Application of an Enzymatic Hydrolysed L-α-Amino Acid Based Biostimulant to Improve Sunflower Tolerance to Imazamox
Abstract
1. Introduction
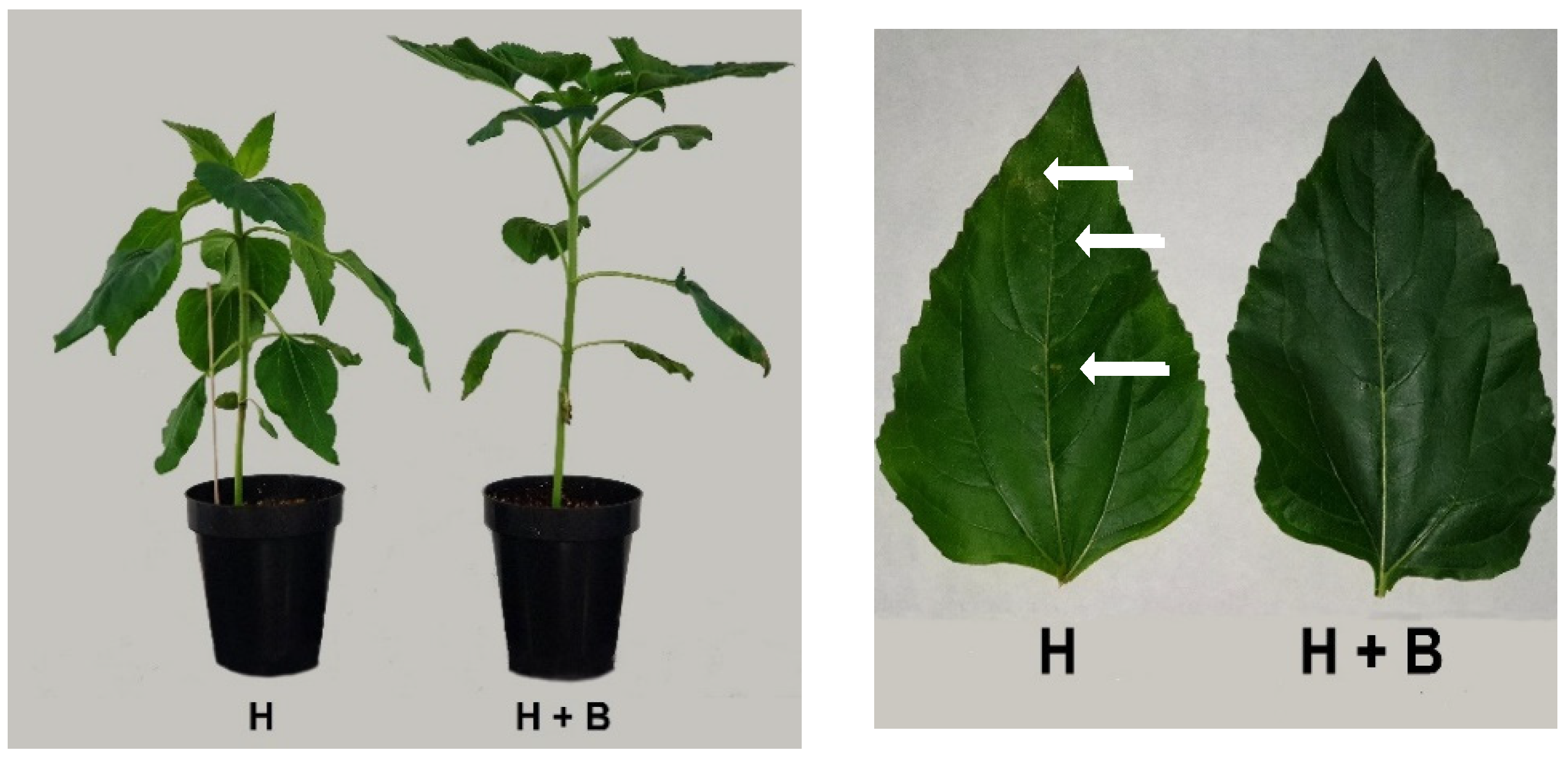
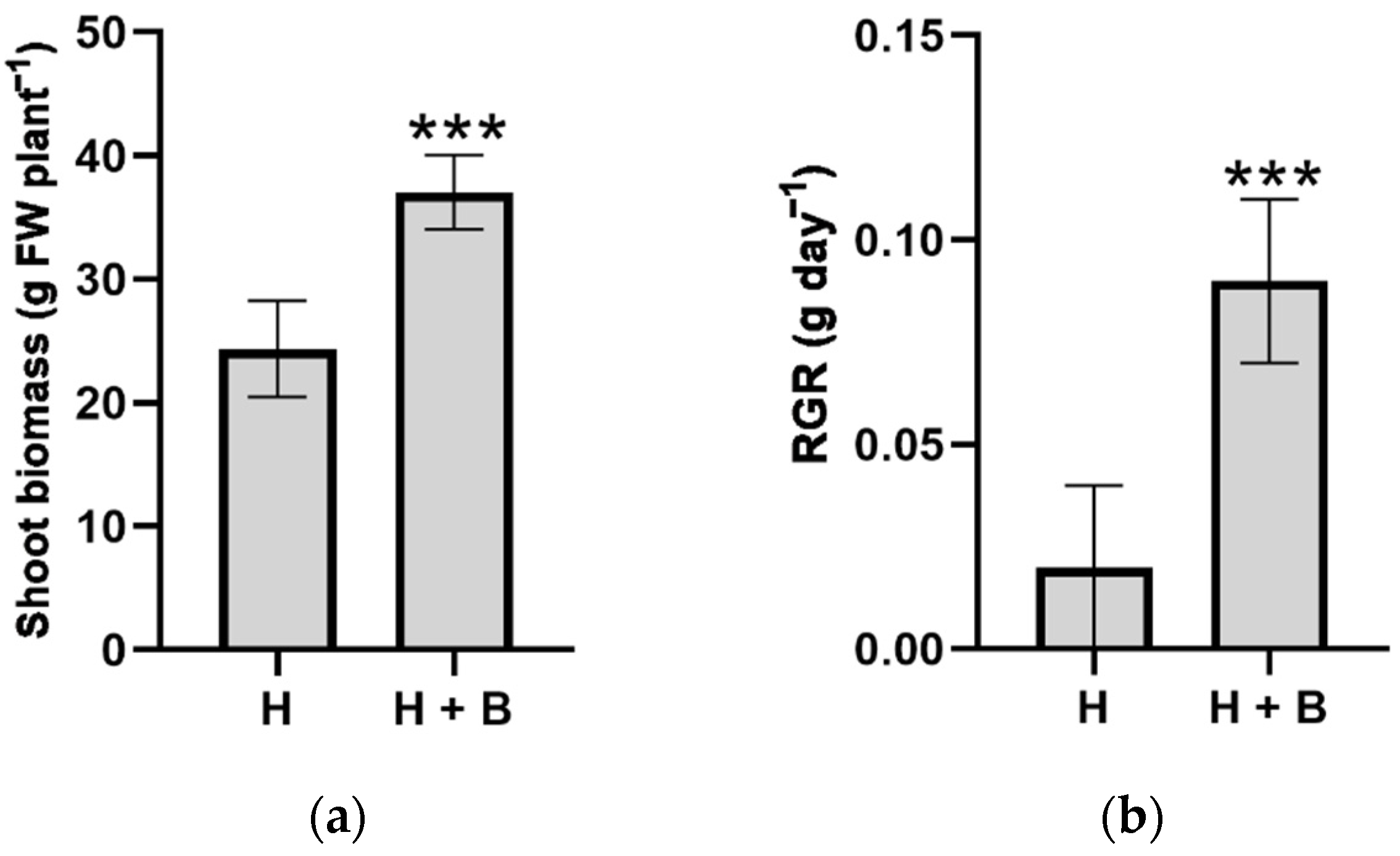
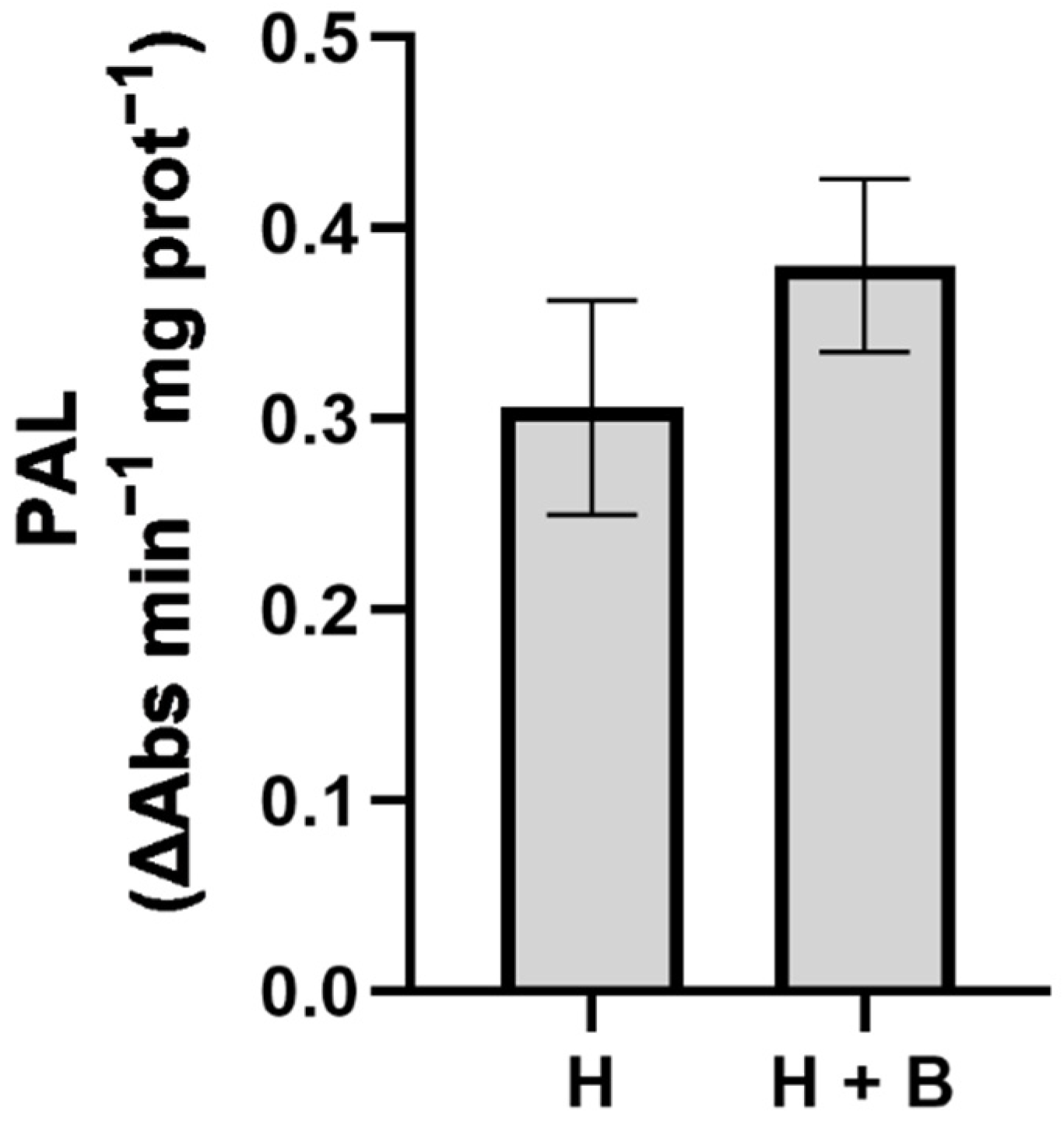
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Experimental Design
3.2. Relative Growth Rate (RGR)
3.3. Determination of Enzymatic Activities
3.4. Aminogram Analysis
3.5. Chl a Fluorescence
3.6. Determination of the Concentration of Oxidative Indicators (Malondialdehyde (MDA), H2O2, and O2−)
3.7. Determination of GSH Concentration
3.8. Determination of the Phenolic Compound Profile
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shennan, C. Biotic Interactions, Ecological Knowledge and Agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 717–739. [Google Scholar] [CrossRef] [PubMed]
- Munsif, F.; Shah, S.; Samuel, A.L.; Amin, M.; Shan, A.; Ahmad, A.; Ali, Z. Integration of Weed Control Methods with Seed Rates for Improving Wheat Yield. Pak. J. Weed Sci. Res. 2014, 20, 155–165. [Google Scholar]
- Pang, S.S.; Guddat, L.W.; Duggleby, R.G. Molecular Basis of Sulfonylurea Herbicide Inhibition of Acetohydroxyacid Synthase. J. Biol. Chem. 2003, 278, 7639–7644. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Evans, R.R.; Dahmer, M.L.; Singh, B.K.; Shaner, D.L. Imidazolinone-Tolerant Crops: History, Current Status and Future. Pest Manag. Sci. 2005, 61, 246–257. [Google Scholar] [CrossRef]
- Rosinger, C. Herbicide Safeners: An Overview. Jul.-Kühn-Arch. 2014, 443, 516–525. [Google Scholar]
- Powles, S.B.; Yu, Q. Evolution in Action: Plants Resistant to Herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Tripthi, D.K.; Varma, R.K.; Singh, S.; Sachan, M.; Guerriero, G.; Kushwaha, B.K.; Bhardwaj, S.; Ramawat, N.; Sharma, S.; Singh, V.P.; et al. Silicon Tackles Butachlor Toxicity in Rice Seedlings by Regulating Anatomical Characteristics, Ascorbate-Glutathione Cycle, Proline Metabolism and Levels of Nutrients. Sci. Rep. 2020, 10, 14078. [Google Scholar] [CrossRef]
- Gomes, M.P.; le Manac’h, S.G.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-Dependent Inhibition of Photosynthesis in Willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, Y.; Luo, Y.; Zou, N.; Liu, W.; Wang, J. Unravelling Mesosulfuron-Methyl Phytotoxicity and Metabolism-Based Herbicide Resistance in Alopecurus Aequalis: Insight into Regulatory Mechanisms Using Proteomics. Sci. Total Environ. 2019, 670, 486–497. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Balabanova, D.A.; Paunov, M.; Goltsev, V.; Cuypers, A.; Vangronsveld, J.; Vassilev, A. Photosynthetic Performance of the Imidazolinone Resistant Sunflower Exposed to Single and Combined Treatment by the Herbicide Imazamox and an Amino Acid Extract. Front. Plant Sci. 2016, 7, 1559. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.F.; Kunz, C.; Peteinatos, G.G.; Santel, H.-J.; Gerhards, R. Utilization of Chlorophyll Fluorescence Imaging Technology to Detect Plant Injury by Herbicides in Sugar Beet and Soybean. Weed Technol. 2017, 31, 523–535. [Google Scholar] [CrossRef]
- Duhoux, A.; Pernin, F.; Desserre, D.; Délye, C. Herbicide Safeners Decrease Sensitivity to Herbicides Inhibiting Acetolactate-Synthase and Likely Activate Non-Target-Site-Based Resistance Pathways in the Major Grass Weed Lolium sp. (Rye-Grass). Front. Plant Sci. 2017, 8, 1310. [Google Scholar] [CrossRef]
- Délye, C.; Gardin, J.A.C.; Boucansaud, K.; Chauvel, B.; Petit, C. Non-Target-Site-Based Resistance Should Be the Centre of Attention for Herbicide Resistance Research: Alopecurus Myosuroides as an Illustration. Weed Res. 2011, 51, 433–437. [Google Scholar] [CrossRef]
- Neshev, N.; Balabanova, D.; Yanev, M.; Mitkov, A. Is the Plant Biostimulant Application Ameliorative for Herbicide-Damaged Sunflower Hybrids? Ind. Crops Prod. 2022, 182, 114926. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhuyan, M.H.M.B.; Oku, H.; Fujita, M. Exogenous Nitric Oxide Pretreatment Protects Brassica napus L. Seedlings from Paraquat Toxicity through the Modulation of Antioxidant Defense and Glyoxalase Systems. Plant Physiol. Biochem. 2018, 126, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Hernández Estévez, I.; Rodríguez Hernández, M. Plant Glutathione S-Transferases: An Overview. Plant Gene 2020, 23, 100233. [Google Scholar] [CrossRef]
- Fayez, K.; Ali, E. Impact of Glyphosate Herbicide and Salicylic Acid on Seed Germination, Cell Structure and Physiological Activities of Faba Bean (Vicia faba L.) Plant. Annu. Res. Rev. Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Deng, X.; Zheng, W.; Zhou, X.; Bai, L. The Effect of Salicylic Acid and 20 Substituted Molecules on Alleviating Metolachlor Herbicide Injury in Rice (Oryza sativa). Agronomy 2020, 10, 317. [Google Scholar] [CrossRef]
- Arda, H.; Kaya, A.; Alyuruk, G. Physiological and Genetic Effects of Imazamox Treatment on Imidazolinone-Sensitive and Resistant Sunflower Hybrids. Water Air Soil Pollut. 2020, 231, 118. [Google Scholar] [CrossRef]
- Sivey, J.D.; Lehmler, H.-J.; Salice, C.J.; Ricko, A.N.; Cwiertny, D.M. Environmental Fate and Effects of Dichloroacetamide Herbicide Safeners: “Inert” yet Biologically Active Agrochemical Ingredients. Environ. Sci. Technol. Lett. 2015, 2, 260–269. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in Agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef]
- Taha, R.S.; Alharby, H.F.; Bamagoos, A.A.; Medani, R.A.; Rady, M.M. Elevating Tolerance of Drought Stress in Ocimum Basilicum Using Pollen Grains Extract; a Natural Biostimulant by Regulation of Plant Performance and Antioxidant Defense System. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- ur Rehman, H.; Alharby, H.F.; Alzahrani, Y.; Rady, M.M. Magnesium and Organic Biostimulant Integrative Application Induces Physiological and Biochemical Changes in Sunflower Plants and Its Harvested Progeny on Sandy Soil. Plant Physiol. Biochem. 2018, 126, 97–105. [Google Scholar] [CrossRef]
- Alharby, H.; Alzahrani, Y.; Rady, M. Seeds Pretreatment with Zeatins or Maize Grain-Derived Organic Biostimulant Improved Hormonal Contents, Polyamine Gene Expression, and Salinity and Drought. Int. J. Agric. Biol. 2020, 24, 714–724. [Google Scholar]
- Desoky, E.-S.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.M.; Selem, E.; Rady, M.M.; Ali, E.F.; Mersal, G.A.M.; Semida, W.M. Application of Biostimulants Promotes Growth and Productivity by Fortifying the Antioxidant Machinery and Suppressing Oxidative Stress in Faba Bean under Various Abiotic Stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Martínez-Esteso, M.; Vilella-Antón, M.; Sellés-Marchart, S.; Martínez-Márquez, A.; Botta-Català, A.; Piñol-Dastis, R.; Bru-Martínez, R. A DIGE Proteomic Analysis of Wheat Flag Leaf Treated with TERRA-SORB® Foliar, a Free Amino Acid High Content Biostimulant. J. Integr. OMICS 2016, 6, 188. [Google Scholar] [CrossRef]
- Gavelienė, V.; Pakalniškytė, L.; Novickienė, L.; Balčiauskas, L. Effect of Biostimulants on Cold Resistance and Productivity Formation in Winter Rapeseed and Winter Wheat. Ir. J. Agric. Food Res. 2018, 57, 71–83. [Google Scholar] [CrossRef]
- Navarro-León, E.; López-Moreno, F.J.; Borda, E.; Marín, C.; Sierras, N.; Blasco, B.; Ruiz, J.M. Effect of l -amino Acid-based Biostimulants on Nitrogen Use Efficiency (NUE) in Lettuce Plants. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Fernández-Escalada, M.; Zulet-González, A.; Gil-Monreal, M.; Royuela, M.; Zabalza, A. Physiological Performance of Glyphosate and Imazamox Mixtures on Amaranthus Palmeri Sensitive and Resistant to Glyphosate. Sci. Rep. 2019, 9, 18225. [Google Scholar] [CrossRef]
- Gil-Monreal, M.; Royuela, M.; Zabalza, A. Hypoxic Treatment Decreases the Physiological Action of the Herbicide Imazamox on Pisum Sativum Roots. Plants 2020, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.; Braun, H.; Hildebrandt, T. The Role of Amino Acid Metabolism during Abiotic Stress Release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.G.; Teicher, H.B.; Streibig, J.C. Linking Fluorescence Induction Curve and Biomass in Herbicide Screening. Pest Manag. Sci. 2003, 59, 1303–1310. [Google Scholar] [CrossRef]
- Linn, A.I.; Mink, R.; Peteinatos, G.G.; Gerhards, R. In-field Classification of Herbicide-resistant Papaver rhoeas and Stellaria media Using an Imaging Sensor of the Maximum Quantum Efficiency of Photosystem II. Weed Res. 2019, 59, 357–366. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An Overview of Plant-Based Natural Biostimulants for Sustainable Horticulture with a Particular Focus on Moringa Leaf Extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Balabanova, D.; Remans, T.; Vassilev, A.; Cuypers, A.; Vangronsveld, J. Possible Involvement of Glutathione S-Transferases in Imazamox Detoxification in an Imidazolinone-Resistant Sunflower Hybrid. J. Plant Physiol. 2018, 221, 62–65. [Google Scholar] [CrossRef]
- Abu-Qare, A.W.; Duncan, H.J. Herbicide Safeners: Uses, Limitations, Metabolism, and Mechanisms of Action. Chemosphere 2002, 48, 965–974. [Google Scholar] [CrossRef]
- DeRidder, B.P.; Dixon, D.P.; Beussman, D.J.; Edwards, R.; Goldsbrough, P.B. Induction of Glutathione S -Transferases in Arabidopsis by Herbicide Safeners. Plant Physiol. 2002, 130, 1497–1505. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Yang, A. Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Rosales, M.A.; Romero, L.; Ruiz, J.M. Genotypic Differences in Some Physiological Parameters Symptomatic for Oxidative Stress under Moderate Drought in Tomato Plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- de la Torre-González, A.; Navarro-León, E.; Albacete, A.; Blasco, B.; Ruiz, J.M. Study of Phytohormone Profile and Oxidative Metabolism as Key Process to Identification of Salinity Response in Tomato Commercial Genotypes. J. Plant Physiol. 2017, 216, 164–173. [Google Scholar] [CrossRef]
- Boudet, A.-M. Evolution and Current Status of Research in Phenolic Compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Bellaloui, N.; Brown, P.H. Cultivar Differences in Boron Uptake and Distribution in Celery (Apium graveolens), Tomato (Lycopersicon esculentum) and Wheat (Triticum aestivum). Plant Soil 1998, 198, 153–158. [Google Scholar] [CrossRef]
- Malkawi, H.I.; Al-Quraan, N.A.; Owais, W.M. Acetolactate Synthase Activity and Chlorsulfuron Sensitivity of Gamma-Irradiated Lentil (Lens culinaris Medik.) Cultivars. J. Agric. Sci. 2003, 140, 83–91. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric Oxide Modulates Antioxidant Defense and the Methylglyoxal Detoxification System and Reduces Salinity-Induced Damage of Wheat Seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and Nitric Oxide Crosstalk: Antagonistic Effects on Cadmium Toxicity in Mung Bean Plants through Upregulating the Metal Detoxification, Antioxidant Defense and Methylglyoxal Detoxification Systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to Cold and Heat Stress: Accumulation of Phenolic Compounds in Tomato and Watermelon Plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Turner, N.A. Separation and Estimation of Amino Acids in Crude Plant Extracts by Thin-Layer Electrophoresis and Chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Strasser, R.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 443–480. [Google Scholar]
- Fu, J.; Huang, B. Involvement of Antioxidants and Lipid Peroxidation in the Adaptation of Two Cool-Season Grasses to Localized Drought Stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized Assay for Hydrogen Peroxide Determination in Plant Tissue Using Potassium Iodide. Am. J. Anal. Chem. 2014, 05, 730–736. [Google Scholar] [CrossRef]
- Xiao, H.; He, W.; Fu, W.; Cao, H.; Fan, Z. A Spectrophotometer Method Testing Oxygen Radicals. Prog. Biochem. Biophys. 1999, 26, 180–182. [Google Scholar]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and Ascorbic Acid in Spinach (Spinacia oleracea) Chloroplasts. The Effect of Hydrogen Peroxide and of Paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.A.; Carvajal, M.; López-Berenguer, C.; García-Viguera, C. Chemical and Biological Characterisation of Nutraceutical Compounds of Broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar] [CrossRef] [PubMed]



| H | H + B | p-Value | |
|---|---|---|---|
| Alanine | 145.69 ± 2.30 | 210.92 ± 3.33 | *** |
| Arginine | 37.26 ± 0.59 | 36.13 ± 0.57 | NS |
| Asparagine | ND | ND | |
| Aspartate | 237.33 ± 2.08 | 306.49 ± 4.85 | *** |
| Glutamate | 381.49 ± 6.03 | 430.49 ± 6.81 | *** |
| Glutamine | ND | ND | |
| Glycine | 169.06 ± 2.67 | 195.13 ± 3.09 | *** |
| Histidine | 11.72 ± 0.22 | 11.49 ± 0.21 | NS |
| Isoleucine | 460.64 ± 7.28 | 751.21 ± 11.88 | *** |
| Leucine | 821.21 ± 12.98 | 1113.13 ± 17.60 | *** |
| Lysine | 7.23 ± 0.11 | 7.30 ± 0.12 | NS |
| Phenylalanine | 479.71 ± 7.58 | 485.64 ± 7.68 | NS |
| Proline | 43.20 ± 0.68 | 62.93 ± 1.00 | *** |
| Serine | 635.08 ± 10.04 | 691.70 ± 10.94 | *** |
| Threonine | 42.86 ± 0.68 | 42.63 ± 0.67 | NS |
| Tryptophan | 355.27 ± 5.62 | 361.70 ± 5.72 | NS |
| Tyrosine | 512.92 ± 8.11 | 514.62 ± 8.14 | NS |
| Valine | 1008.64 ± 15.95 | 1475.79 ± 23.33 | *** |
| Methionine | 75.51 ± 1.19 | 75.88 ± 1.20 | NS |
| Cysteine | 7.39 ± 0.13 | 7.23 ± 0.12 | NS |
| MDA (μg g−1 FW) | O2− (μg g−1 FW) | H2O2 (μg g−1 FW) | |
|---|---|---|---|
| H | 9.68 ± 0.49 | 1.64 ± 0.35 | 12.64 ± 1.47 |
| H + B | 8.41 ± 0.35 | 1.08 ± 0.27 | 8.33 ± 1.81 |
| p-value | *** | * | *** |
| GSH (mg g−1 FW) | GST (ΔAbs min−1 mg prot−1) | GPX (ΔAbs min−1 mg prot−1) | Gly I (ΔAbs min−1 mg prot−1) | Gly II (ΔAbs min−1 mg prot−1) | |
|---|---|---|---|---|---|
| H | 0.14 ± 0.02 | 0.32 ± 0.07 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| H + B | 0.27 ± 0.05 | 1.04 ± 0.27 | 0.07 ± 0.01 | 0.15 ± 0.03 | 0.10 ± 0.02 |
| p-value | *** | ** | *** | *** | *** |
| H | H + B | p-Value | |
|---|---|---|---|
| 5-CQA (chlorogenic acid) | 3.17 ± 0.10 | 3.17 ± 0.13 | NS |
| 5-CQA-Isomer | 4.03 ± 0.05 | 4.03 ± 0.05 | NS |
| Di-caffeoylquinic acid | 10.56 ± 2.10 | 10.56 ± 2.02 | NS |
| SA (µg g−1 DW) | 4.03 ± 0.06 | 4.07 ± 0.06 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-León, E.; Borda, E.; Marín, C.; Sierras, N.; Blasco, B.; Ruiz, J.M. Application of an Enzymatic Hydrolysed L-α-Amino Acid Based Biostimulant to Improve Sunflower Tolerance to Imazamox. Plants 2022, 11, 2761. https://doi.org/10.3390/plants11202761
Navarro-León E, Borda E, Marín C, Sierras N, Blasco B, Ruiz JM. Application of an Enzymatic Hydrolysed L-α-Amino Acid Based Biostimulant to Improve Sunflower Tolerance to Imazamox. Plants. 2022; 11(20):2761. https://doi.org/10.3390/plants11202761
Chicago/Turabian StyleNavarro-León, Eloy, Elisabet Borda, Cándido Marín, Nuria Sierras, Begoña Blasco, and Juan M. Ruiz. 2022. "Application of an Enzymatic Hydrolysed L-α-Amino Acid Based Biostimulant to Improve Sunflower Tolerance to Imazamox" Plants 11, no. 20: 2761. https://doi.org/10.3390/plants11202761
APA StyleNavarro-León, E., Borda, E., Marín, C., Sierras, N., Blasco, B., & Ruiz, J. M. (2022). Application of an Enzymatic Hydrolysed L-α-Amino Acid Based Biostimulant to Improve Sunflower Tolerance to Imazamox. Plants, 11(20), 2761. https://doi.org/10.3390/plants11202761







