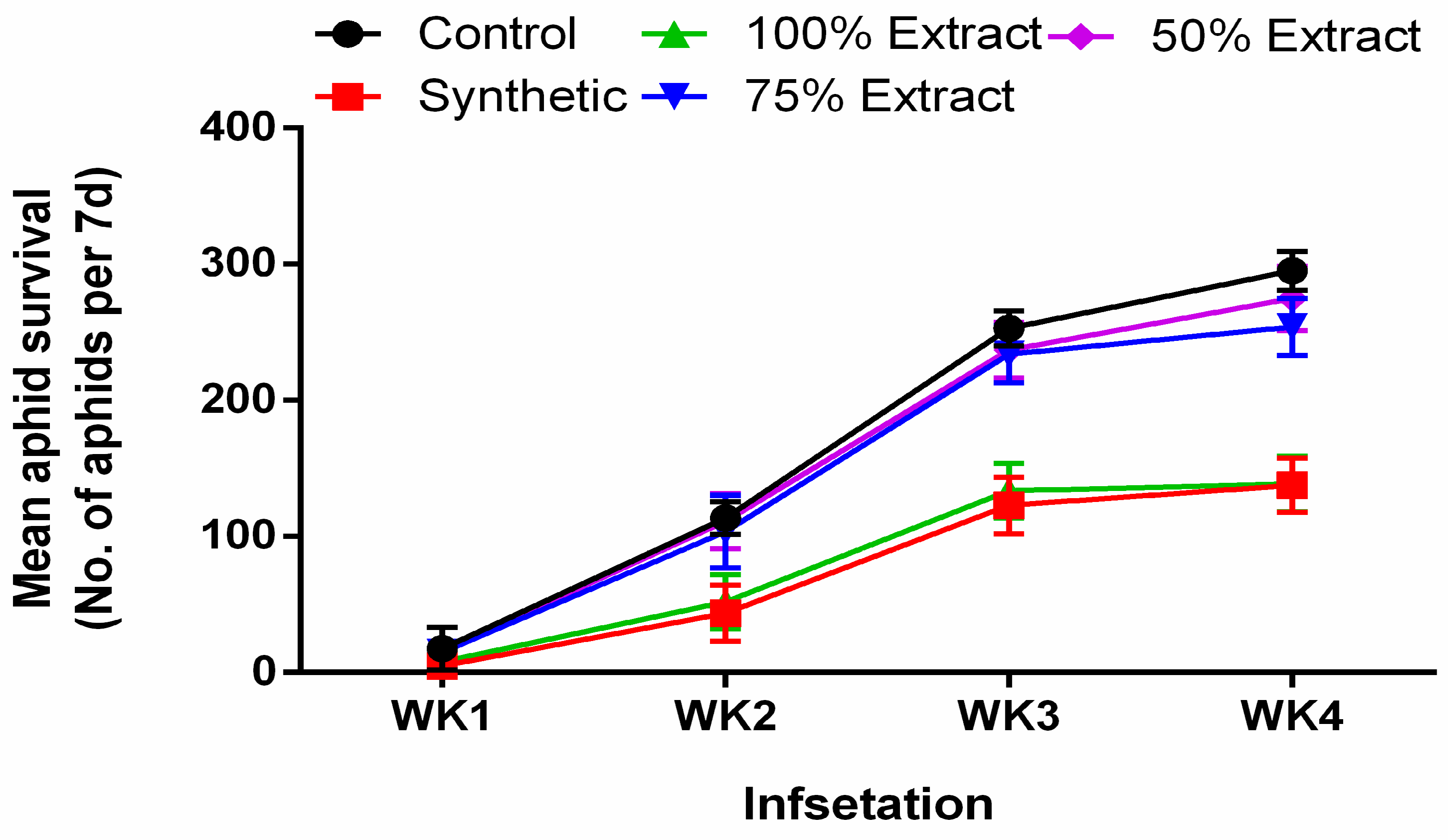
2.1. The Effect of Potato Leaf Extract on Mean Aphid Survival
2.2. The Effect of Potato Leaf Extract on Aphid Reproductive Performance
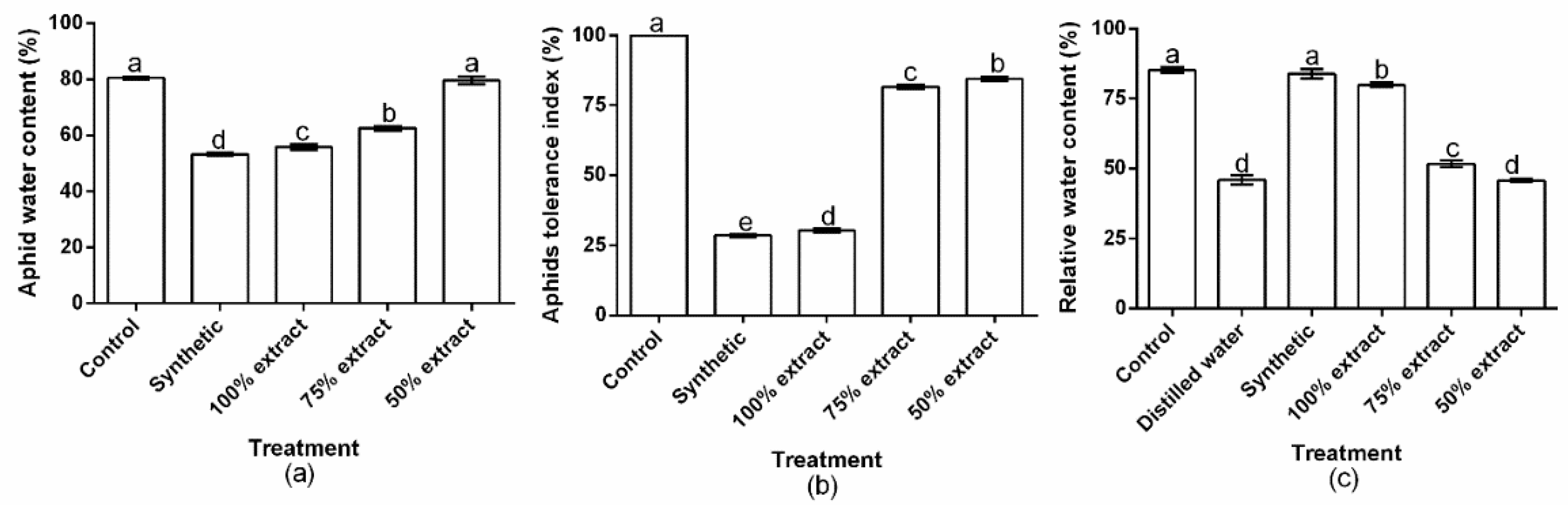
2.3. The Effect of Potato Extract on Aphid Water Content, the Aphid Tolerance Index, and Relative Water Content
2.4. The Effect of Potato Extract on H2O2 and MDA Content in the Green Peach Aphid
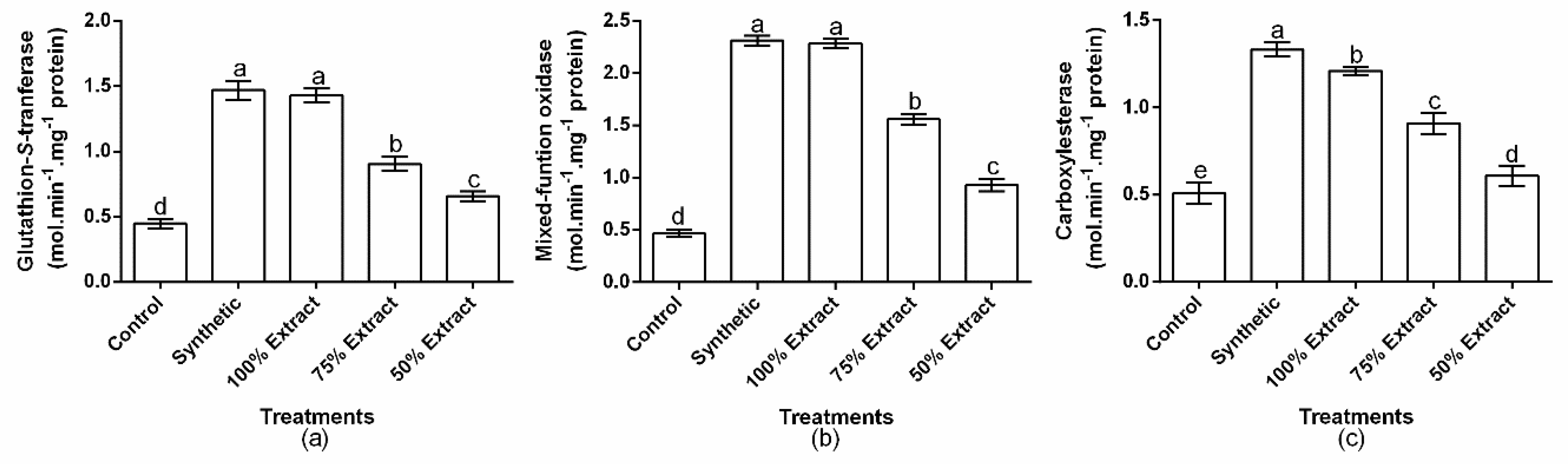
2.5. The Effect of Potato Extract on Green Peach Aphid Detoxifying Enzymes
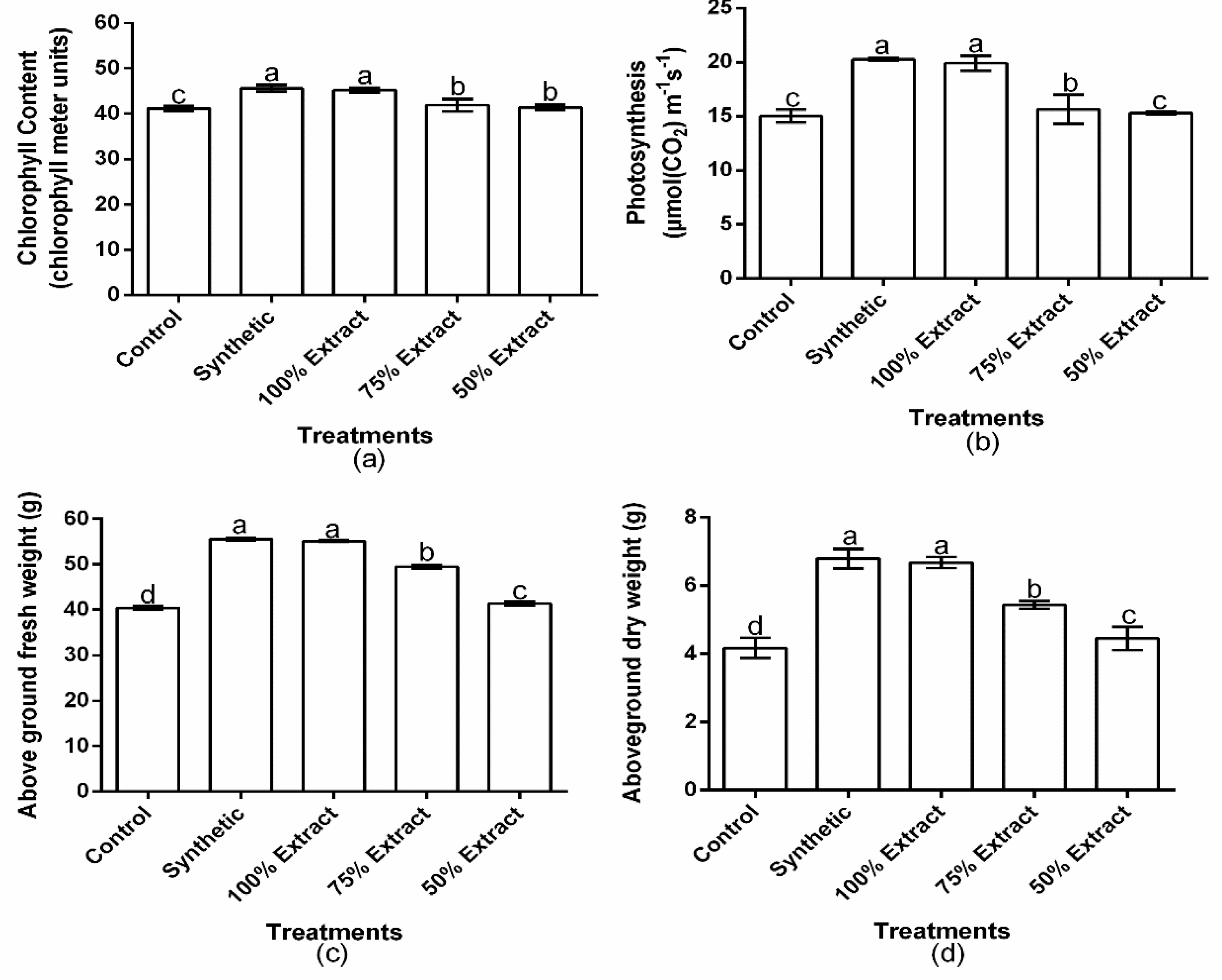
2.6. The Effect of Potato Leaf Extract on the Physiology of the Host Plant
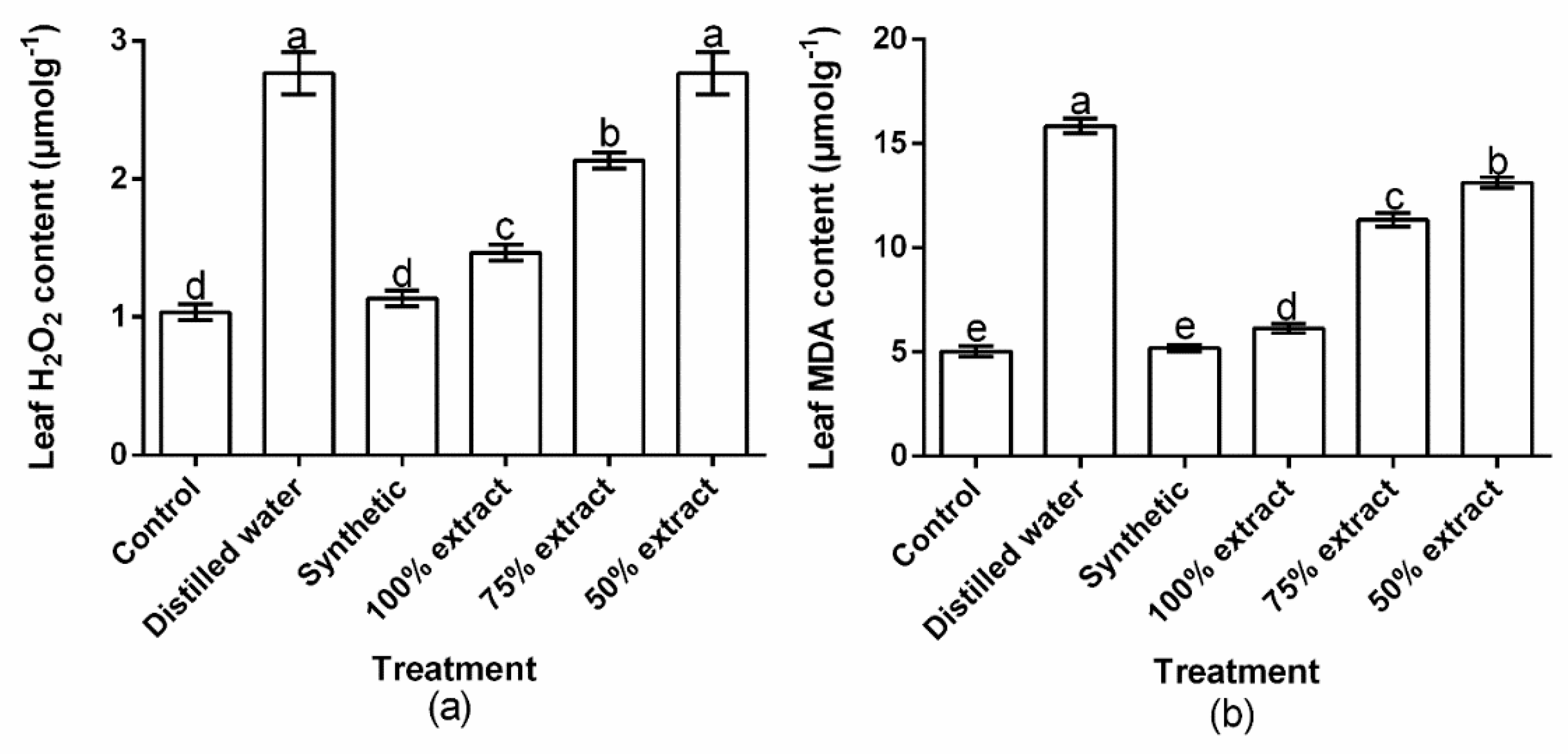
2.7. Effect of Potato Extract on H2O2 and MDA Content in the Host Plant
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Planting Materials
4.2. Aphid Culture
4.3. Experimental Design and Treatments
4.4. Preparation and Application of the Potato Leaf Extract
4.5. Determination of Aphid Performance
4.6. Aphid Water Content (AWC) and the Aphid Tolerance Index (ATI)
4.7. Determination of Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Content in Aphids
4.8. Determination of Aphids’ Detoxifying Enzymes
4.9. Leaf Relative Water Content (RWC)
4.10. Chlorophyll Content and Net Photosynthesis
4.11. Determination of the Aboveground Biomass
4.12. Determination of H2O2 Content in Leaves
4.13. Determination of MDA Content in Leaves
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenstein, M. Natural solutions for agricultural productivity. Nature 2020, 588, S58–S59. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, E.; Armero, C.; Roselló, J.; Serra, J.; Muñoz, M.J.; Canet, R.; Galipienso, L.; Rubio, L. Comparison of viral infection risk between organic and conventional crops of tomato in Spain. Eur. J. Plant Pathol. 2019, 155, 1145–1154. [Google Scholar] [CrossRef]
- Ugurlu, S.; Gurkan, M.O. Insecticide Resistance in Helicoverpa armigera from Cottongrowing Areas in Turkey. Phytoparasitica 2007, 35, 376–379. [Google Scholar] [CrossRef]
- Duke, S.O.; Cantrell, C.L.; Meepagala, K.M.; Wedge, D.E.; Tabanca, N.; Schrader, K.K. Natural Toxins for Use in Pest Management. Toxins 2010, 2, 1943–1962. [Google Scholar] [CrossRef] [Green Version]
- Ignatowicz, S.; Wesolowska, B. Potential of Common Herbs as Grain Protectants: Repellent Effect of Herb Extracts on the Granary Weevil, Sitophilus granarius L. In Proceedings of the 6th International Working Conference on Stored-products Protection, Canberra, Australia, 17–23 April 1994; Volume 2, pp. 790–794. [Google Scholar]
- Wakeil, N.E. Botanical Pesticides and Their Mode of Action. Gesunde Pflanzen. 2013, 65, 125–149. [Google Scholar] [CrossRef]
- Rezaei, S.S.; Dehghanifard, E.; Noorisepehr, M.; Ghadirinejad, K.; Kakavandi, B.; Esfahani, A.R. Efficient clean-up of waters contaminated with diazinon pesticide using photo-decomposition of peroxymonosulfate by ZnO decorated on a magnetic core/shell structure. J. Environ. Manag. 2019, 250, 109–472. [Google Scholar] [CrossRef] [PubMed]
- Parizad, S.; Bera, S. The effect of organic farming on water reusability, sustainable ecosystem, and food toxicity. Environ. Sci. Pollut. Res. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Alotaiba, S.; Elsayed, G. Recent knowledge about the relation between allelochemicals in plants and insects. World J. Zool. 2007, 2, 1–8. [Google Scholar]
- Friedman, M.J. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S. Comparing impacts of plant extracts and pure allelochemicals and implications for pest control. CAB Rev. Perspect Agr. Vet. Sci. Nut. Nat. Res. 2009, 4, 1–30. [Google Scholar]
- Nenaah, G.E. Individual and synergistic toxicity of solanaceous glycoalkaloids against two coleopteran stored-product insects. J. Pest Sci. 2011, 84, 77–86. [Google Scholar] [CrossRef]
- Büyükgüzel, E.; Büyükgüzel, K.; Erdem, M.; Adamski, Z.; Marciniak, P.; Ziemnicki, K.; Ventrella, E.; Scrano, L.; Bufo, S.A. The influence of dietary α-solanine on the waxmoth Galleria mellonella L. Arch. Insect Biochem. Physiol. 2013, 83, 15–24. [Google Scholar] [CrossRef]
- Dao, L.; Friedman, M. Comparison of glycoalkaloid content of fresh and freeze-dried potato leaves determined by HPLC and colorimetry. J. Agric. Food Chem. 1996, 44, 2287–2291. [Google Scholar] [CrossRef]
- Ventrella, E.; Marciniak, P.; Adamski, Z.; Rosiński, G.; Chowański, S.; Falabella, P.; Scrano, L.; Bufo, S.A. Cardioactive properties of solanaceae plant pure glycoalkaloids on Zophobas atratus. Insect Sci. 2014, 3, 2–28. [Google Scholar] [CrossRef]
- Bokov, A.; Chaudhuri, A.; Richardson, A. The role of oxidative damage and stress in aging. Mech. Age Dev. 2004, 125, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Senhal, F. Compartmentalization of oxidative stress and antioxidant defense in the larval gut of Spodoptera littoralis. Arch. Insect Biochem. Physiol. 2006, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Che-Mendoza, A.R.; Penilla, P.; Rodriguez, D.A. Insecticide resistance and glutathione Stransferases in mosquitoes: A review. Afr. J. Biotechonol. 2009, 8, 1386–1397. [Google Scholar]
- Bale, J.S.; Ponder, K.L.; Pritchard, J. Coping with Stress. Aphids as Crop Pests (ed. by H. F. Van Emden and R. Harrington). CAB Int. UK 2007, 287, 309. [Google Scholar]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H. Physiological response to drought stress in Camptotheca acuminata seedlings from two provenances. Front. Plant Sci. 2015, 6, 361. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, P.; Jaleel, C.A.; Somasundaram, R.; Panneerselvam, R. Osmoregulation and antioxidant metabolism in drought stressed Helianthus annuus under triadimefon drenching. C. R. Biol. 2008, 331, 418–425. [Google Scholar] [CrossRef]
- Carena, M.; Glogoza, P. Resistance of maize to the corn leaf aphid:A review. Maydica 2004, 49, 241–254. [Google Scholar]
- Quandahor, P.; Lin, C.; Gou, Y.; Coulter, A.J.; Liu, C. Leaf Morphological and Biochemical Responses of Three Potato (Solanum tuberosum L.) Cultivars to Drought Stress and Aphid (Myzus persicae Sulzer) Infestation. Insects 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.Z.; Fu, W.Y.; Li, N.; Zhang, F.; Liu, T.X. Antioxidant responses of Propylaea japonica, (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for sustainable global food security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, J.; Yang, Y.; Tang, H.; Lu, H.; Fan, M.; Shi, Y.; Dong, D.; Wang, G.; Wang, W.; et al. Status of Major Diseases and Insect Pests of Potato and Pesticide Usage in China. Sci. Agric. Sin. 2019, 2, 2800–2808. [Google Scholar]
- Nikolakakis, N.N.; Margaritopoulos, J.T.; Tsitsipis, J.A. Performance of Myzus persicae (Hemiptera: Aphididae) clones on different host-plants and their host preference. Bull. Entomol. Res. 2003, 93, 235–242. [Google Scholar] [CrossRef]
- Silva, A.X.; Jander, G.; Samaniego, H.; Ramsey, J.S.; Figueroa, C.C. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS ONE 2012, 7, e36366. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; · Liu, Y.; Donkersley, P.; Dong, Y.; Chen, X.; Zang, Y.; Xu, P.; Ren, G. Preference of the aphid Myzus persicae (Hemiptera: Aphididae) for tobacco plants at specific stages of potato virus Y infection. Arch. Virol. 2019, 164, 1567–1573. [Google Scholar] [CrossRef]
- Bera, S.; Arena, G.D.; Ray, S.; Flannigan, S.; Casteel, C.L. The Potyviral Protein 6K1 Reduces Plant Proteases Activity during Turnip mosaic virus Infection. Viruses 2022, 14, 1341. [Google Scholar] [CrossRef]
- Berlandier, F.A. Aphids on the world’s crops. An information and identification guide. Aust. J. Entomol. 2010, 39, 354–355. [Google Scholar]
- Murphy, A.F.; Rondon, I.; Moreno, A.; Fereres, A. Effect of Potato virus Y presence in Solanum tuberosum (Solanales: Solanaceae) and Chenopodium album on aphid (Hemiptera: Aphididae) behavior. Environ. Entomol. 2018, 47, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Science of the total environment current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Liu, N.; Zhou, Q.; Ding, X.; Zhan, J.; Cheng, X.; Huang, J.; Lu, Y.; Yang, Y. Current status and management strategies for potato insect pests and diseases in China. Plant Prot. 2019, 45, 106–111. [Google Scholar]
- Talukder, F.; Islam, M.; Hossain, M.; Rahman, M.; Alam, M. Toxicity effects of botanicals and synthetic insecticides on Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.). Bangladesh J. Environ. Sci. 2004, 10, 365–371. [Google Scholar]
- Quandahor, P.; Gou, Y.; Lin, C.; Dawuda, M.M.; Coulter, J.A.; Liu, C. Phytohormone Cross-Talk Synthesizes Glycoalkaloids in Potato (Solanum tuberosum L.) in Response to Aphid (Myzus persicae Sulzer) Infestation under Drought Stress. Insects 2020, 11, 724. [Google Scholar] [CrossRef]
- Rajashekar, Y.; Bakthavatsalam, N.; Shivanandappa, T. Botanicals as grain protectants. Psyche 2012, 8, 646740. [Google Scholar] [CrossRef]
- Bahlai, C.A.; Xue, Y.; McCreary, C.M.; Schaafsma, A.W.; Hallett, R.H. Choosing organic pesticides over synthetic pesticides may not effectively mitigate environmental risk in soybeans. PLoS ONE 2010, 5, 11–250. [Google Scholar] [CrossRef]
- Ndakidemi, B.; Mtei, K.; Ndakidemi, P.A. Impacts of synthetic and botanical pesticides on beneficial insects. Agric. Sci. 2016, 7, 364–372. [Google Scholar] [CrossRef]
- El Aalaoui, M.; Bouharroud, R.; Sbaghi, M.; El Bouhssini, M.; Hilali, L.; Dari, K. Comparative toxicity of different chemical and biological insecticides against the scale insect Dactylopius opuntiae and their side effects on the predator Cryptolaemus montrouzieri. Arch. Für Phytopathol. Und Pflanzenschutz 2019, 52, 155–169. [Google Scholar] [CrossRef]
- Loko, L.Y.; Alagbe, O.; Dannon, E.A.; Datinon, B.; Orobiyi, A.; Thomas-Odjo, A.; Tamo, M. Repellent effect and insecticidal activities of Bridelia ferruginea, Blighia sapida, and Khaya senegalensis leaves powders and extracts against Dinoderus porcellus in infested dried yam chips. Psyche 2017, 18, 59–64. [Google Scholar]
- Mathur, Y.K.; Shankar, K.; Ram, S. Evaluation of some grain protectants against Callosobruchus chinensis (L.) on black gram. Bull. Grain Technol. 1985, 23, 253–259. [Google Scholar]
- Abdullah, F.; Subramanian, P.; Ibrahim, H.; Abdul Malek, S.N.; Lee, G.S.; Hong, S.L. Chemical composition, antifeedant, repellent, and toxicity activities of the rhizomes of galangal, Alpinia galanga Against Asian Subterranean Termites, Coptotermes gestroi and Coptotermes curvignathus (Isoptera: Rhinotermitidae). J. Insect Sci. 2017, 15, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Aslanturk, A.; Kalender, S.; Uzunhisarcikli, M.; Kalender, Y. Effects of methidathion on antioxidant enzyme activities and malondialdehyde level in midgut tissues of Lymantria dispar (Lepidoptera) larvae. J. Entomol. Res. Soc. 2011, 13, 27–38. [Google Scholar]
- Kaspi, R.; Madar, R.; Domeradzki, S. Acaricides compatibility with the armored scale predator Rhyzobius lophanthae. Biol. Control 2019, 132, 42–48. [Google Scholar] [CrossRef]
- Quesada, C.R.; Sadof, C.S. Residual toxicity of insecticides to Chrysoperla rufilabris and Rhyzobius lophanthae predators as biocontrol agents of pine needle scale. Crop Prot. 2020, 130, 10–14. [Google Scholar] [CrossRef]
- Palma-Onetto, V.; Oliva, D.; González-Teuber, M. Lethal and oxidative stress side effects of organic and synthetic pesticides on the insect scale predator Rhyzobius lophanthae. Entomol. Gen. 2021, 1, 345–355. [Google Scholar] [CrossRef]
- Kim, S.I.; Roh, J.Y.; Kim, D.H.; Lee, H.S.; Ahn, Y.J. Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J. Stored Prod. Res. 2003, 39, 293–303. [Google Scholar] [CrossRef]
- Wang, Z.L.; Huang, B.R. Physiological recovery of Kentucky bluegrass from simultaneous drought and heat stress. Crop Sci. 2004, 44, 1729–1736. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffei, M.E.; Mithofer, A.; Arimura, G.; Uchtenhagen, H.; Bossi, S.; Bertea, C.M.; Cucuzza, L.S.; Novero, M.; Volpe, V.; Quadro, S.; et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006, 140, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, P.; Adamski, Z.; Bednarz, P.; Slocinska, M.; Ziemnicki, K.; Lelario, F.; Scrano, L.; Bufo, S.A. Cardioinhibitory properties of potato glycoalkaloids in beetles. Bull. Environ. Contam. Toxicol. 2010, 84, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sun, Y.; Peng, X.; Wang, Q.; Harris, M.; Ge, F. Up regulation of abscisic acid signaling pathway facilitates aphid xylem absorption and osmoregulation under droughtstress. J. Exp. Bot. 2015, 67, 681–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, D.A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Zhu, G.; Xue, M.; Luo, J.; Guixia, G.; Liu, F.; Zhao, H.; Sun, X. Effects of short-term heat shock and physiological responses to heat stress in two Bradysia adults, Bradysia odoriphaga and Bradysia difformis. Sci. Rep. 2017, 7, 79–81. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Li, M.X.; Chang, H.J.; Mao, Y.; Zhang, H.Y.; Lu, L.X.; Yan, S.G.; Lang, M.L.; Qiao, C.L. Carboxylesterase-mediated insecticide resistance: Quantitative increase induces broader metabolic resistance than qualitative change. Pestic. Biochem. Physiol. 2014, 121, 88–96. [Google Scholar] [CrossRef]
- Sun, X.Q.; Zhang, M.X.; Yu, J.Y.; Jin, Y.; Ling, B.; Du, J.P.; Cai, Q.N. Glutathione S-transferase of brown planthoppers (Nilaparvata lugens) is essential for their adaptation to gramine-containing host plants. PLoS ONE 2013, 8, e64026. [Google Scholar] [CrossRef] [Green Version]
- Sintim, H.O.; Tashiro, T.; Motoyama, N.E. Effect of sesame leaf diet on detoxification activities of insects with different feeding behavior. Arch. Biochem. Physiol. 2012, 81, 148–159. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Bañón, S.; Ochoa, J.; Franco, J.A.; Alarcón, J.J.; Sánchez-Blanco, M.J. Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ. Exp. Bot. 2006, 56, 36–43. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Su, X.; Xia, Z. Grafting improves drought tolerance by regulating antioxidant enzyme activities and stressresponsive gene expression in tobacco. Environ. Exp. Bot. 2014, 107, 173–179. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quandahor, P.; Gou, Y.; Lin, C.; Liu, C. Potato (Solanum tuberosum L.) Leaf Extract Concentration Affects Performance and Oxidative Stress in Green Peach Aphids (Myzus persicae (Sulzer). Plants 2022, 11, 2757. https://doi.org/10.3390/plants11202757
Quandahor P, Gou Y, Lin C, Liu C. Potato (Solanum tuberosum L.) Leaf Extract Concentration Affects Performance and Oxidative Stress in Green Peach Aphids (Myzus persicae (Sulzer). Plants. 2022; 11(20):2757. https://doi.org/10.3390/plants11202757
Chicago/Turabian StyleQuandahor, Peter, Yuping Gou, Chunyan Lin, and Changzhong Liu. 2022. "Potato (Solanum tuberosum L.) Leaf Extract Concentration Affects Performance and Oxidative Stress in Green Peach Aphids (Myzus persicae (Sulzer)" Plants 11, no. 20: 2757. https://doi.org/10.3390/plants11202757
APA StyleQuandahor, P., Gou, Y., Lin, C., & Liu, C. (2022). Potato (Solanum tuberosum L.) Leaf Extract Concentration Affects Performance and Oxidative Stress in Green Peach Aphids (Myzus persicae (Sulzer). Plants, 11(20), 2757. https://doi.org/10.3390/plants11202757







