Phytochemical Characterization of Water Avens (Geum rivale L.) Extracts: Structure Assignment and Biological Activity of the Major Phenolic Constituents
Abstract
:1. Introduction
2. Results
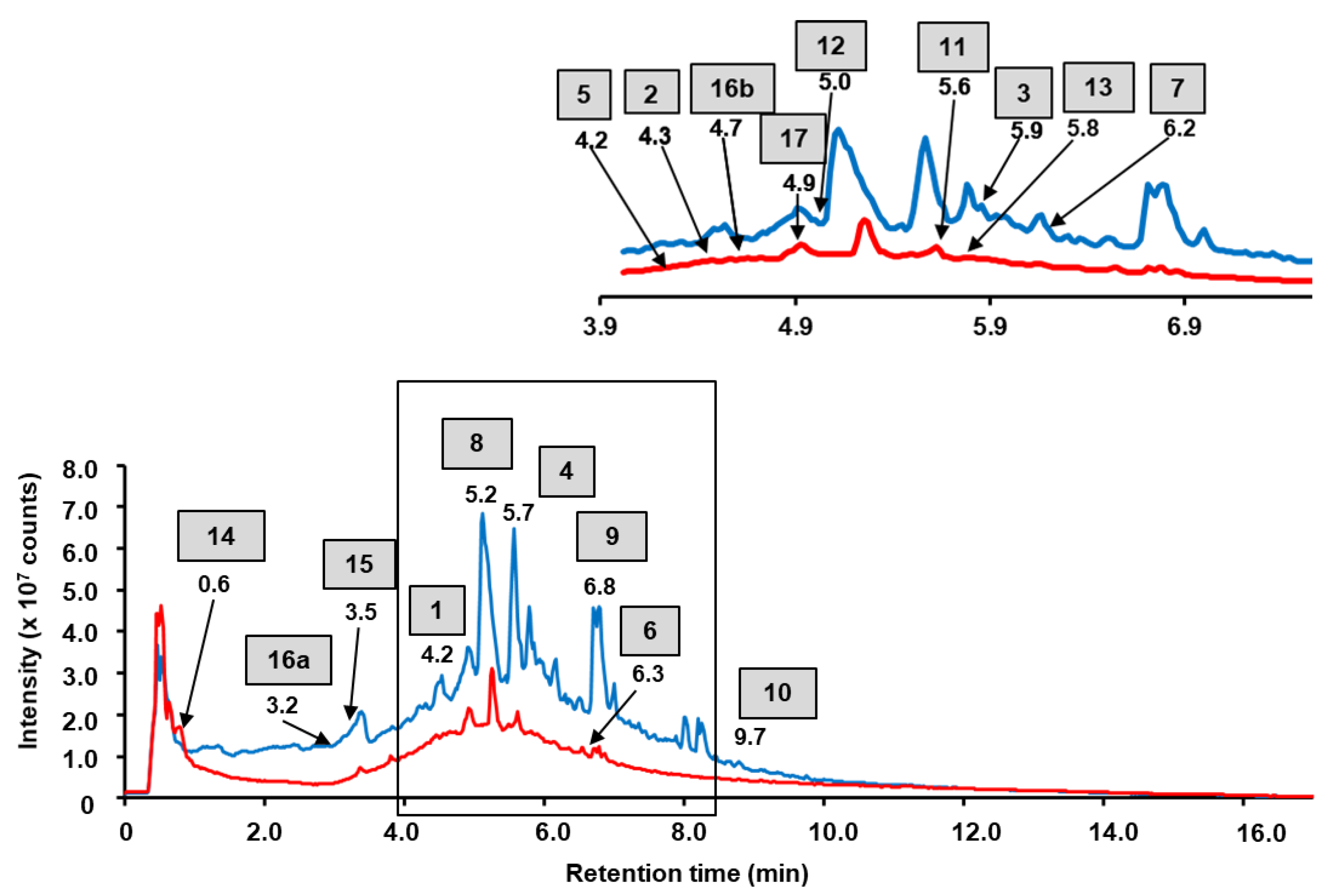
2.1. RP-UHPLC-ESI-MS and MSn
2.1.1. Phenolic Acid Derivatives
2.1.2. Flavonoids and Their Derivatives
2.1.3. Tannins
2.2. Biological Activity of the Aqueous and Ethyl Acetate Fractions of the Total Extract
2.2.1. Antioxidant Effects
2.2.2. Antibacterial Activity
2.2.3. Cytotoxicity Assay
2.2.4. Neuroprotective (Antineurodegenerative) Activity of G. rivale L. Fractions of Total Extracts in a Cellular Model of Alzheimer’s Disease
2.2.5. Neuroprotective Activity of Aqueous and Ethyl Acetate Fractions of the Total G. rivale Ethanolic Extract in a Cellular Model of Parkinson Disease
2.3. Isolation of the Major Phenolic Constituents of the G. rivale L. Fractions of Total Extract in Their Individual Form
2.4. Quantification of the Major Phenolic Constituents in the Aqueous and Ethyl Acetate Fractions of the Total G. rivale Extract
2.5. Biological Activities of Individual Metabolites Isolated from the Aqueous and Ethyl Acetate Fractions of the Total G. rivale L. Extract
2.5.1. Antioxidant Activity of G. rivale L. Fractions of Total Extract and Isolated Compounds
2.5.2. Antibacterial Activity of the Compounds Isolated from G. rivale L.
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Materials
4.3. Extraction, Fractionation, Isolation and Structure Elucidation
4.4. Metabolite Profiling
4.5. Targeted Tandem Mass Spectrometry (MSn) Experiments
4.6. Absolute Quantification of Phenolic Metabolites with Identified Structures
4.7. Antioxidant Assays
4.7.1. DPPH Free Radical Scavenging Effect
4.7.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
4.7.3. Assessment of Extract Capacity to Scavenge Superoxide Anion Radicals (NBT Assay)
4.8. Antibacterial Assays
4.9. Assessment of Anti-Neurodegenerative Effects
4.9.1. Cell Culture
4.9.2. Synthesis and Aggregation of Aβ25–35 Amyloid Peptide
4.9.3. Anti-Alzheimer Assay
4.9.4. Cell Viability Assay of Paraquat Treated SH-SY5Y Cells
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abidullah, S.; Rauf, A.; Khan, S.W.; Ayaz, A.; Liaquat, F.; Saqib, S. A comprehensive review on distribution, paharmacological uses and biological activities of Argyrolobium roseum (Cambess.) Jaub. & Spach. Acta Ecol. Sin. 2021, 42, 198–205. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombe, B.K.; Winand, L.; Diettrich, J.; Töbermann, M.; Hiller, W.; Kaiser, M.; Nett, M. Discovery, biosynthetic origin, and heterologous production of massinidine, an antiplasmodial alkaloid. Org. Lett. 2022, 24, 2935–2939. [Google Scholar] [CrossRef]
- Cuenca-León, K.; Pacheco-Quito, E.-M.; Granda-Granda, Y.; Vélez-León, E.; Zarzuelo-Castañeda, A. Phytotherapy: A Solution to Decrease Antifungal Resistance in the Dental Field. Biomolecules 2022, 12, 789. [Google Scholar] [CrossRef]
- Vaidyanathan, V.; Naidu, V.; Jabed, A.; Tran, K.; Kallingappa, P.; Kao, C.H.-J.; Wang, A.; Karunasinghe, N.; Pallati, R.; Marlow, G.; et al. Modern molecular biology technologies and higher usability of ancient knowledge of medicinal plants for treatment of human diseases. In Plant and Human Health; Ozturk, M., Hakeem, K., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, pp. 173–205. [Google Scholar] [CrossRef]
- Kaur, H.; Mukhtar, H.M.; Singh, A.; Mahajan, A. Antiplasmodial medicinal plants: A literature review on efficacy, selectivity and phytochemistry of crude plant extracts. J. Biol. Act. Prod. Nat. 2019, 8, 272–294. [Google Scholar] [CrossRef]
- Rex, J.R.S.; Muthukumar, N.M.S.A.; Selvakumar, P.M. Phytochemicals as a potential source for anti-microbial, anti-oxidant and wound healing—A review. MOJ Biorg Org Chem. 2018, 2, 61–70. [Google Scholar] [CrossRef]
- Owolabi, T.A.; Ezenwa, K.C.; Olayioye, E.Y.; Iyorhibe, O.C.; Amodu, E.; Aferuan, O.F.; Okubor, P.C.; Ayinde, B.A.; Okogun, J.I. Adaptogenic (anti-stress) effect of aqueous Musanga cecropioides (Urticaceae). Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2558–2565. [Google Scholar] [CrossRef]
- Khatri, S.; Paramanya, A.; Ali, A. Phenolic acids and their health-promoting activity. In Plant and Human Health; Ozturk, M., Hakeem, K., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, pp. 661–680. [Google Scholar] [CrossRef]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Stuppner, H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hano, C.; Tungmunnithum, D. Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Salazar, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perne, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The Effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef] [PubMed]
- Cipolletti, M.; Solar Fernandez, V.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the antioxidant activity of dietary polyphenols in cancer: The modulation of estrogen receptors (ERs) signaling. Int. J. Mol. Sci. 2018, 19, 2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018, 11, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.A.; Sharief, N.H.; Mohamed, Y.S. Hepatoprotective activity of some medicinal plants in Sudan. Evid.-Based Complement. Altern. Med. 2019, 2019, 2196315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birnesser, H.; Stolt, P. The homeopathic antiarthritic preparation Zeel comp. N: A review of molecular and clinical data. EXPLORE 2007, 3, 16–22. [Google Scholar] [CrossRef]
- Orlova, A.A.; Whaley, A.K.; Ponkratova, A.O.; Balabas, O.A.; Smirnov, S.N.; Povydysh, M.N. Two new flavonol-bis-3,7-glucuronides from Geum rivale L. Phytochem. Lett. 2021, 42, 41–44. [Google Scholar] [CrossRef]
- Panizzi, L.; Catalano, S.; Miarelli, C.; Cioni, P.L.; Campeol, E. In vitro antimicrobial activity of extracts and isolated constituents of Geum rivale. Phytother. Res. 2000, 14, 561–653. [Google Scholar] [CrossRef]
- Tunon, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef]
- Owczarek, A.; Gudej, J.; Olszewska, M.A. Antioxidant activity of Geum rivale L. and Geum urbanum L. Acta Pol. Pharm. 2015, 72, 1239–1244. [Google Scholar]
- Spínola, V.; Pinto, J.; Llorent-Martínez, E.J.; Tomás, H.; Castilho, P.C. Evaluation of Rubus grandifolius L. (wild blackberries) activities targeting management of type-2 diabetes and obesity using in vitro models. Food Chem. Toxicol. 2019, 123, 443–452. [Google Scholar] [CrossRef]
- Duckstein, S.M.; Lorenz, P.; Stintzing, F.C. Conversion of Phenolic Constituents in Aqueous Hamamelis virginiana Leaf Extracts During Fermentation. Phytochem. Anal. 2012, 23, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Pukalskienė, M.; Venskutonis, P.R.; Pukalskas, A. Phytochemical composition and antioxidant properties of Filipendula vulgaris as a source of healthy functional ingredients. J. Funct. Foods 2015, 15, 233–242. [Google Scholar] [CrossRef]
- Manurung, J.; Kappen, J.; Schnitzler, J.; Frolov, A.; Wessjohann, L.A.; Agusta, A.; Muellner-Riehl, A.N.; Franke, K. Analysis of unusual sulfated constituents and anti-infective properties of two Indonesian mangroves, Lumnitzera littorea and Lumnitzera racemosa (Combretaceae). Separations 2021, 8, 82. [Google Scholar] [CrossRef]
- Ren, Q.; Li, Y.; Wu, C.; Wang, C.; Jin, Y.; Zhang, J. Metabolism of secondary metabolites isolated from Tartary buckwheat and its extract. Food Chem. 2014, 154, 134–144. [Google Scholar] [CrossRef]
- Bujor, O.-C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.; Al-Saleem, M.S.M.; Basudan, O.A.; Abdel-Mageed, W.M. Flavonoid dimers from the aerial parts of Conyza stricta. Biochem. Syst. Ecol. 2019, 87, 103959. [Google Scholar] [CrossRef]
- March, R.E.; Miao, X.-S. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Montero, L.; Meckelmann, S.W.; Kim, H.; Ayala-Cabrera, J.F.; Schmitz, O.J. Differentiation of industrial hemp strains by their cannabinoid and phenolic compounds using LC × LC-HRMS. Anal. Bioanal. Chem. 2022, 414, 5445–5459. [Google Scholar] [CrossRef]
- Duckstein, S.M.; Lotter, E.M.; Meyer, U.; Lindequist, U.; Stintzing, F.C. Phenolic Constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at Different Dates of Harvest. Z. Naturforsch. C J. Biosci. 2012, 67, 529–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faugno, S.; Piccolella, S.; Sannino, M.; Principio, L.; Crescente, G.; Baldi, G.M.; Fiorentino, N.; Pacifico, S. Can agronomic practices and cold-pressing extraction parameters affect phenols and polyphenols content in hempseed oils? Indl. Crops Prod. 2019, 130, 511–519. [Google Scholar] [CrossRef]
- Su, S.; Guo, J.; Duan, J.; Wang, T.; Qian, D.; Shang, E.; Tang, Y. Ultra-performance liquid chromatography–tandem mass spectrometry analysis of the bioactive components and their metabolites of Shaofu Zhuyu decoction active extract in rat plasma. J. Chromatogr. B 2010, 878, 355–362. [Google Scholar] [CrossRef]
- Silva, L.N.; Rigo, G.V.; Silva, D.B.; Carollo, C.A.; Trentin, D.S.; Silva, M.V.; Macedo, A.J. Hydrolyzable tannins from Poincianella (Caesalpinia) microphylla fruits: Metabolite profiling and anti-Trichomonas vaginalis activity. Food Res. Int. 2020, 134, 109236. [Google Scholar] [CrossRef] [PubMed]
- Finimundy, T.C.; Karkanis, A.; Fernandes, Â.; Petropoulos, S.A.; Calhelha, R.; Petrović, J.; Ferreira, I.C.F.R. Bioactive properties of Sanguisorba minor L. cultivated in central Greece under different fertilization regimes. Food Chem. 2020, 327, 127043. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Li, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1604, 460472. [Google Scholar] [CrossRef]
- Zhu, M.; Dong, X.; Guo, M. Phenolic profiling of Duchesnea indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules 2015, 20, 22463–22475. [Google Scholar] [CrossRef] [Green Version]
- Blinova, K.F. Gravilates as Tannid Plants; Collection of Scientific Works of the Leningrad Chemical-Pharmaceutical Institute; Publishing House of the Leningrad Chemical-Pharmaceutical Institute: Leningrad, Russia, 1957; pp. 80–90. (In Russian) [Google Scholar]
- Lazarev, A.V.; Burchenko, T.V. Chemical composition of seeds of Geum urbanum L. depending on growing conditions. Bull. Krasn. State Agrar. Univ. 2010, 7, 96–100. [Google Scholar]
- Vollmann, C.; Schultze, W. Composition of the root essential oils of several Geum species and related members of the subtribus Geinae (Rosaceae). Flavour Fragr. J. 1995, 10, 173–178. [Google Scholar] [CrossRef]
- Owczarek, A.; Gudej, J.; Kicel, A. Composition of essential oil from aerial and underground parts of Geum rivale and G. urbanum growing in Poland. Nat. Prod. Commun. 2013, 8, 505–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.-R.; Jin, H.-Z.; Qin, J.-J.; Fu, J.-J.; Zhang, W.-D. Chemical constituents of plants from the genus Geum. Chem. Biodivers. 2011, 8, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, A.; Gudej, J. Investigation into biological active constituents of Geum rivale L. Acta Pol. Pharm. 2013, 70, 111–114. [Google Scholar] [PubMed]
- Ming, D.-S.; Jiang, R.-W.; But, P.P.-H.; Towers, G.H.N.; Yu, D.-Q. A new compound from Geum rivale L. J. Asian Nat. Prod. Res. 2002, 4, 217–220. [Google Scholar] [CrossRef]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Moilanen, J.; Koskinen, P.; Salminen, J.-Р. Distribution and content of ellagitannins in Finnish plant species. Phytochemistry 2015, 116, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Nausch, H.; Dorn, M.; Frolov, A.; Hoedtke, S.; Wolf, P.; Broer, I. Direct delivery of health promoting β-Asp-Arg dipeptides via stable co-expression of cyanophycin and the cyanophycinase CphE241 in tobacco plants. Front. Plant. Sci. 2020, 11, 842. [Google Scholar] [CrossRef]
- Owczarek, A.; Olszewska, M.A.; Gudej, J. Quantitative determination of ellagic acid and gallic acid in Geum rivale L. and G. urbanum L. Acta Biol. Crac. Ser. Bot. 2015, 56, 74–78. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Cichoż-Lach, H. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granica, S.; Czerwińska, M.E.; Żyżyńska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Herranz-López, M.; Beltrán-Debón, R.; Borrás-Linares, I.; Barrajón-Catalán, E.; Joven, J.; Fernandez-Gutierrez, A.; Segura-Carreteri, A.; Micol, V. Bioavailability study of a polyphenol-enriched extract from Hibiscus sabdariffain rats and associated antioxidant status. Mol. Nutr. Food Res. 2012, 56, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Hevesi Tóth, B.; Blazics, B.; Kéry, Á. Polyphenol composition and antioxidant capacity of Epilobium species. J. Pharm. Biomed. Anal. 2009, 49, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food. Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- Jabeur, I.; Martins, N.; Barros, L.; Calhelha, R.C.; Vaz, J.; Achour, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Contribution of the phenolic composition to the antioxidant, anti-inflammatory and antitumor potential of Equisetum giganteum L. and Tilia platyphyllos Scop. Food Funct. 2017, 8, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Kang, Q.; Yang, X.; Wu, S.; Ma, Y.; Li, L.; Shen, Y. Chemical constituents from the stem bark of Trewia nudiflora L. and their antioxidant activities. Planta Med. 2008, 74, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Lee, Y.G.; Lee, S.H.; Kim, W.-S.; Park, K.-H.; Moon, J.-H. An ether and three ester derivatives of phenylpropanoid from pear (Pyrus pyrifolia Nakai cv. Chuhwangbae) fruit and their radical-scavenging activity. Food Sci. Biotechnol. 2014, 23, 253–259. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Fu, Z.-M.; Deng, G.; Guo, R.; Chen, D.-F. Free radical scavenging potency of ellagic acid and its derivatives in multiple H+/e− processes. Phytochemistry 2020, 180, 112517. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Panahi, Y.; Javadi, B.; Sehabkar, A. The underlying role of oxidative stress in neurodegeneration: A mechanistic review. CNS Neurol. Disord. Drug Targets 2018, 17, 207–215. [Google Scholar] [CrossRef]
- Zdunić, G.; Aradski, A.A.; Gođevac, D.; Živković, J.; Laušević, S.D.; Milošević, D.K.; Šavikin, K. In vitro hypoglycemic, antioxidant and antineurodegenerative activity of chokeberry (Aronia melanocarpa) leaves. Ind. Crops Prod. 2020, 148, 112328. [Google Scholar] [CrossRef]
- Ayeni, E.A.; Gong, Y.; Yuan, H.; Hu, Y.; Bai, X.; Liao, X. Medicinal plants for anti-neurodegenerative diseases in West Africa. J. Ethnopharmacol. 2021, 285, 114468. [Google Scholar] [CrossRef]
- Ngoungoure, V.L.N.; Mfotie, N.E.; Ngamli, F.S.; Ella, A.F.; McGaw, L.J.; Moundipa, P.F. Acetylcholinesterase inhibitory, anti-inflammatory and antioxidant properties of some Cameroonian medicinal plants used to treat some neurological disorders. Investig. Med. Chem. Pharmacol. 2019, 2, 33. [Google Scholar] [CrossRef]
- Fan, D.; Liu, L.; Wu, Z.; Cao, M. Combating neurodegenerative diseases with the plant alkaloid berberine: Molecular mechanisms and therapeutic potential. Curr. Neuropharmacol. 2019, 17, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y. Novel targets of pentacyclic triterpenoids in Staphylococcus aureus: A systematic review. Phytomedicine 2019, 73, 152933. [Google Scholar] [CrossRef]
- Cruz, A.B.; Cruz, R.C.B.; Kanegusuku, M.; Filho, V.C.; Yunes, F.A.; delle Monache, F.; Niero, R. Antimicrobial activity of Rubus imperialis (Rosaceae). Acta Farm. Bonaer. 2006, 25, 256–259. [Google Scholar]
- Leonova, T.; Popova, V.; Tsarev, A.; Henning, C.; Antonova, K.; Rogovskaya, N.; Vikhnina, M.; Baldensperger, T.; Soboleva, A.; Dinastia, E.; et al. Does protein glycation impact on the drought-related changes in metabolism and nutritional properties of mature pea (Pisum sativum L.) seeds? Int. J. Mol. Sci. 2020, 21, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masci, A.; Mattioli, R.; Costantino, P.; Baima, S.; Morelli, G.; Punzi, P.; Giordano, C.; Pinto, A.; Donini, L.M.; d’Erme, M.; et al. Neuroprotective effect of Brassica oleracea sprouts crude juice in a cellular model of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2015, 2015, 781938. [Google Scholar] [CrossRef] [Green Version]
- Saqib, S.; Nazeer, A.; Ali, M.; Zaman, W.; Younas, M.; Shahzad, A.; Nisar, S.; Nisar, M. Catalytic potential of endophytes facilitates synthesis of biometallic zinc oxide nanoparticles for agricultural application. Biometals 2022, 35, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Approved Standard, M07-A; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018. [Google Scholar]



| # | tR a (min) | [M−H]−obs b (m/z) | [M−H]−calc c (m/z) | EC d | Fragmentation Patterns e | Δm (ppm) | Assignment | Extract Fraction | Ref. f |
|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids derivatives | |||||||||
| 1 | 4.2 | 635.0908 | 635.0890 | C27H25O18 | 169.0125 (5%), 313.0553 (7%), 465.0676 (20%), 483.0735 (4%), 635.0908 (100%) | −2.6 | Trigalloyl hexose | EA | [25,26,27] |
| 2 | 4.3 | 457.0907 | 457.0988 | C19H21O13 | 179.0333 (100%), 341.0802 (70%), 457.0907 (30%) | 17.7 | Caffeoyl-hexoside malate | A | [25] |
| 3 | 5.9 | 300.9959 | 300.9999 | C14H6O8 | 117.0333 (9%), 145.0282 (8%), 173.0226 (15%), 185.0230 (20%), 201.0170 (20%), 229.0110 (23%), 245.0060 (18%), 283.9932 (40%), 299.9874 (42%), 300.9959 (100%) | 10 | Ellagic acid | EA | [28] |
| 4 | 5.7 | 433.0366 | 433.0412 | C19H14O12 | 299.9888 (40%), 300.9965 (100%), 313.0684 (25%), 343.0769 (10%), 433.0359 (50%) | 10.6 | Ellagic acid pentoside | EA | [25] |
| Flavonoids and their derivatives | |||||||||
| 5 | 4.2 | 653.0976 | 653.0996 | C27H26O19 | 301.0343 (10%), 477.0649 (13%), 653.0976 (100%) | 2.6 | Quercetin-bis-hexuronide | A | [27] |
| 6 | 6.3 | 477.0638 | 477.0675 | C21H18O13 | 301.0376 (100%), 477.0638 (5%) | 5.6 | Quercetin-hexuronide | A | [25,29] |
| 7 | 6.2 | 953.1265 | 953.1266 | C42H34O26 | 301.0336 (5%), 477.0647 (100%), 953.1265 (70%) | −6.8 | Quercetin-hexuronide dimer | EA | [30] |
| 8 | 5.2 | 637.1003 | 637.1046 | C27H27O18 | 285.0384 (17%), 461.0686 (45%), 637.1003 (100%) | −1.0 | Kaempferol-bis-hexuronide | EA | [20] |
| 9 | 6.8 | 923.1519 | 923.1523 | C42H35O24 | 285.0394 (10%), 461.0708 (100%), 923.1519 (70%) | 4.2 | Dihydrokaempferol-kaempferol-hexuronide dimer | EA | [30] |
| 10 | 9.7 | 285.0344 | 285.0405 | C15H10O6 | 93.0333 (22%), 108.0190 (10%), 117.0321 (8%), 154.0374 (9%), 159.0433 (15%), 169.0626 (10%), 185.0576 (25%), 187.0360 (17%), 211.0351 (10%), 227.0295 (13%), 239.0295 (15%), 285.0344 (100%) | 21.7 | Kaempferol | EA | [31] |
| 11 | 5.6 | 461.0692 | 461.0725 | C21H18O22 | 285.0385 (100%), 461.0692 (15%) | 3.2 | Kaempferol-hexuronide | A | [32,33] |
| 12 | 5.0 | 667.1106 | 667.1152 | C28H28O19 | 315.0487 (15%), 491.0797 (30%), 667.1106 (100%) | 6.8 | Isorhamnetin-bis-hexuronide | A | [20] |
| 13 | 5.8 | 491.0818 | 491.0831 | C22H20O13 | 300.0261 (10%), 315.0501 (100%), 491.0818 (6%), | 2.6 | Isorhamnetin-hexuronide | A | [34,35] |
| Tannins | |||||||||
| 14 | 0.6 | 481.0615 | 481.0624 | C20H19O14 | 300.9988 (70%), 481.0606 (100%), 275.0195 (40%), 133.0143 (20%) | 1.9 | HHDP-hexoside | EA | [25,36,37] |
| 15 | 3.5 | 577.1263 | 577.1351 | C30H26O12 | 289.0673 (16%), 407.0704 (18%), 425.0803 (30%), 577.1263 (100%) | 15.3 | Proantocyanidin dimer | EA | [25,38] |
| 16a | 3.2 | 783.0634 | 783.0686 | C34H25O22 | 300.9967 (4%), 783.0634 (100%) | 6.6 | Pedunculagin I | EA | [25,36,37] |
| 16b | 4.7 | 783.0680 | 783.0686 | C34H25O22 | 300.9982 (100%), 633.0714 (75%), 783.0680 (100%) | 0.7 | Pedunculagin I | A | [25,36,37] |
| 17 | 4.9 | 935.0780 | 935.0796 | C41H28O26 | 300.9988 (30%), 767.0745 (2%), 935.0780 (100%) | 1.7 | Galloyl-bis-HHDP-hexose | A | [25,36,37] |
| Plant Isolate | DPPH Normalized Activity, % | TEAC, μmol/L Trolox eq./μg | NBT Assay, nmol of O2•−/min |
|---|---|---|---|
| Aqueous fraction | 98.23 ± 1.11 | 3.70 ± 0.01 | 3.22 ± 0.48 |
| Ethyl acetate fraction | 94.65 ± 0.29 | 0.03 ± 0.02 | 6.01 ± 0.46 |
| Microorganism Strain | Activity (MICs, µg/mL) | |
|---|---|---|
| Aqueous Fraction | Ethyl Acetate Fraction | |
| Escherichia coli ATCC 25922 | 62.5 | 15.6 |
| Pseudomonas aeruginosa ATCC 27853 | 62.5 | 31.2 |
| Staphylococcus aureus SG-511 | 31.2 | 15.6 |
| Staphylococcus aureus ATCC 25923 | 62.5 | 15.6 |
| MRSA ATCC 33591 | 62.5 | 31.2 |
| Micrococcus luteus CIP A270 | >250 | 62.5 |
| Listeria monocytogenes EGD | >250 | 62.5 |
| Major Phenolic Constituents | Contents (µg/mg d.w.) | |
|---|---|---|
| Ethyl Acetate Fraction | Aqueous Fraction | |
| Isorhamnetin-3-O-β-D-glucuronide | 13.74 ± 1.22 | 1.40 ± 0.22 |
| Kaempferol-3-O-β-D-glucuronide | 6.33 ± 0.37 | 0.80 ± 0.16 |
| Isorhamnetin-bis-3,7-O-β-D-glucuronide | 0.48 ± 0.04 | 1.88 ± 0.07 |
| Kaempferol-bis-3,7-O-β-D-glucuronide | 0.23 ± 0.03 | 1.22 ± 0.16 |
| 6″-(4-Hydroxycinnamoyl)-astragalin | 9.76 ± 1.75 | b.l.q. |
| Caffeoyl malate | 16.44 ± 0.38 | 8.25 ± 0.19 |
| 3-O-Methylellagic acid | 39.23 ± 1.45 | 0.0043 ± 0.0007 |
| Compound | DPPH Normalized Activity, % | TEAC, μmol/L Trolox eq./μg | NBT Assay, nmol of O2•−/min |
|---|---|---|---|
| Kaempferol-3-O-β-D-glucuronide | 76.452 ± 0.890 | 0.519 ± 0.057 | 7.667 ± 0.96 |
| Isorhamnetin-bis-3,7-O-β-D-glucuronide | 75.557 ± 1.784 | 0.611 ± 0.039 | 3.477 ± 0.576 |
| 3-O-Methylellagic acid | 93.675 ± 0.890 | 2.963 ± 0.024 | 6.027 ± 0.870 |
| 6″-(4-hydroxycinnamoyl)-astragalin | 80.827 ± 1.250 | 0.796 ± 0.024 | 1.813 ± 0.540 |
| Kaempferol-bis-3,7-O-β-D-glucuronide | 99.483 ± 2.512 | 0.815 ± 0.029 | 4.649 ± 0.598 |
| Isorhamnetin-3-O-β-D-glucuronide | 86.993 ± 1.867 | 0.019 ± 0.014 | 7.387 ± 0.563 |
| Caffeoyl malate | 80.469 ± 4.012 | 3.074 ± 0.115 | 7.076 ± 0.890 |
| DMSO (negative control) | - | - | 8.373 ± 0.076 |
| Microorganism Strain | Minimal Inhibitory Concentrations (MICs, µg/mL) | |||||
|---|---|---|---|---|---|---|
| 11 | 12 | 3-O-Methylellagic Acid | 8 | 13 | Caffeoyl Malate | |
| Escherichia coli ATCC 25922 | 500 | >500 | 125 | 500 | >125 | 500 |
| Pseudomonas aeruginosa ATCC 27853 | 250 | 250 | 62.5 | 250 | 125 | 125 |
| Staphylococcus aureus SG-511 | 500 | 250 | 31.2 | 250 | >125 | 125 |
| Staphylococcus aureus ATCC 25923 | 500 | >500 | 62.5 | >500 | >125 | 250 |
| Staphylococcus aureus MRSA ATCC 33591 | 500 | 500 | 250 | 500 | >125 | 500 |
| Micrococcus luteus CIP A270 | >500 | >500 | 250 | >500 | >125 | >500 |
| Listeria monocytogenes EGD | >500 | >500 | 125 | >500 | >125 | >500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlova, A.; Kysil, E.; Tsvetkova, E.; Meshalkina, D.; Whaley, A.; Whaley, A.O.; Laub, A.; Francioso, A.; Babich, O.; Wessjohann, L.A.; et al. Phytochemical Characterization of Water Avens (Geum rivale L.) Extracts: Structure Assignment and Biological Activity of the Major Phenolic Constituents. Plants 2022, 11, 2859. https://doi.org/10.3390/plants11212859
Orlova A, Kysil E, Tsvetkova E, Meshalkina D, Whaley A, Whaley AO, Laub A, Francioso A, Babich O, Wessjohann LA, et al. Phytochemical Characterization of Water Avens (Geum rivale L.) Extracts: Structure Assignment and Biological Activity of the Major Phenolic Constituents. Plants. 2022; 11(21):2859. https://doi.org/10.3390/plants11212859
Chicago/Turabian StyleOrlova, Anastasia, Elana Kysil, Elena Tsvetkova, Darya Meshalkina, Andrei Whaley, Anastasiia O. Whaley, Annegret Laub, Antonio Francioso, Olga Babich, Ludger A. Wessjohann, and et al. 2022. "Phytochemical Characterization of Water Avens (Geum rivale L.) Extracts: Structure Assignment and Biological Activity of the Major Phenolic Constituents" Plants 11, no. 21: 2859. https://doi.org/10.3390/plants11212859








